Redox Reactions Chapter 4 Redox Reactions l OxidationReduction






- Slides: 14

Redox Reactions Chapter 4

Redox Reactions l Oxidation-Reduction (Redox) Reactions l Redox reactions involve an electron transfer. l These reaction occur between metals and nonmetals. l Ionic reactions are redox reactions.

The name l There are two parties in a redox reaction. l The one that is gaining electron(s). l This one gets reduced. l Think of the charge, it gets lower. l The one that is losing electron(s). l It gets oxidized.

Oil Rig l Oxidation l Is l Losing an electron l Reduction l Is l Gaining an electron

What makes this a redox reaction? l Follow the electron l Ag. Cl + Cu Ag + Cu. Cl l Ag+ + Cl- + Cu Ag + Cl- +Cu+ e- l Copper starts out neutral, Ag is +, Cl is -. The compound is neutral but they still have their charges l Then the reaction. l Copper gives an electron to silver making silver neutral, and copper positive.

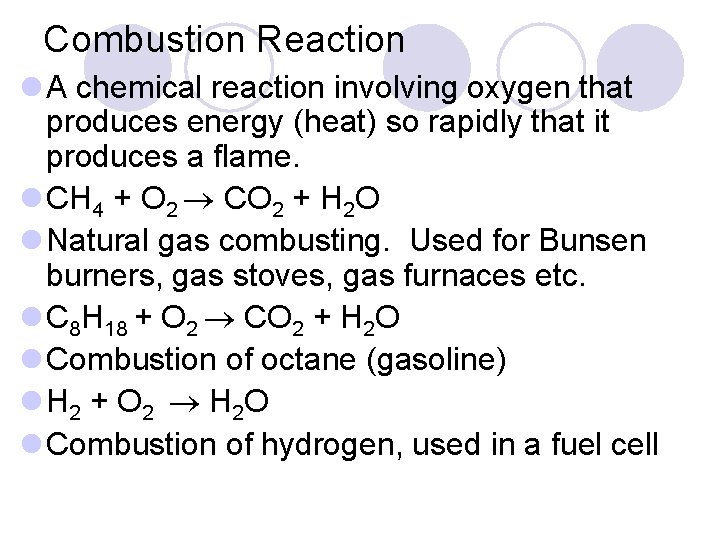
Combustion Reaction l A chemical reaction involving oxygen that produces energy (heat) so rapidly that it produces a flame. l CH 4 + O 2 CO 2 + H 2 O l Natural gas combusting. Used for Bunsen burners, gas stoves, gas furnaces etc. l C 8 H 18 + O 2 CO 2 + H 2 O l Combustion of octane (gasoline) l H 2 + O 2 H 2 O l Combustion of hydrogen, used in a fuel cell

4 general forms of reactions l Almost all reactions can fit into one of 4 general forms l Single replacement l Double replacement l Synthesis l Decomposition

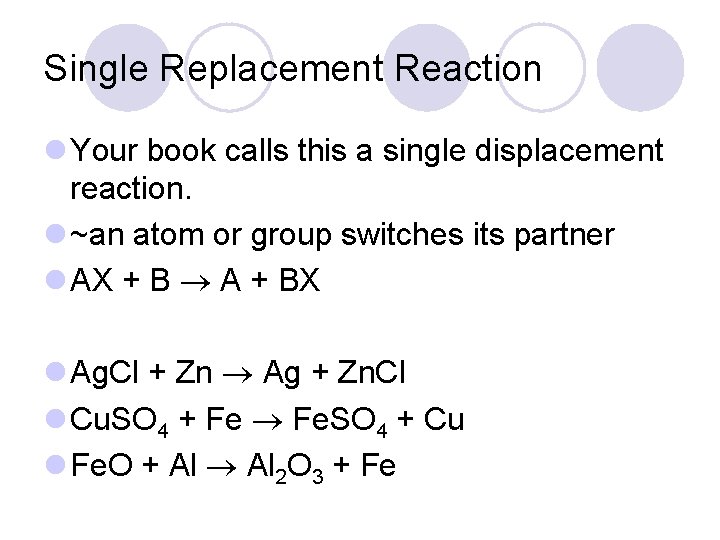
Single Replacement Reaction l Your book calls this a single displacement reaction. l ~an atom or group switches its partner l AX + B A + BX l Ag. Cl + Zn Ag + Zn. Cl l Cu. SO 4 + Fe Fe. SO 4 + Cu l Fe. O + Al 2 O 3 + Fe

Double Replacement Reaction l ~two atoms or groups switches their partners l AX + BY AY + BX l 2 Ni. NO 3 + Ca. SO 4 Ca(NO 3)2 + Ni 2 SO 4 l Na. Cl + Ag. I Na. I + Ag. Cl l All of the precipitation reactions/net ionic equations we have been doing have been double replacement reactions.

