4 9 OxidationReduction Reactions Redox reactions reactions in











- Slides: 22

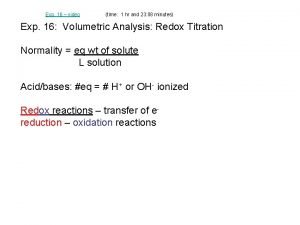
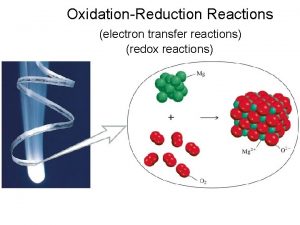
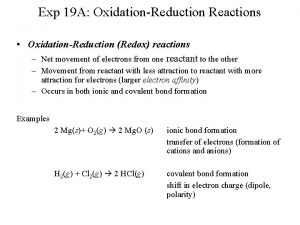
4. 9 Oxidation-Reduction Reactions � Redox reactions- reactions in which one or more electrons is transferred

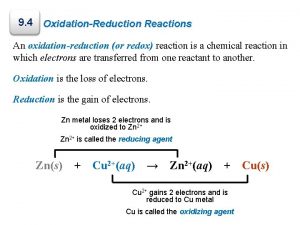
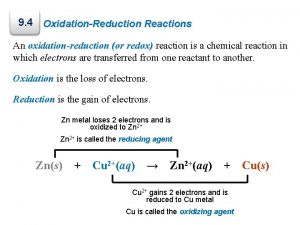
4. 9 Oxidation-Reduction Reactions Oxidation and Reduction When a metal undergoes corrosion it loses electrons to form cations: Ca(s) +2 H+(aq) Ca 2+(aq) + H 2(g) � Oxidized: atom, molecule, or ion becomes more positively charged. � � Oxidation � is the loss of electrons. Reduced: atom, molecule, or ion becomes less positively charged. � Reduction is the gain of electrons.

Oxidation Numbers � Vanadium compounds having different oxidation numbers or oxidation states

Oxidation Numbers � How do maximum and minimum values of the oxidation number correlate with the position in the periodic table?

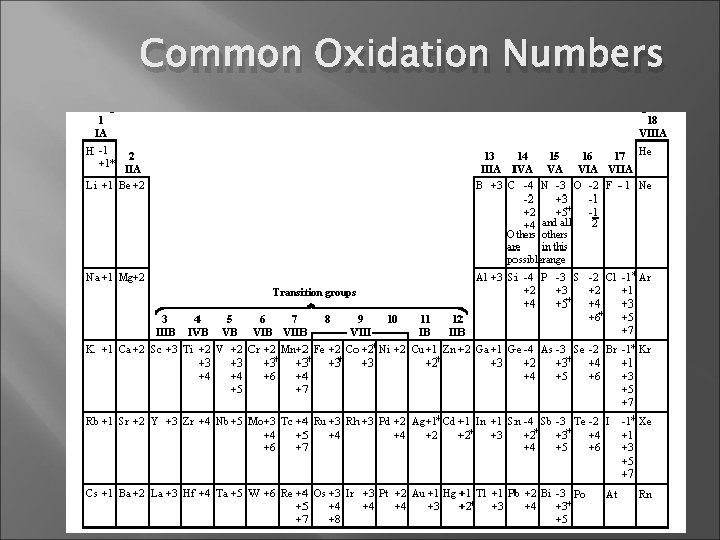
Common Oxidation Numbers

Rules for Assigning Oxidation Numbers The oxidation number of an atom in an element is 0. Ex: Na (s), O 2 (g), Hg (l) The oxidation state of a monatomic ion is the same as its charge Ex. Na + = +1 Cl - = -1 Fluorine has an oxidation number of -1 in its compounds

Rules for Assigning Oxidation Numbers Oxygen usually has an oxidation number of -2 in its compounds. There are some exceptions: a. Oxygen has an oxidation number of -1 in peroxides, which contain the O 22 - ion. b. Oxygen has an oxidation number of -1/2 in superoxides, which contain the O 2 - ion. Hydrogen has an oxidation number of +1 unless it is combined with metals, in which case it has an oxidation number of -1. The sum of the oxidation numbers of all atoms in a substance must equal the total charge on the species: 0 for molecules; the ionic charge for ions.

