Redox and Electrochemistry Redox Reactions Reduction Oxidation reactions
















- Slides: 25

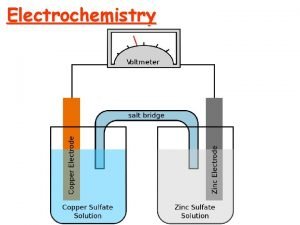
Redox and Electrochemistry

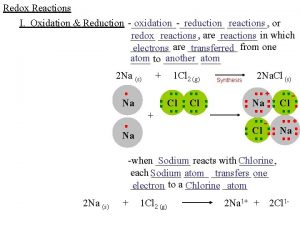
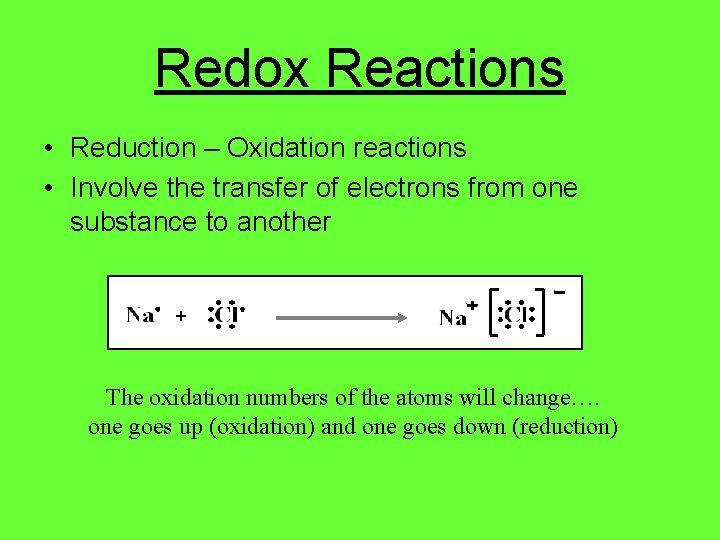
Redox Reactions • Reduction – Oxidation reactions • Involve the transfer of electrons from one substance to another + The oxidation numbers of the atoms will change…. one goes up (oxidation) and one goes down (reduction)

Oxidation Number (Oxidation State) • Used to keep track of the transfer of electrons • Number is assigned to every atom in a chemical formula, in accordance with certain rules • NOT an ionic charge, but is often the same as the ionic charge – Possible oxidation states are given on the periodic table (upper right hand corner)

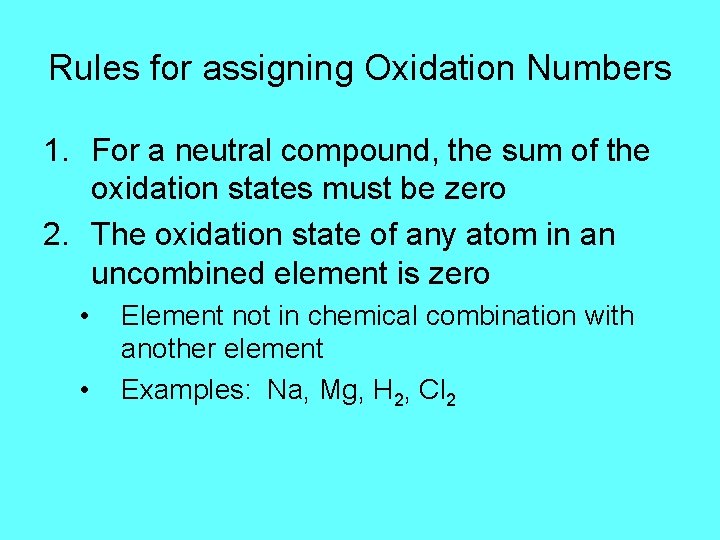
Rules for assigning Oxidation Numbers 1. For a neutral compound, the sum of the oxidation states must be zero 2. The oxidation state of any atom in an uncombined element is zero • • Element not in chemical combination with another element Examples: Na, Mg, H 2, Cl 2

