9 4 OxidationReduction Reactions An oxidationreduction or redox



- Slides: 22

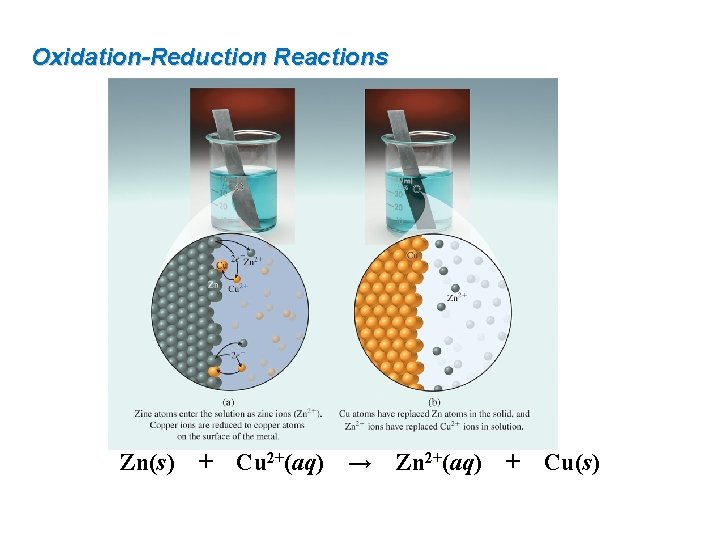
9. 4 Oxidation-Reduction Reactions An oxidation-reduction (or redox) reaction is a chemical reaction in which electrons are transferred from one reactant to another. Oxidation is the loss of electrons. Reduction is the gain of electrons. Zn metal loses 2 electrons and is oxidized to Zn 2+ is called the reducing agent Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu 2+ gains 2 electrons and is reduced to Cu metal Cu is called the oxidizing agent Cu(s)

Oxidation-Reduction Reactions Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s)

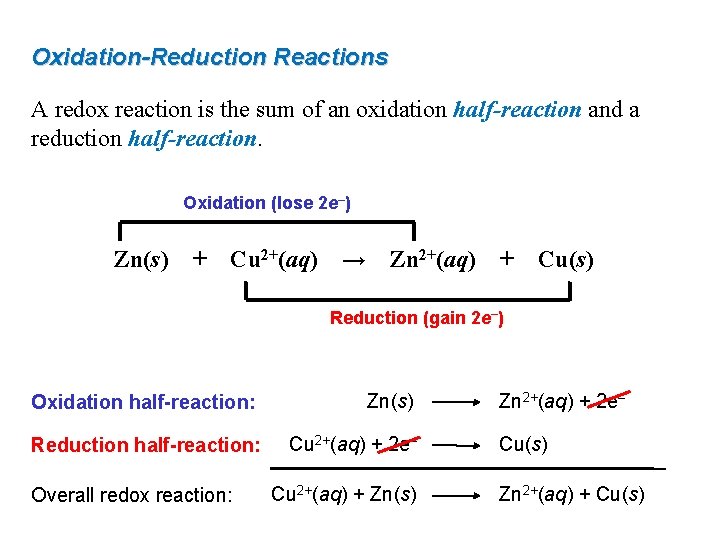
Oxidation-Reduction Reactions A redox reaction is the sum of an oxidation half-reaction and a reduction half-reaction. Oxidation (lose 2 e–) Zn(s) + Cu 2+(aq) → Zn 2+(aq) + Cu(s) Reduction (gain 2 e–) Oxidation half-reaction: Zn(s) Reduction half-reaction: Cu 2+(aq) + 2 e– Overall redox reaction: Cu 2+(aq) + Zn(s) Zn 2+(aq) + 2 e– Cu(s) Zn 2+(aq) + Cu(s)