A guide to the Rules The position of the element in the periodic table may be useful: a. Group IA (1) elements have oxidation numbers of +1 in their compounds. b. Group IIA (2) elements have oxidation numbers of +2 in their compounds. c. Group VIIA (17) elements have oxidation numbers of -1 unless combined with oxygen or a halogen closer to the top of the group. d. In binary compounds, Group VIA (16) elements have oxidation numbers of -2, unless combined with oxygen or halogens.

A guide to the Rules General Summary Element: 0 Fluorine: -1 Oxygen: -2 Hydrogen: +1

Example � � � H 2 SO 4 H is +1 S is -2 unless combined with oxygen or a halogen, so leave this for last O is -2 Use summation rule for S: 2(+1) + 1(S) + 4(-2) = 0 � S = 0 - 2 + 8 = +6

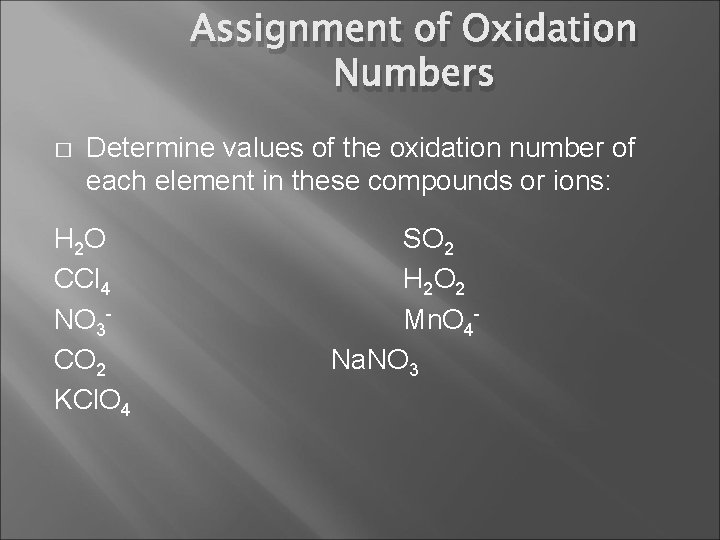
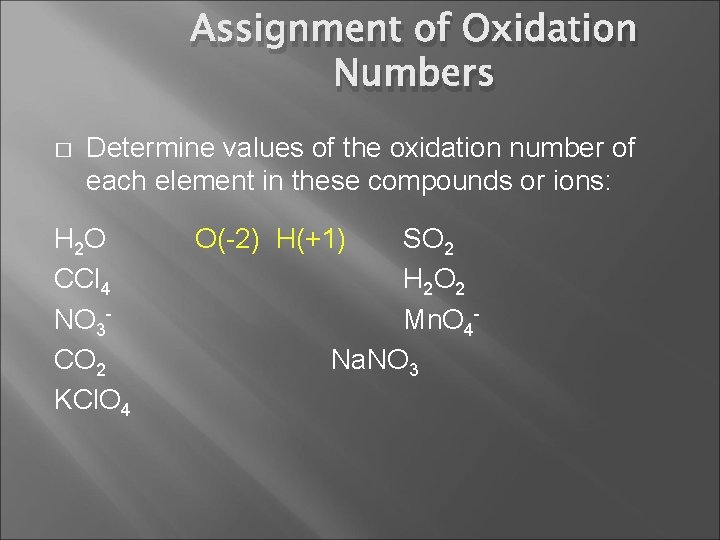
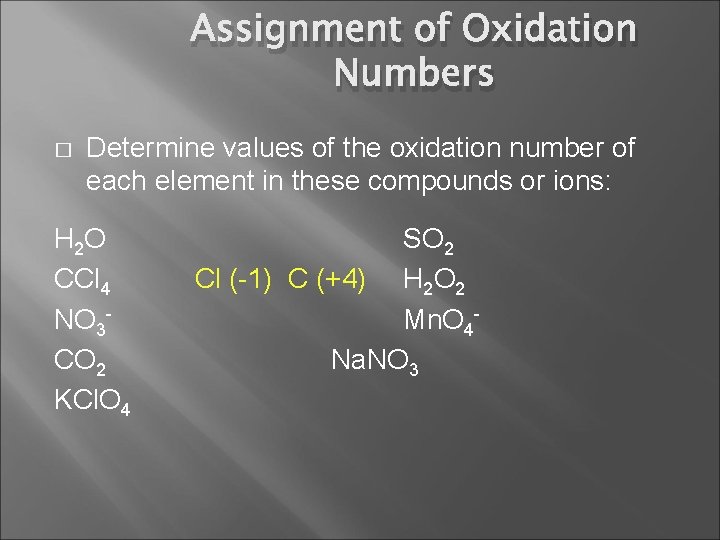
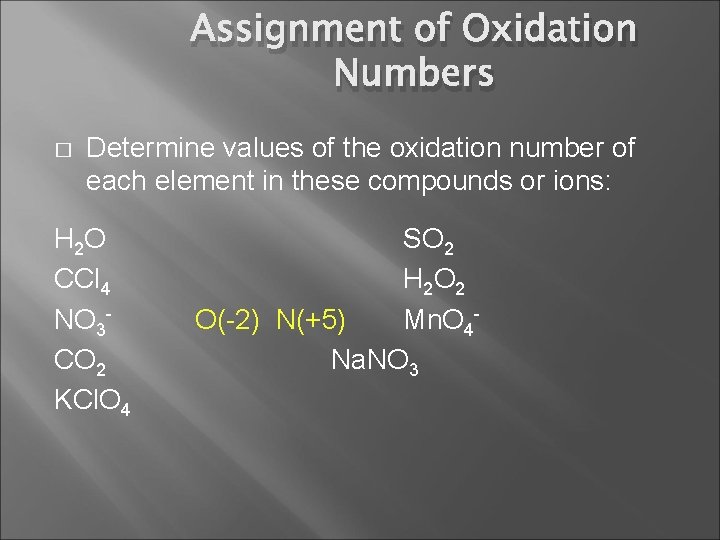
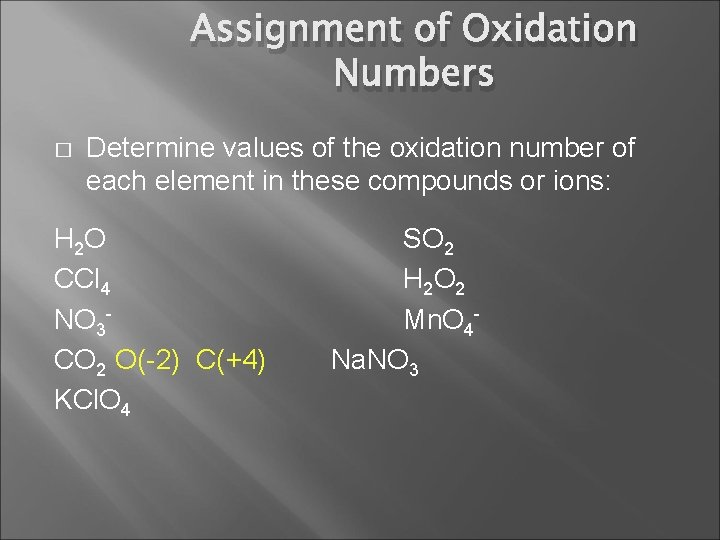
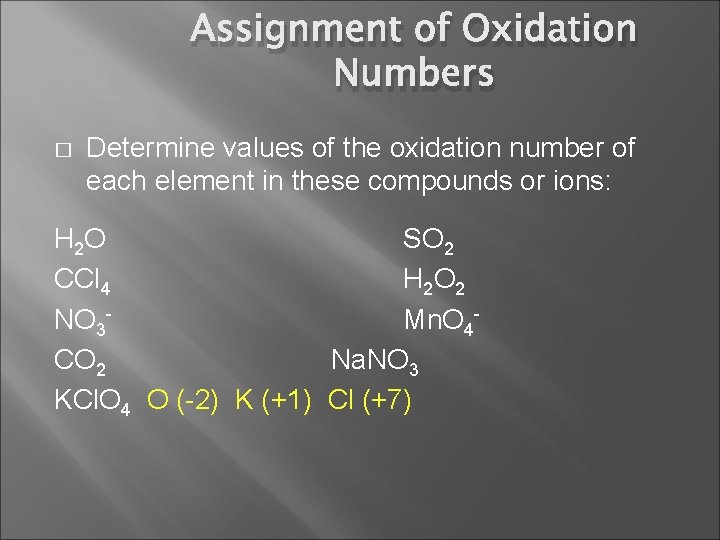
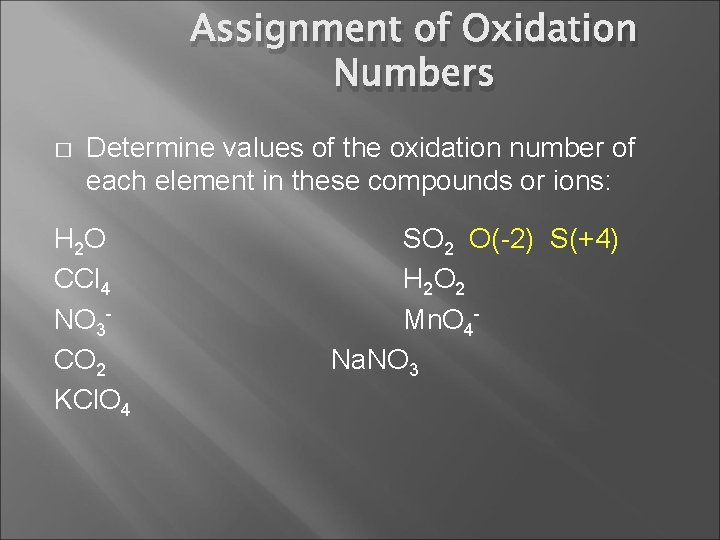
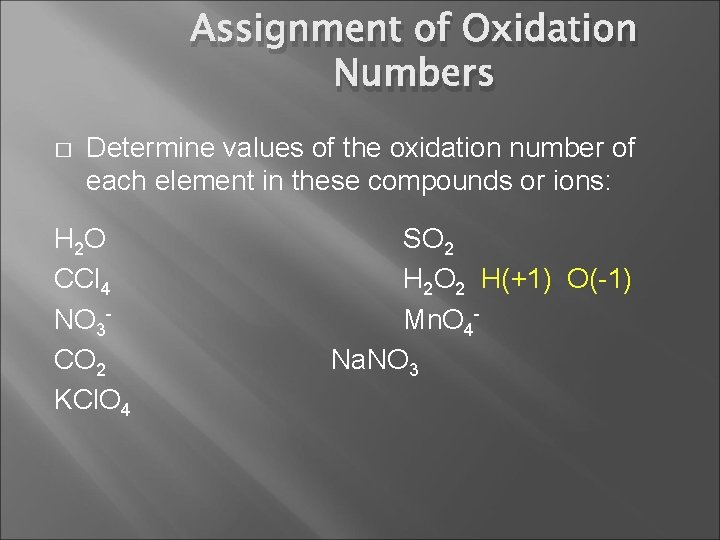
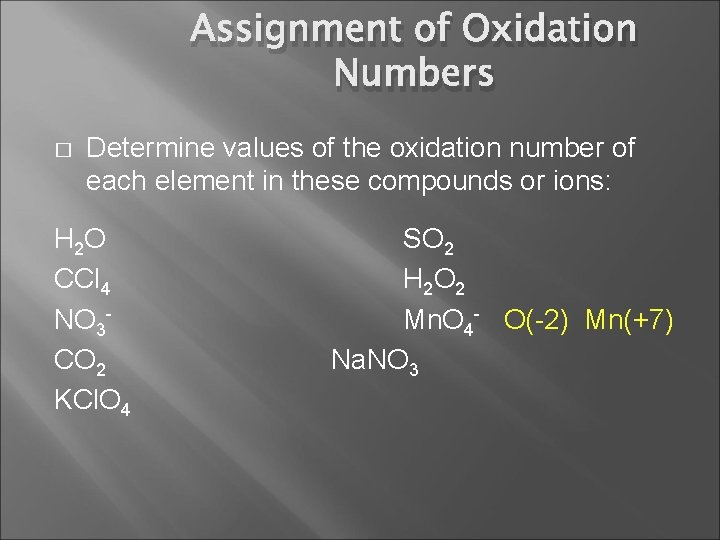