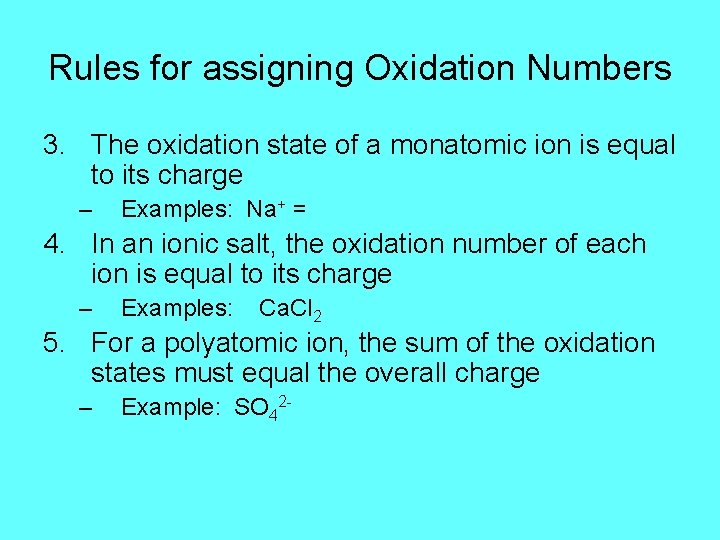
Rules for assigning Oxidation Numbers 3. The oxidation state of a monatomic ion is equal to its charge – Examples: Na+ = 4. In an ionic salt, the oxidation number of each ion is equal to its charge – Examples: Ca. Cl 2 5. For a polyatomic ion, the sum of the oxidation states must equal the overall charge – Example: SO 42 -

Rules for assigning Oxidation Numbers 6. Metals of group 1 always have an oxidation number of +1 7. Metals of groups 2 always have an oxidation number of +2 8. Fluorine is always -1, other halogens are usually -1 9. Aluminum is always +3

Rules for assigning Oxidation Numbers 10. Oxygen is usually -2 Exceptions: – When paired with F (OF 2), oxygen will be +2 – Peroxides (H 2 O 2), oxygen will be -1 11. Hydrogen is usually +1 Exceptions: – Metal hydrides (Group 1 or 2 metals paired with hydrogen), Li. H, Ca. H 2, hydrogen will be -1

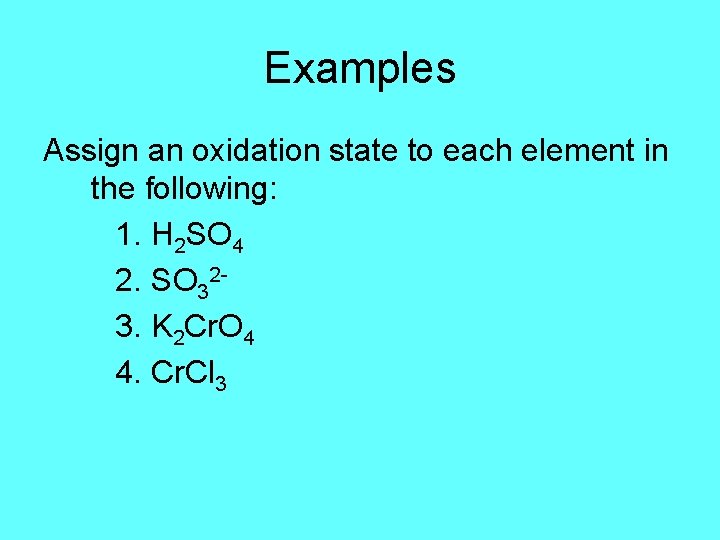
Examples Assign an oxidation state to each element in the following: 1. H 2 SO 4 2. SO 323. K 2 Cr. O 4 4. Cr. Cl 3