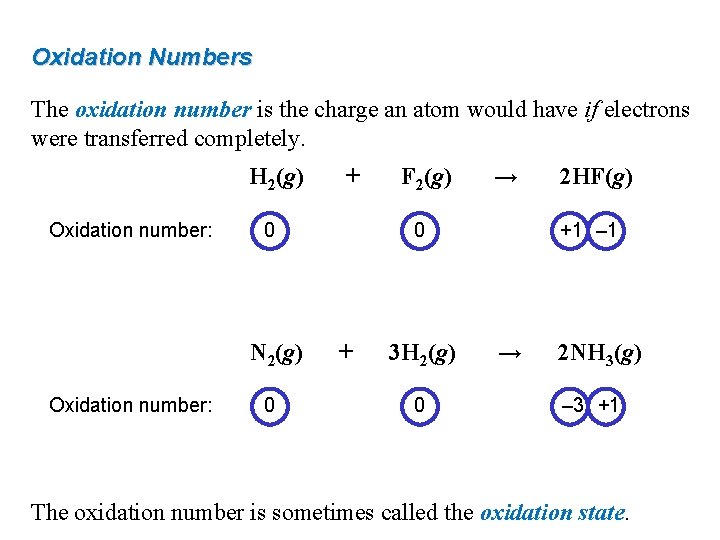
Oxidation Numbers The oxidation number is the charge an atom would have if electrons were transferred completely. H 2(g) Oxidation number: 0 N 2(g) Oxidation number: + 0 F 2(g) → 0 + 3 H 2(g) 0 2 HF(g) +1 – 1 → 2 NH 3(g) – 3 +1 The oxidation number is sometimes called the oxidation state.

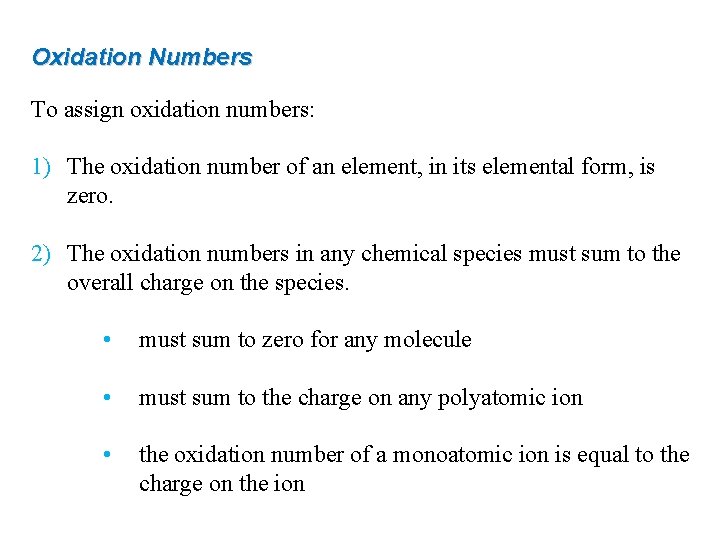
Oxidation Numbers To assign oxidation numbers: 1) The oxidation number of an element, in its elemental form, is zero. 2) The oxidation numbers in any chemical species must sum to the overall charge on the species. • must sum to zero for any molecule • must sum to the charge on any polyatomic ion • the oxidation number of a monoatomic ion is equal to the charge on the ion

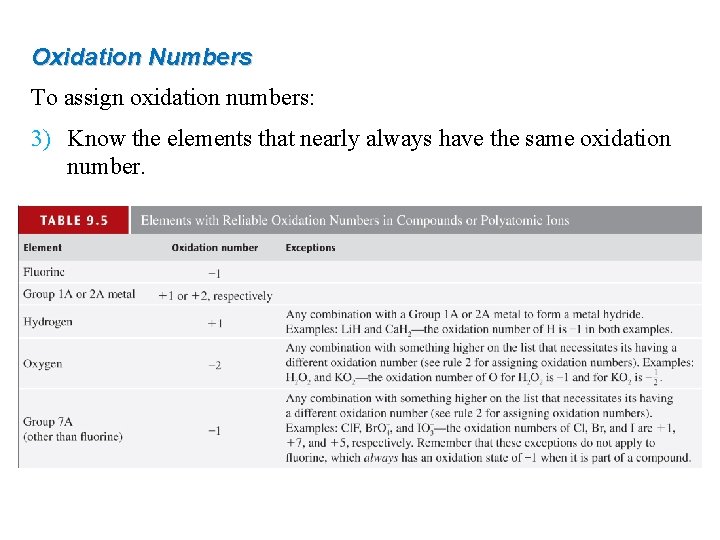
Oxidation Numbers To assign oxidation numbers: 3) Know the elements that nearly always have the same oxidation number.