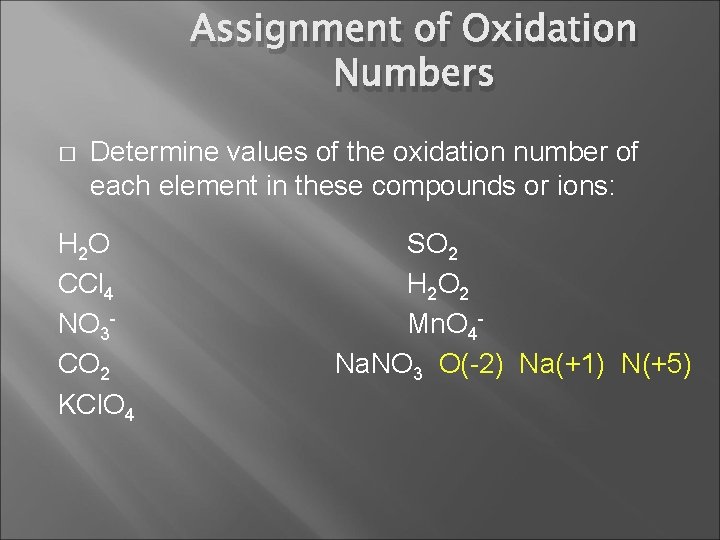
Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 H 2 O 2 Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 O(-2) H(+1) SO 2 H 2 O 2 Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 Cl (-1) C (+4) H 2 O 2 Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 H 2 O 2 O(-2) N(+5) Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 O(-2) C(+4) KCl. O 4 SO 2 H 2 O 2 Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O SO 2 CCl 4 H 2 O 2 NO 3 Mn. O 4 CO 2 Na. NO 3 KCl. O 4 O (-2) K (+1) Cl (+7)

