CHEMISTRY Matter and Change Chapter 19 Redox Reactions

































- Slides: 48

CHEMISTRY Matter and Change Chapter 19: Redox Reactions

CHAPTER 19 Table Of Contents Section 19. 1 Oxidation and Reduction Section 19. 2 Balancing Redox Equations Click a hyperlink to view the corresponding slides. Exit

1 9. 1 SECTION Oxidation and Reduction • Describe the processes of oxidation and reduction. • Identify oxidizing and reducing agents. • Determine the oxidation number of an element in a compound. • Interpret redox reactions in terms of change in oxidation state. spectator ion: an ion that does not participate in a reaction and is not usually shown in an ionic equation

Oxidation and Reduction 1 9. 1 SECTION oxidation-reduction reaction reduction redox reaction oxidizing agent oxidation reducing agent Oxidation and reduction are complementary—as an atom is oxidized, another atom is reduced.

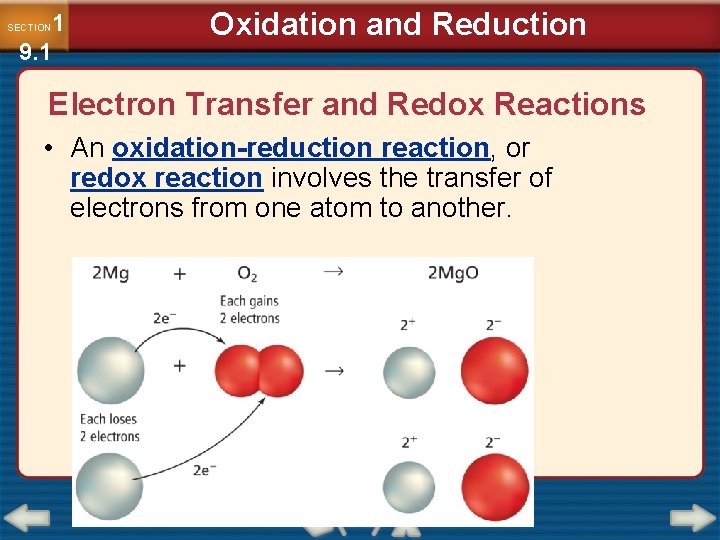
1 9. 1 SECTION Oxidation and Reduction Electron Transfer and Redox Reactions • An oxidation-reduction reaction, or redox reaction involves the transfer of electrons from one atom to another.

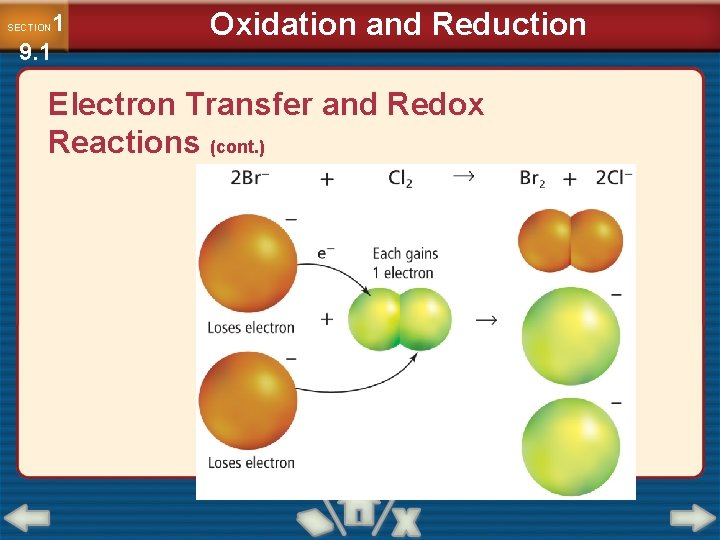
1 9. 1 SECTION Oxidation and Reduction Electron Transfer and Redox Reactions (cont. )