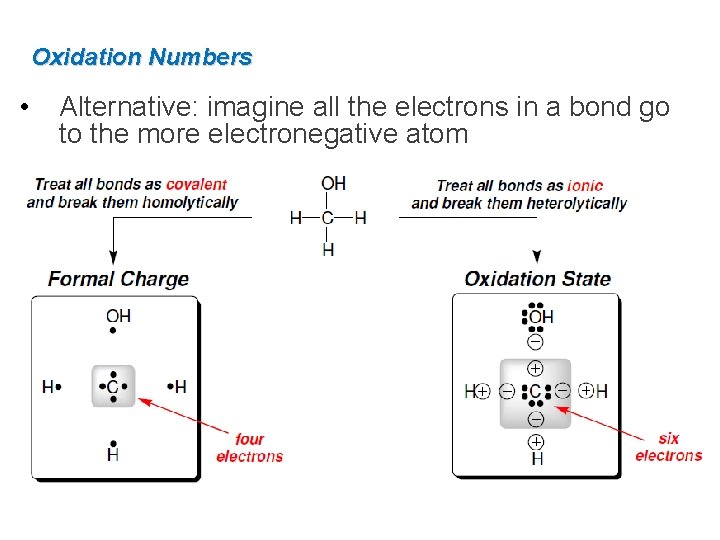
Oxidation Numbers • Alternative: imagine all the electrons in a bond go to the more electronegative atom

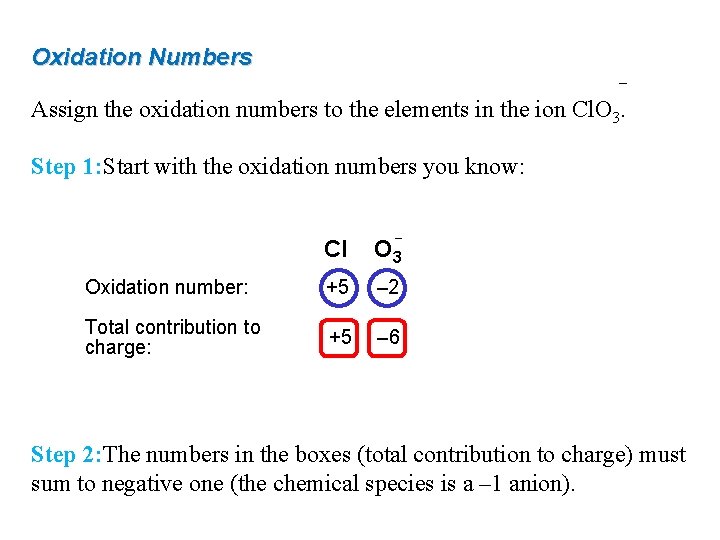
Oxidation Numbers – Assign the oxidation numbers to the elements in the ion Cl. O 3. Step 1: Start with the oxidation numbers you know: – Cl O 3 Oxidation number: +5 – 2 Total contribution to charge: +5 – 6 Step 2: The numbers in the boxes (total contribution to charge) must sum to negative one (the chemical species is a – 1 anion).