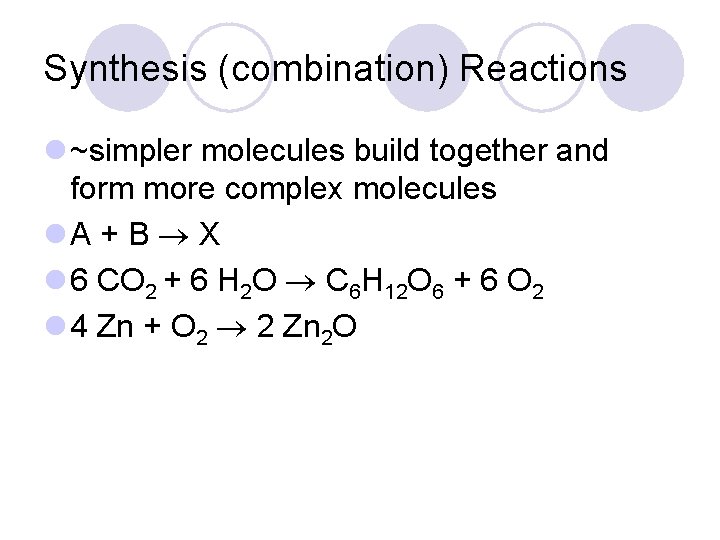
Synthesis (combination) Reactions l ~simpler molecules build together and form more complex molecules l. A + B X l 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 l 4 Zn + O 2 2 Zn 2 O

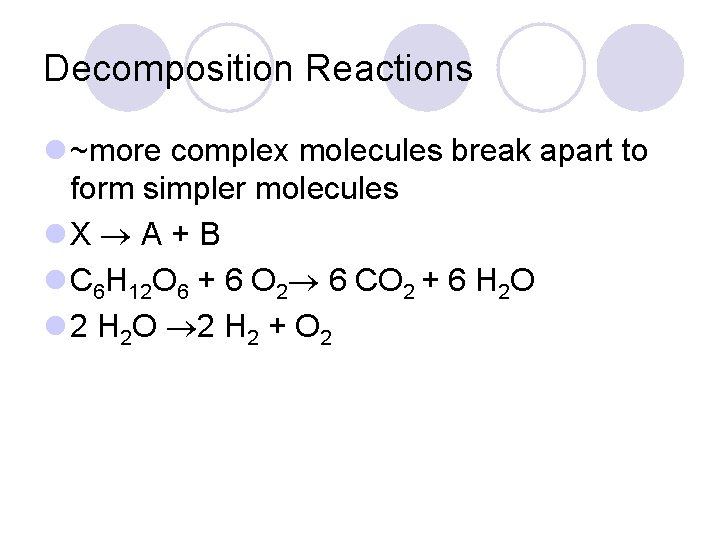
Decomposition Reactions l ~more complex molecules break apart to form simpler molecules l. X A + B l C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O l 2 H 2 O 2 H 2 + O 2