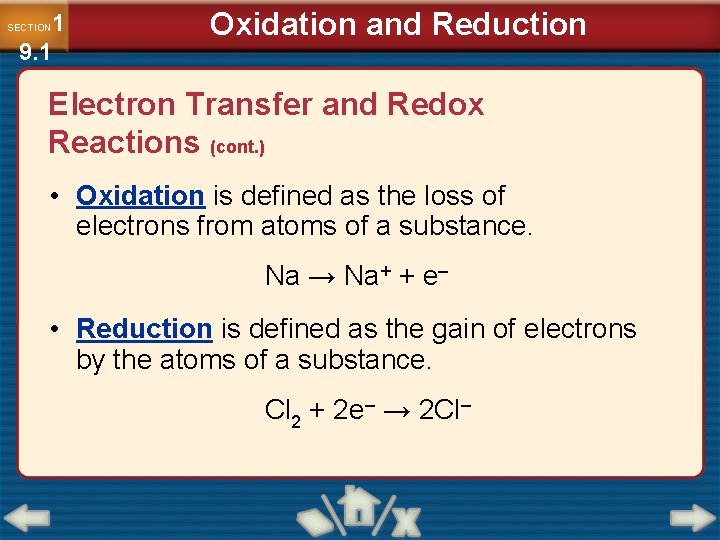
1 9. 1 SECTION Oxidation and Reduction Electron Transfer and Redox Reactions (cont. ) • Oxidation is defined as the loss of electrons from atoms of a substance. Na → Na+ + e– • Reduction is defined as the gain of electrons by the atoms of a substance. Cl 2 + 2 e– → 2 Cl–

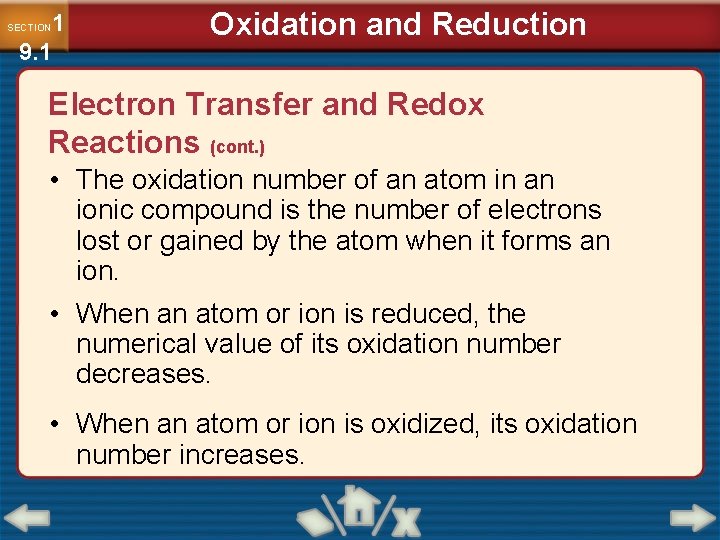
1 9. 1 SECTION Oxidation and Reduction Electron Transfer and Redox Reactions (cont. ) • The oxidation number of an atom in an ionic compound is the number of electrons lost or gained by the atom when it forms an ion. • When an atom or ion is reduced, the numerical value of its oxidation number decreases. • When an atom or ion is oxidized, its oxidation number increases.

1 9. 1 SECTION Oxidation and Reduction Electron Transfer and Redox Reactions (cont. ) • Oxidation numbers are tools that scientists use to keep track of the movement of electrons in a redox reaction.

1 9. 1 SECTION Oxidation and Reduction Oxidizing and Reducing Agents • The substance that oxidizes another substance by accepting its electrons is called an oxidizing agent. • The oxidizing agent is the substance that is reduced in a redox reaction.

1 9. 1 SECTION Oxidation and Reduction Oxidizing and Reducing Agents (cont. ) • The substance that reduces another substance by losing its electrons is the reducing agent. • The reducing agent is the substance that is oxidized in a redox reaction.

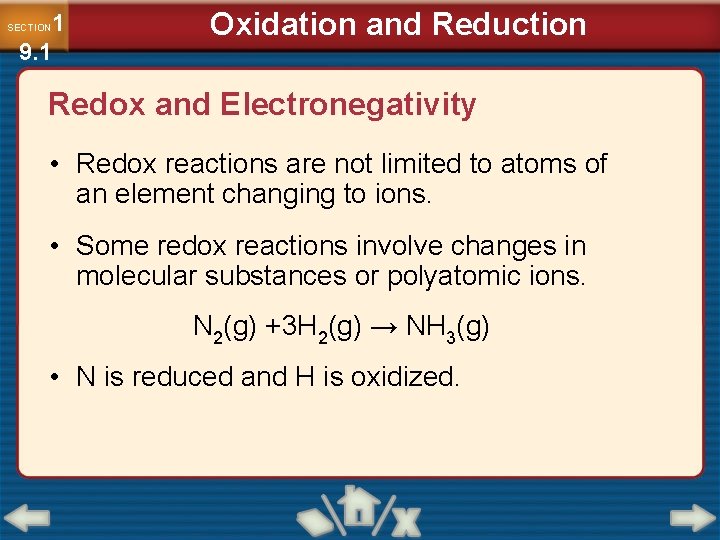
1 9. 1 SECTION Oxidation and Reduction Redox and Electronegativity • Redox reactions are not limited to atoms of an element changing to ions. • Some redox reactions involve changes in molecular substances or polyatomic ions. N 2(g) +3 H 2(g) → NH 3(g) • N is reduced and H is oxidized.