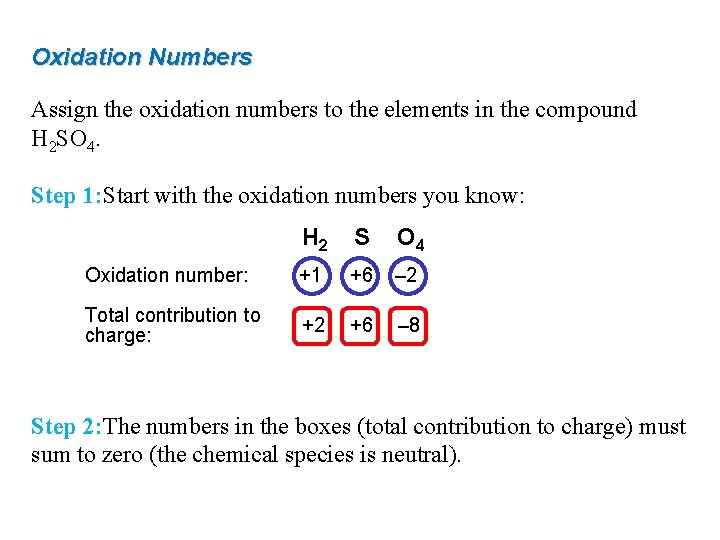
Oxidation Numbers Assign the oxidation numbers to the elements in the compound H 2 SO 4. Step 1: Start with the oxidation numbers you know: H 2 S O 4 Oxidation number: +1 +6 – 2 Total contribution to charge: +2 +6 – 8 Step 2: The numbers in the boxes (total contribution to charge) must sum to zero (the chemical species is neutral).

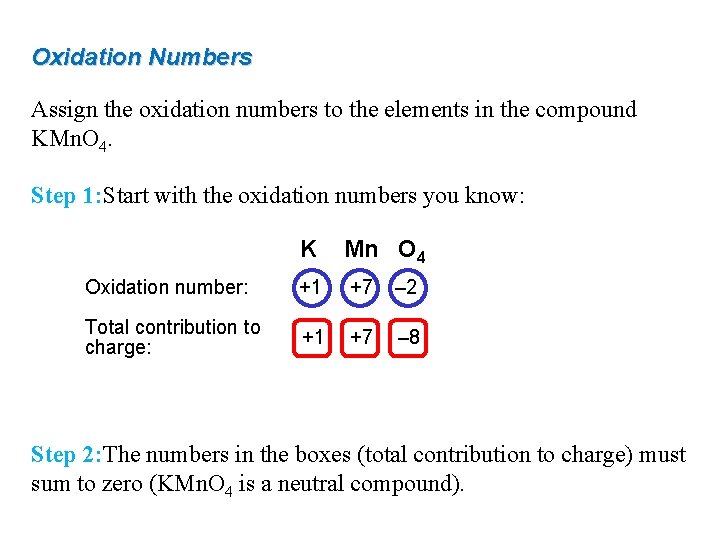
Oxidation Numbers Assign the oxidation numbers to the elements in the compound KMn. O 4. Step 1: Start with the oxidation numbers you know: K Mn O 4 Oxidation number: +1 +7 – 2 Total contribution to charge: +1 +7 – 8 Step 2: The numbers in the boxes (total contribution to charge) must sum to zero (KMn. O 4 is a neutral compound).

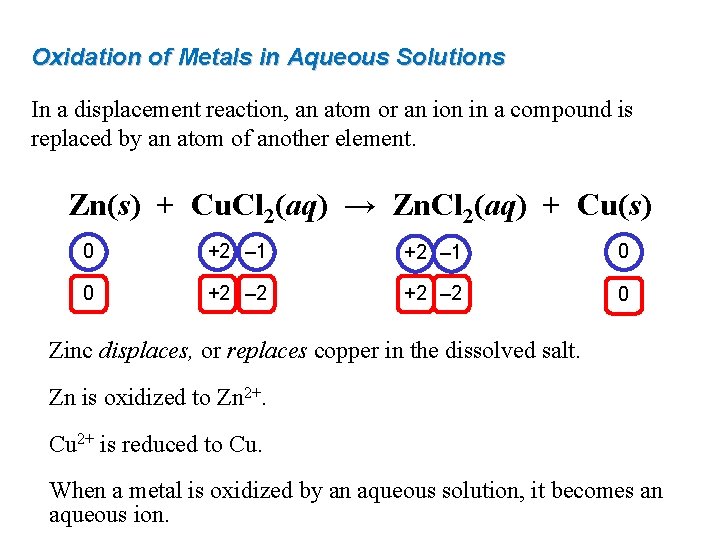
Oxidation of Metals in Aqueous Solutions In a displacement reaction, an atom or an ion in a compound is replaced by an atom of another element. Zn(s) + Cu. Cl 2(aq) → Zn. Cl 2(aq) + Cu(s) 0 +2 – 1 0 0 +2 – 2 0 Zinc displaces, or replaces copper in the dissolved salt. Zn is oxidized to Zn 2+. Cu 2+ is reduced to Cu. When a metal is oxidized by an aqueous solution, it becomes an aqueous ion.