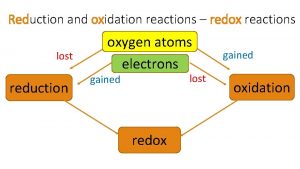
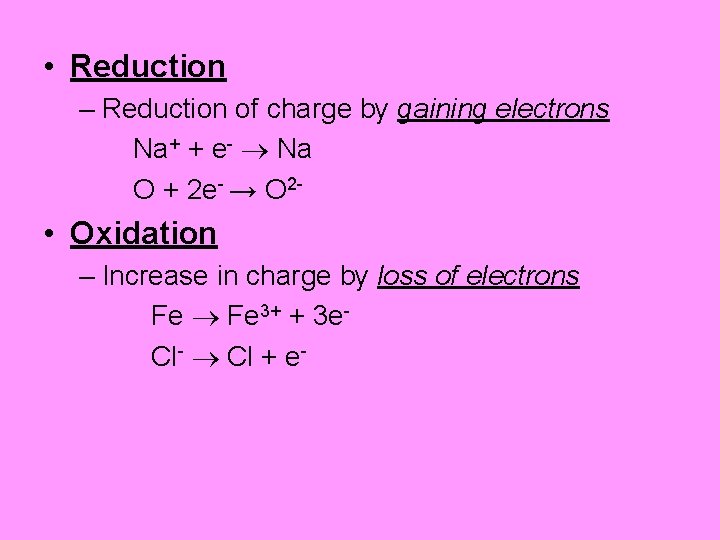
• Reduction – Reduction of charge by gaining electrons Na+ + e- Na O + 2 e- → O 2 - • Oxidation – Increase in charge by loss of electrons Fe 3+ + 3 e. Cl- Cl + e-

LEO the lion says GER Losing Electrons Oxidation Gaining Electrons Reduction

Conservation of Matter/ Conservation of Charge • Mass must be conserved – Mass on both sides must be the same (balanced) • Charge must be conserved – Net charge on both sides must be the same (balanced) – add electrons to the higher side • Reduction and Oxidation reactions must occur together (REDOX reactions)

Half Reactions • Every Redox reaction consists of a reduction and oxidation reaction • Each reaction is called a ½ reaction • A separate equation can be written for each ½ reaction

Half Reactions • Net charge and mass must be the same on both sides of the equation • The number of electrons must balance out, electrons do not appear in the net equation • One ½ reaction is reduction and the other is oxidation

Spectator Ion • Does not change oxidation states in the reaction, same oxidation state on both sides of the equation • Not every species in an equation is oxidized or reduced, some are spectator ions

1. Assign oxidation states to each element in the reaction 2. Identify the 2 substances that are changing oxidation states 3. Write the half reactions • • Balance the mass Balance the charge (add electrons to the higher side)

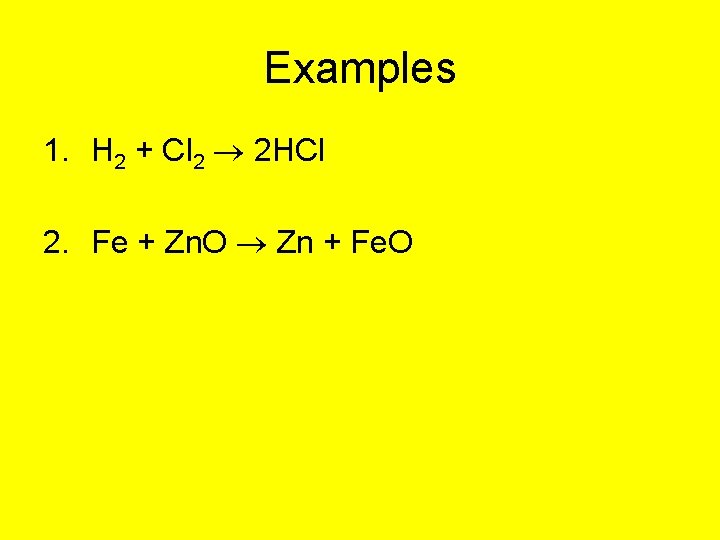
Examples 1. H 2 + Cl 2 2 HCl 2. Fe + Zn. O Zn + Fe. O

Reducing Agent • Substance which is oxidized – Serves as a source of electrons to make the reduction reaction occur – Good reducing agents are substances that lose (donate) electrons easily – elements with low ionization energies Examples: group 1 and 2 metals

Oxidizing Agent • Substance which is reduced – Accepts (gains electrons) – Good oxidizing agents are substances that gain electrons (highly electronegative elements) Examples: Group 17 elements