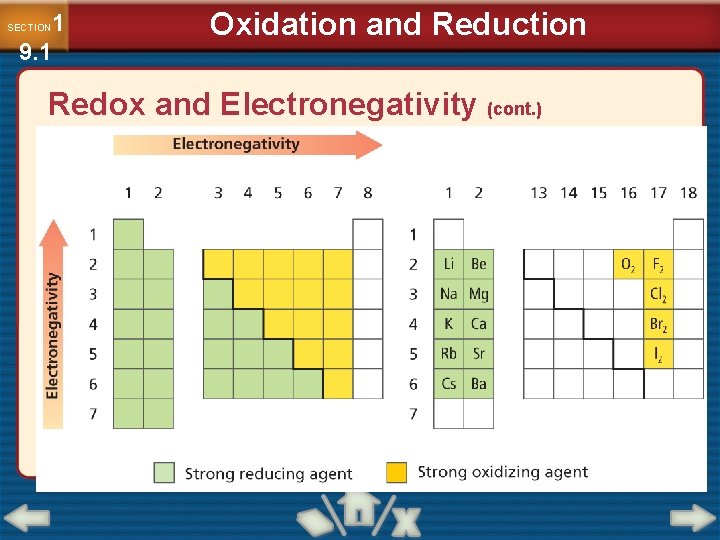
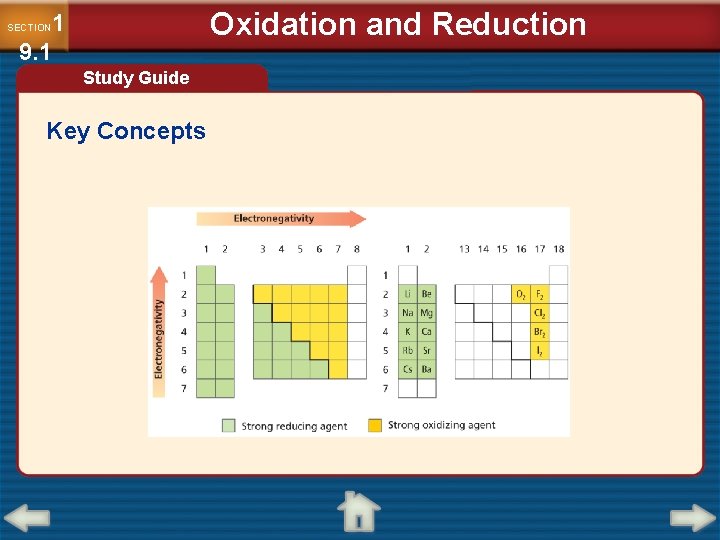
1 9. 1 SECTION Oxidation and Reduction Redox and Electronegativity (cont. ) • To determine which was oxidized and which was reduced, you must know which atom is more electronegative. • Elements with high electronegativity are strong oxidizing agents.

1 9. 1 SECTION Oxidation and Reduction Redox and Electronegativity (cont. )

1 9. 1 SECTION Oxidation and Reduction Determining Oxidation Numbers • To understand all types of redox reactions, the oxidation number of the atoms involved in the reaction must be determined.

1 9. 1 SECTION Oxidation and Reduction Determining Oxidation Numbers (cont. )