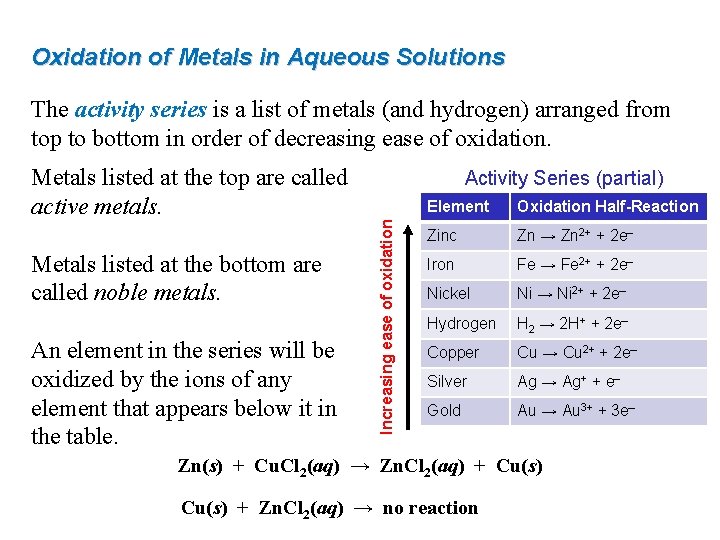
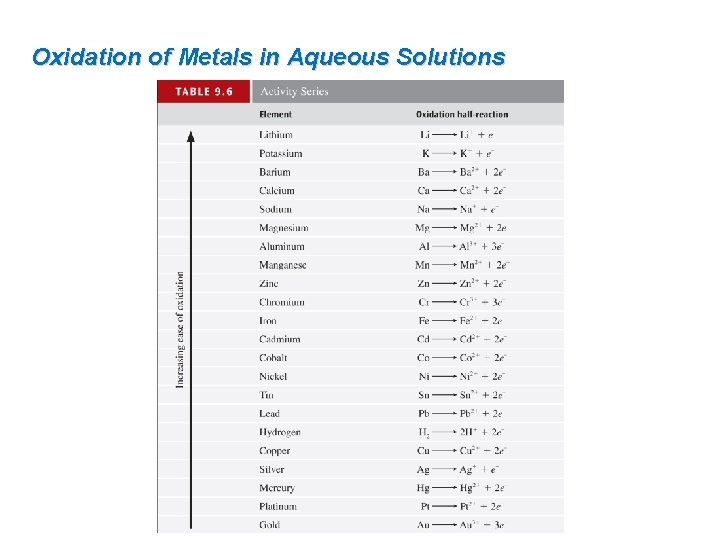
Oxidation of Metals in Aqueous Solutions The activity series is a list of metals (and hydrogen) arranged from top to bottom in order of decreasing ease of oxidation. Metals listed at the top are called active metals. An element in the series will be oxidized by the ions of any element that appears below it in the table. Increasing ease of oxidation Metals listed at the bottom are called noble metals. Activity Series (partial) Element Oxidation Half-Reaction Zinc Zn → Zn 2+ + 2 e– Iron Fe → Fe 2+ + 2 e– Nickel Ni → Ni 2+ + 2 e– Hydrogen H 2 → 2 H+ + 2 e– Copper Cu → Cu 2+ + 2 e– Silver Ag → Ag+ + e– Gold Au → Au 3+ + 3 e– Zn(s) + Cu. Cl 2(aq) → Zn. Cl 2(aq) + Cu(s) + Zn. Cl 2(aq) → no reaction