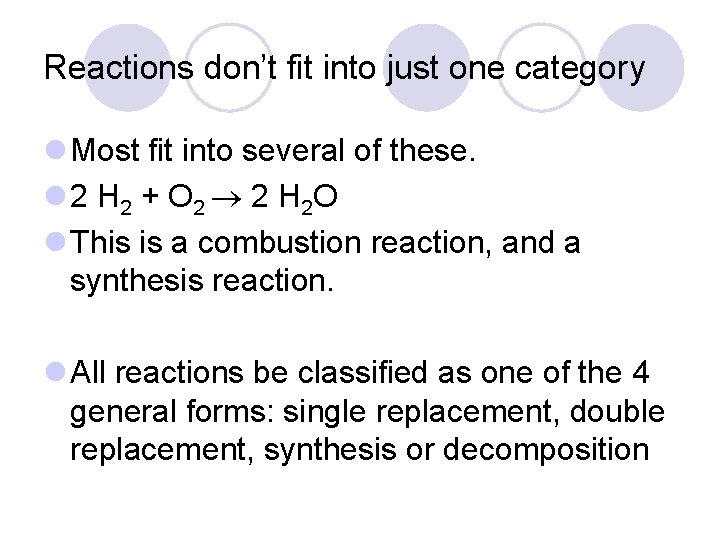
Reactions don’t fit into just one category l Most fit into several of these. l 2 H 2 + O 2 2 H 2 O l This is a combustion reaction, and a synthesis reaction. l All reactions be classified as one of the 4 general forms: single replacement, double replacement, synthesis or decomposition

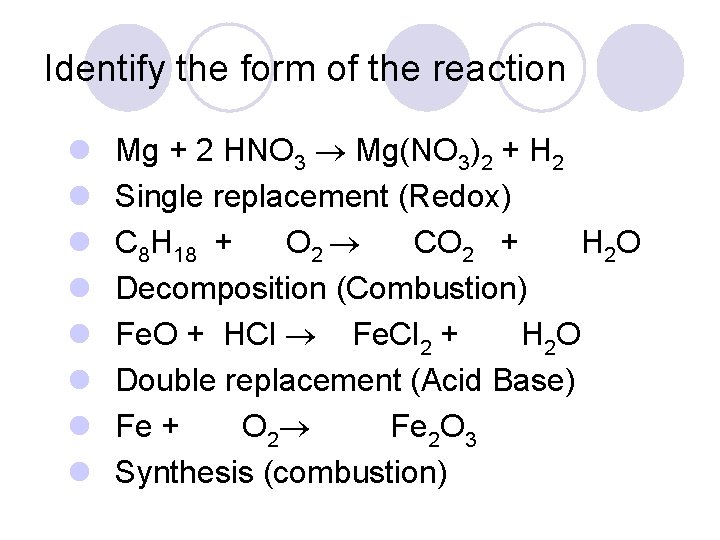
Identify the form of the reaction l l l l Mg + 2 HNO 3 Mg(NO 3)2 + H 2 Single replacement (Redox) C 8 H 18 + O 2 CO 2 + H 2 O Decomposition (Combustion) Fe. O + HCl Fe. Cl 2 + H 2 O Double replacement (Acid Base) Fe + O 2 Fe 2 O 3 Synthesis (combustion)

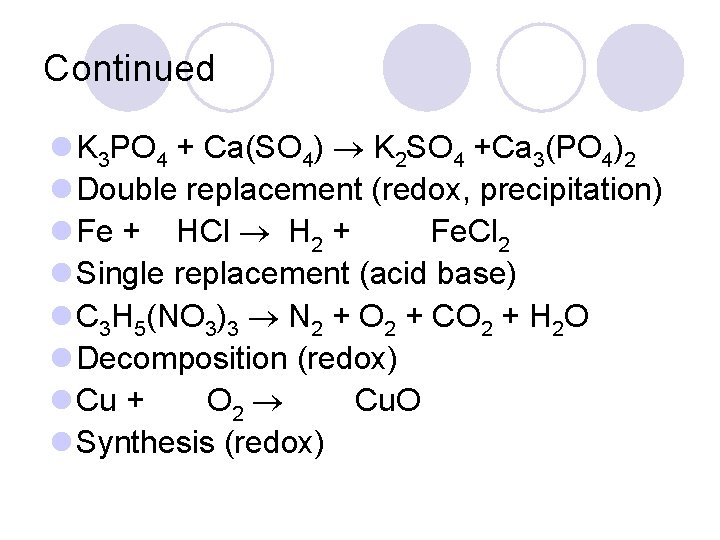
Continued l K 3 PO 4 + Ca(SO 4) K 2 SO 4 +Ca 3(PO 4)2 l Double replacement (redox, precipitation) l Fe + HCl H 2 + Fe. Cl 2 l Single replacement (acid base) l C 3 H 5(NO 3)3 N 2 + O 2 + CO 2 + H 2 O l Decomposition (redox) l Cu + O 2 Cu. O l Synthesis (redox)