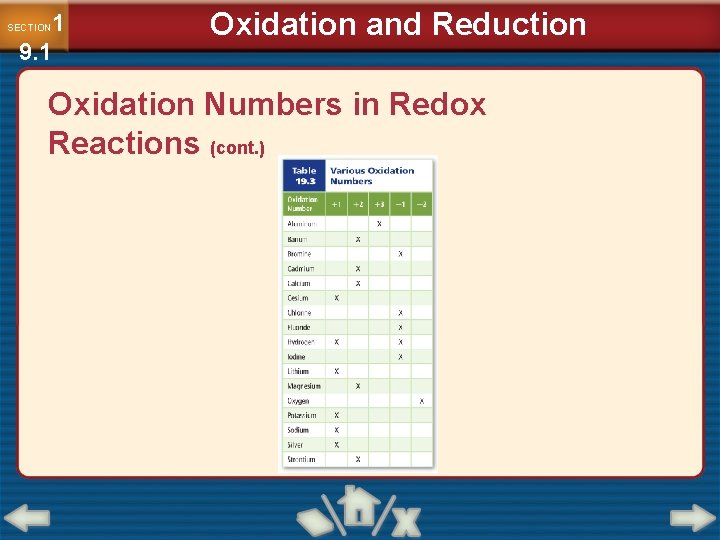
1 9. 1 SECTION Oxidation and Reduction Oxidation Numbers in Redox Reactions • Oxidation-reduction reactions feature changes in oxidation number. • Atoms that are reduced have their oxidation number decreased. • Atoms that are oxidized have their oxidation number increased.

1 9. 1 SECTION Oxidation and Reduction Oxidation Numbers in Redox Reactions (cont. )

1 9. 1 SECTION Section Check In a redox reaction, the reducing agent is: A. the substance that is reduced B. the substance that is oxidized C. the substance that gains electrons D. none of the above

1 9. 1 SECTION Section Check In redox reactions, more electronegative elements tend to: A. be reduced B. be reducing agents C. lose electrons D. not change


1 9. 2 SECTION Balancing Redox Reactions • Relate changes in oxidation number to the transfer of electrons. • Use changes in oxidation number to balance redox equations. • Balance net ionic redox equations using the oxidation-number method. net ionic equation: an ionic equation that includes only the particles that participate in the reaction

1 9. 2 SECTION Balancing Redox Reactions oxidation-number method species half-reaction Redox equations are balanced when the total increase in oxidation numbers equals the total decrease in oxidation numbers of the atoms involved in the reaction.

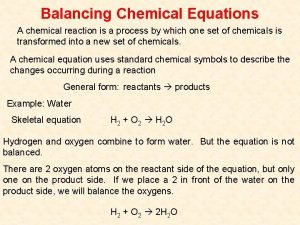
1 9. 2 SECTION Balancing Redox Reactions The Oxidation-Number Method • Chemical equations must be balanced to show the correct quantities of reactants and products. • The number of electrons transferred from atoms must equal the number of electrons accepted by other atoms.

1 9. 2 SECTION Balancing Redox Reactions The Oxidation-Number Method (cont. ) • The total increase in oxidation numbers must equal the total decrease in oxidation numbers in the reaction. • This method is called the oxidation number method.

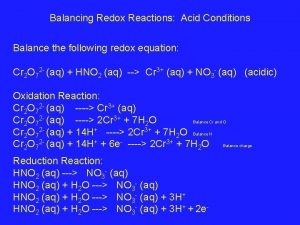
1 9. 2 SECTION Balancing Redox Reactions Balancing Net Ionic Redox Equations • Sometimes it is preferred to express redox reactions in the simplest possible terms, showing only the oxidation and reduction processes. • When balancing equations in acidic solution, hydrogen ions (H+) or water molecules can be added to either side of the equation. • When balancing equations in basic solution, hydroxide ions (OH–) or water molecules can be added to either side of the equation.

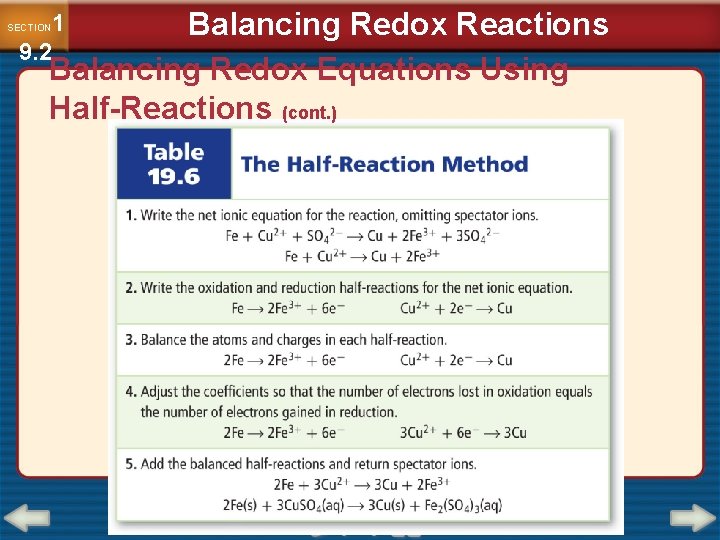
1 9. 2 SECTION Balancing Redox Reactions Balancing Redox Equations Using Half-Reactions (cont. ) • In chemistry, a species is any kind of chemical unit involved in a process. • Oxidation-reduction reactions occur whenever a species that can give up electrons comes in contact with another species that can accept them.