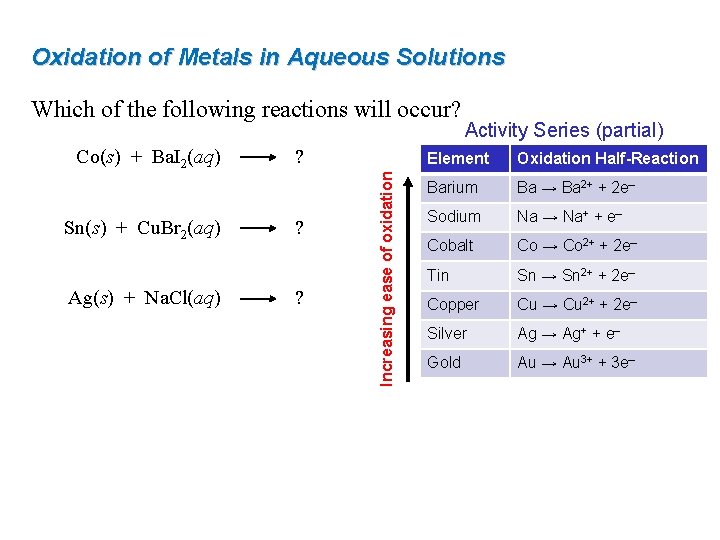
Oxidation of Metals in Aqueous Solutions Which of the following reactions will occur? Sn(s) + Cu. Br 2(aq) Ag(s) + Na. Cl(aq) ? ? ? Increasing ease of oxidation Co(s) + Ba. I 2(aq) Activity Series (partial) Element Oxidation Half-Reaction Barium Ba → Ba 2+ + 2 e– Sodium Na → Na+ + e– Cobalt Co → Co 2+ + 2 e– Tin Sn → Sn 2+ + 2 e– Copper Cu → Cu 2+ + 2 e– Silver Ag → Ag+ + e– Gold Au → Au 3+ + 3 e–

Oxidation of Metals in Aqueous Solutions

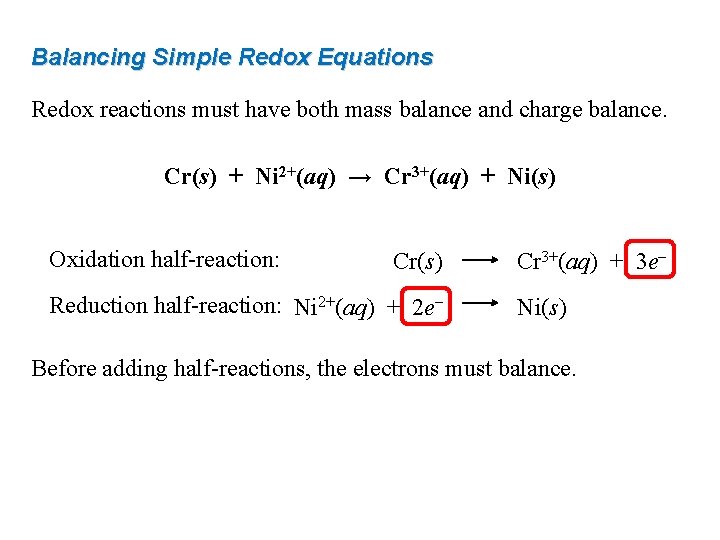
Balancing Simple Redox Equations Redox reactions must have both mass balance and charge balance. Cr(s) + Ni 2+(aq) → Cr 3+(aq) + Ni(s) Oxidation half-reaction: Cr(s) Reduction half-reaction: Ni 2+(aq) + 2 e– Cr 3+(aq) + 3 e– Ni(s) Before adding half-reactions, the electrons must balance.