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 O(-2) S(+4) H 2 O 2 Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 H 2 O 2 H(+1) O(-1) Mn. O 4 Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 H 2 O 2 Mn. O 4 - O(-2) Mn(+7) Na. NO 3

Assignment of Oxidation Numbers � Determine values of the oxidation number of each element in these compounds or ions: H 2 O CCl 4 NO 3 CO 2 KCl. O 4 SO 2 H 2 O 2 Mn. O 4 Na. NO 3 O(-2) Na(+1) N(+5)

4. 9 Oxidation-Reduction Reactions � Oxidation- increase in oxidation state � Loss � Reduction- decrease in oxidation state � Gain � of electrons LEO goes GER

4. 9 Oxidation-Reduction Reactions 2 Na(s) + Cl 2(g) 2 Na. Cl(s) � What substances are being oxidized and reduced?
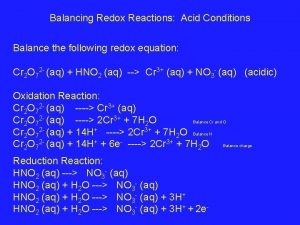
 Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Balancing redox reactions
Balancing redox reactions Balance redox reactions
Balance redox reactions Nernst equation simplified
Nernst equation simplified How redox reactions work
How redox reactions work Leo says ger
Leo says ger Types of chemical reactions redox
Types of chemical reactions redox Balancing redox reactions
Balancing redox reactions Activity series oxidation
Activity series oxidation Half reduction reaction
Half reduction reaction Spontaneity of redox reaction
Spontaneity of redox reaction Concept map of oxidative phosphorylation
Concept map of oxidative phosphorylation Complex redox reactions
Complex redox reactions How do oxidation numbers work
How do oxidation numbers work Redox table
Redox table How to balance an equation step by step
How to balance an equation step by step Redox reactions ncea level 2
Redox reactions ncea level 2 Redox reactions examples
Redox reactions examples Electrolysis khan academy
Electrolysis khan academy Balancing redox reactions in acid
Balancing redox reactions in acid Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Writing half equations chemsheets answers
Writing half equations chemsheets answers How to write redox half reactions
How to write redox half reactions