1 9. 2 SECTION Balancing Redox Reactions Balancing Redox Equations Using Half-Reactions (cont. ) • A half-reaction is one of the two parts of a redox reaction—the oxidation half of the reduction half.

Balancing Redox Reactions Balancing Redox Equations Using Half-Reactions (cont. ) 1 9. 2 SECTION

1 9. 2 SECTION Section Check A redox reaction can be split into two parts called ____. A. net reactions B. oxidation-reactions C. half-reactions D. reduction-reactions

1 9. 2 SECTION Section Check In acid solution, what would you use to balance the charge in a redox-reaction? A. electrons B. hydrogen ions and water C. hydroxide ions and water D. hydrogen ions and hydroxide ions


Redox Reactions CHAPTER 19 Resources Chemistry Online Study Guide Chapter Assessment Standardized Test Practice

Oxidation and Reduction 1 9. 1 SECTION Study Guide Key Concepts • Oxidation-reduction reactions involve the transfer of electrons from one atom to another. • When an atom or ion is reduced, its oxidation number is lowered. When an atom or ion is oxidized, its oxidation number is raised. • In oxidation-reduction reactions involving molecular compounds (and polyatomic ions with covalent bonds), the more-electronegative atoms are treated as if they are reduced. The less-electronegative atoms are treated as if they are oxidized.

Oxidation and Reduction 1 9. 1 SECTION Study Guide Key Concepts

1 9. 2 SECTION Balancing Redox Reactions Study Guide Key Concepts • Redox equations in which the same element appears in several reactants and products can be difficult to balance using the conventional method. • The oxidation-number method is based on the number of electrons transferred from atoms equaling the number of electrons accepted by other atoms. • To balance equations for reactions in an acid solution, add enough hydrogen ions and water molecules to balance the equation.

1 9. 2 SECTION Balancing Redox Reactions Study Guide Key Concepts • To balance equations for reactions in a basic solution, add enough hydroxide ions and water molecules to balance the equation. • A half-reaction is one of the two parts of a redox reaction.

CHAPTER 19 Redox Reactions Chapter Assessment What type of reaction involves the transfer of electrons from one atom to another? A. synthesis B. decomposition C. double replacement D. redox

CHAPTER 19 Redox Reactions Chapter Assessment Less electronegative atoms in redox reactions are most often ____. A. oxidized B. reduced C. oxidizing agents D. neutral

CHAPTER 19 Redox Reactions Chapter Assessment Any chemical unit involved in a process is called a(n) ____. A. atom B. type C. species D. ion

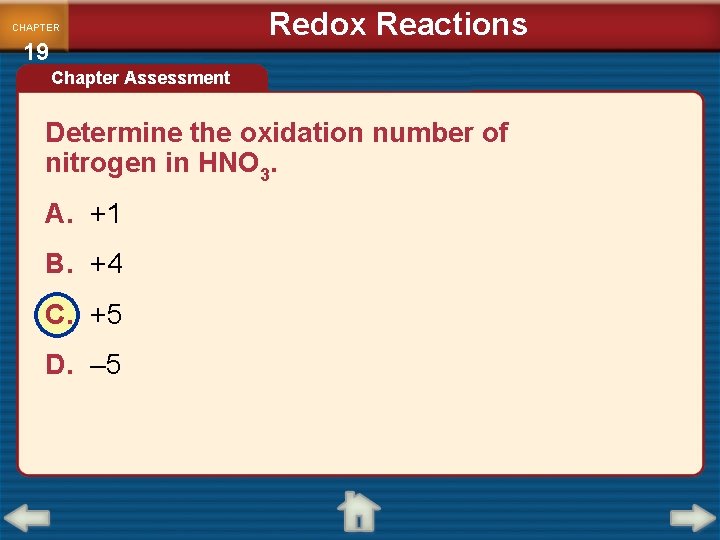
CHAPTER 19 Redox Reactions Chapter Assessment Determine the oxidation number of oxygen in HNO 3. A. +3 B. – 3 C. +2 D. – 2