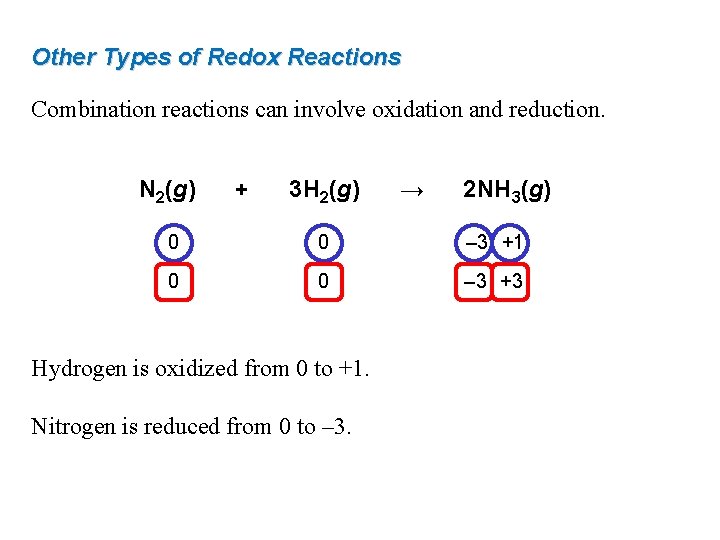
Other Types of Redox Reactions Combination reactions can involve oxidation and reduction. N 2(g) + 3 H 2(g) → 2 NH 3(g) 0 0 – 3 +1 0 0 – 3 +3 Hydrogen is oxidized from 0 to +1. Nitrogen is reduced from 0 to – 3.

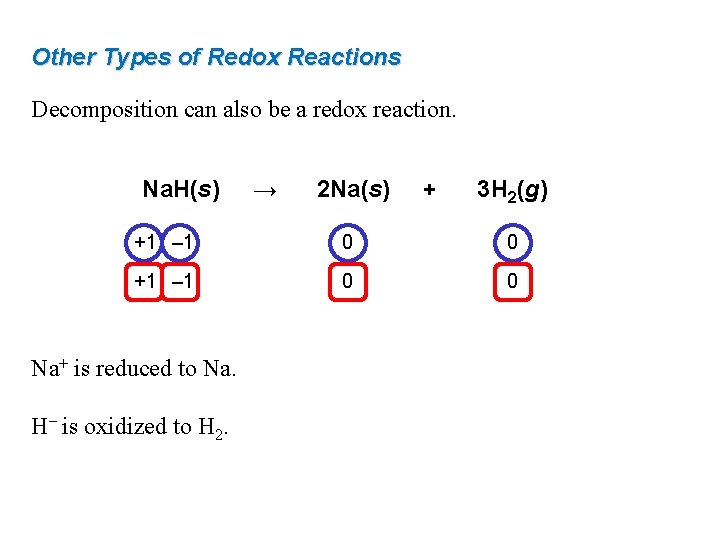
Other Types of Redox Reactions Decomposition can also be a redox reaction. Na. H(s) → 2 Na(s) + 3 H 2(g) +1 – 1 0 0 Na+ is reduced to Na. H– is oxidized to H 2.

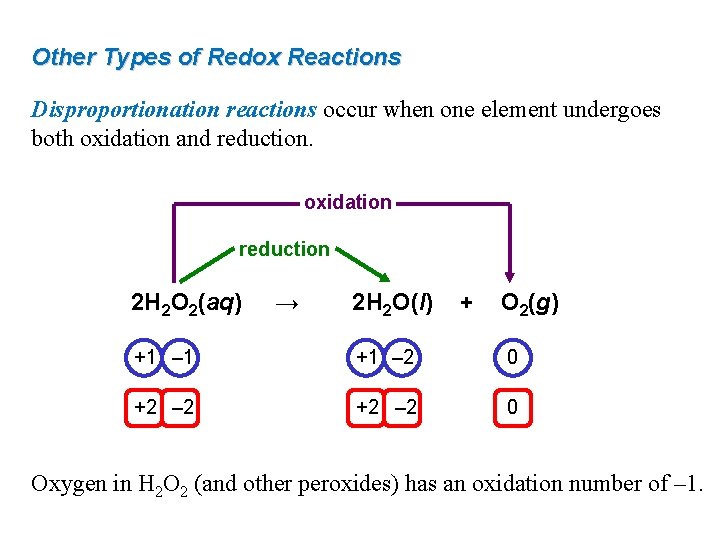
Other Types of Redox Reactions Disproportionation reactions occur when one element undergoes both oxidation and reduction. oxidation reduction 2 H 2 O 2(aq) → 2 H 2 O(l) + O 2(g) +1 – 1 +1 – 2 0 +2 – 2 0 Oxygen in H 2 O 2 (and other peroxides) has an oxidation number of – 1.

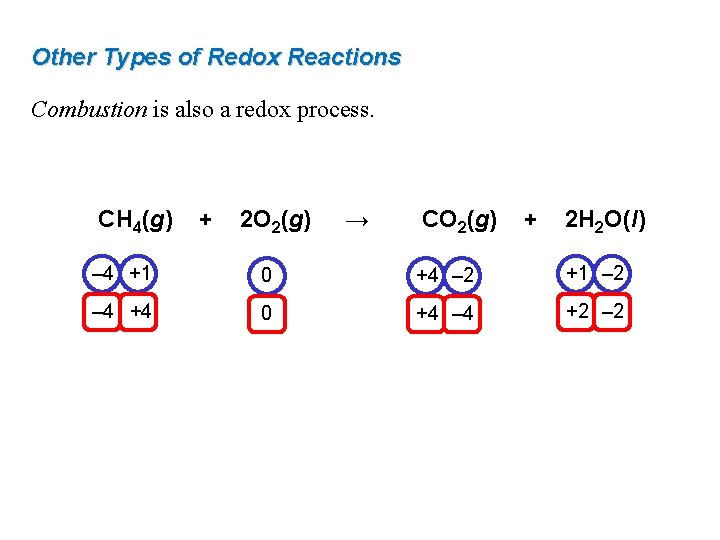
Other Types of Redox Reactions Combustion is also a redox process. CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) – 4 +1 0 +4 – 2 +1 – 2 – 4 +4 0 +4 – 4 +2 – 2

Study Guide for Section 9. 4 DAY 24, Terms to know: Section 9. 4 oxidation, reduction, redox, half-reaction, oxidation number or oxidation state, activity series DAY 24, Specific outcomes and skills that may be tested on exam 4: • Given a redox reaction, be able to identify which reactant is oxidized and which is reduced, and be able to identify which reactant is the oxidizing agent and which is the reducing reagent • Given a simple redox reaction, be able to write two correct half reactions that add together to give the net equation • Given a compound, be able to determine the oxidation number for each atom in the formula • Given a reaction, be able to determine how the oxidation number changes or remains the same for each atom involved • Be able to use the activity series to predict products in redox reactions and whether the reaction will be product or reactant favored

Extra Practice Problems for Section 9. 4 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 9. 43 9. 45 9. 47 9. 49 9. 147 9. 157

Prep for Day 25 Must Watch videos: https: //www. youtube. com/watch? v=AN 4 Kif. V 12 DA (aq solutions, crash course chemistry) http: //www. youtube. com/watch? v=0 c. PFx 0 w. Fu. Vs (solvation, Tyler De. Witt) https: //www. youtube. com/watch? v=cn. STs. Lvyu. Ok (dissolving, Tyler De. Witt) https: //www. youtube. com/watch? v=wxv. ERNl. Ud. BQ (equations, Bozeman) https: //www. youtube. com/watch? v=IIu 16 dy 3 Th. I (ppt reactions, crash course chemistry) Other helpful videos: http: //www. learnerstv. com/video/Free-video-Lecture-29341 -Chemistry. htm (solutions, Bozeman) http: //www. youtube. com/watch? v=gxmpr 09 Xk 4 Y (solvation, Brightstorm) https: //www. youtube. com/watch? v=9 h 2 f 1 Bjr 0 p 4 (solutions, crash course chem) https: //www. youtube. com/watch? v=Q 8 cx. Pof. GKCM (solubility, Isaacs) http: //ps. uci. edu/content/chem-1 p-preparation-general-chemistry (UC-Irvine, lectures 16 -17) Read Sections 13. 2, 13. 1, 13. 4, 9. 1 -9. 2