CHAPTER 19 Redox Reactions Chapter Assessment Determine the oxidation number of nitrogen in HNO 3. A. +1 B. +4 C. +5 D. – 5

CHAPTER 19 Redox Reactions Standardized Test Practice In the reaction Mg. I 2 + Br 2 → Mg. Br 2 + I 2, what is the oxidation number of I 2? A. – 1 B. +1 C. 0 D. +½

CHAPTER 19 Redox Reactions Standardized Test Practice Which is NOT an oxidizing agent in a redox reaction? A. substance reduced B. electron acceptor C. oxidizer of another substance D. electron donor

CHAPTER 19 Redox Reactions Standardized Test Practice How does the oxidation number change in sodium in the following equation? 2 Na. I(aq) + Cl 2(aq) → 2 Na. Cl(aq) + I 2(aq) A. It changes from 0 to – 1. B. It changes from – 1 to 0. C. It changes from 2 to – 2. D. no change

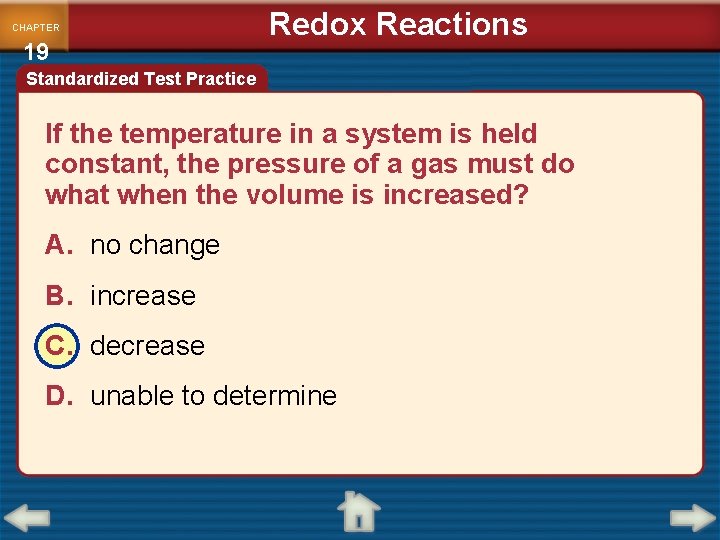
CHAPTER 19 Redox Reactions Standardized Test Practice If the temperature in a system is held constant, the pressure of a gas must do what when the volume is increased? A. no change B. increase C. decrease D. unable to determine

CHAPTER 19 Redox Reactions Standardized Test Practice What is the oxidizing agent in the following equation? Na 2 SO 4 + 4 C → Na 2 S + 4 CO A. C B. S C. O D. Na

END OF SLIDE SHOW
 Example of oxidation reduction reaction
Example of oxidation reduction reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key Oxidation reduction reactions chapter 19 review
Oxidation reduction reactions chapter 19 review Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Different types of redox reactions
Different types of redox reactions Oxidation state
Oxidation state Balancing redox reactions in basic solution
Balancing redox reactions in basic solution Oxidized meaning
Oxidized meaning Ecell vs log cu2+
Ecell vs log cu2+ Downs cell
Downs cell Redox balancing
Redox balancing Electrochemistry balancing equations
Electrochemistry balancing equations Balancing redox reactions in acid
Balancing redox reactions in acid Balance redox reactions
Balance redox reactions Balancing redox reactions
Balancing redox reactions How do you balance a chemical equation
How do you balance a chemical equation How to write redox half reactions
How to write redox half reactions Basic redox reactions
Basic redox reactions Redox reactions ncea level 2
Redox reactions ncea level 2 Chemsheets combining half equations
Chemsheets combining half equations How redox reactions work
How redox reactions work Concept map about oxidative phosphorylation.
Concept map about oxidative phosphorylation. Nernst equation khan academy
Nernst equation khan academy Balancing redox reactions
Balancing redox reactions Balancing redox reactions
Balancing redox reactions Redox table
Redox table Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Chapter 1 review matter and change
Chapter 1 review matter and change Chapter 1 matter and change worksheet answers
Chapter 1 matter and change worksheet answers Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures Grey vs white matter
Grey vs white matter Primary taste cortex
Primary taste cortex Gray matter and white matter
Gray matter and white matter What is gray matter in the brain
What is gray matter in the brain Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Methane oxygen reaction
Methane oxygen reaction