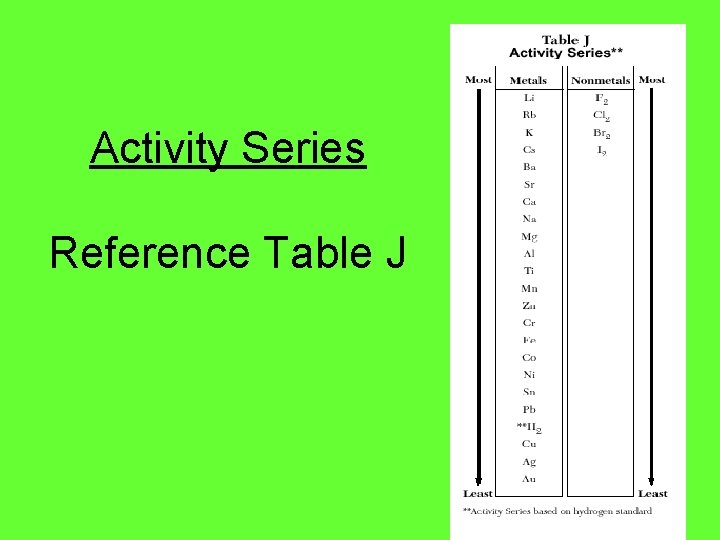
Activity Series Reference Table J

Metals • The most reactive metals are listed at the top • A reaction will occur spontaneously if the metal is higher than the metal ion that it is trying to replace • Reactive metals lose electrons easily (low ionization energy) • Higher on the table = More likely to be oxidized

Examples Ba + Zn. Cl 2 → Zn + Ba. Cl 2 • Ba will replace Zn because Ba is above Zn – Ba is more reactive than Zn • More reactive means that it loses electrons easier

Nonmetals • For the halogen nonmetals listed in Table J, the most reactive ones are at the top • For nonmetals, high reactivity means that they are likely to gain electrons (high electronegativity) • Higher on the table = More likely to be reduced Example: F 2 will replace any other halogen (it is the most reactive)

Examples 1. Which metal is most reactive? a. Fe b. Zn Cu 2. Will Ba react with Mn 2+? 3. Will Na+ react with Cr? 4. Will this reaction occur spontaneously? Mg + Co(NO 3)2 → 5. If this reaction does occur, what products would be made?

Balancing Equations 1. Assign oxidation numbers to all substances in the equation 2. Write the oxidation and reduction ½ reactions 3. Balance (cancel out) the electrons in the ½ reaction 4. Balance the rest of the equation 5. Check

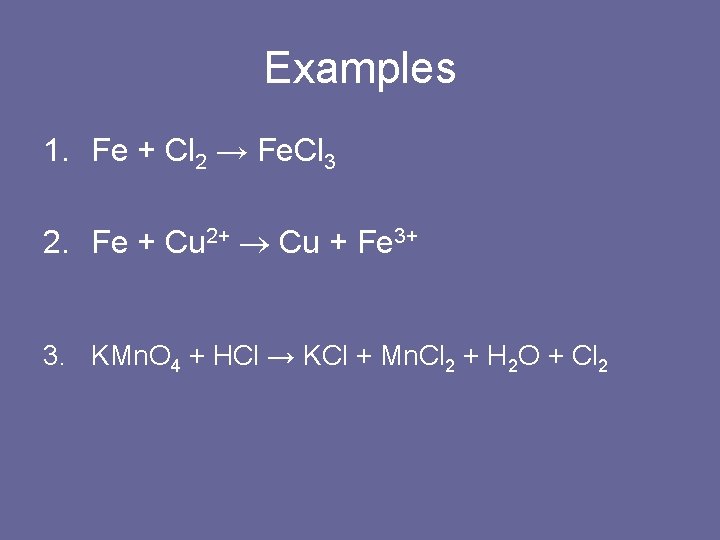
Examples 1. Fe + Cl 2 → Fe. Cl 3 2. Fe + Cu 2+ Cu + Fe 3+ 3. KMn. O 4 + HCl → KCl + Mn. Cl 2 + H 2 O + Cl 2
 Explain oxidation
Explain oxidation Chapter 19 redox reactions answers
Chapter 19 redox reactions answers Reducing agent strength table
Reducing agent strength table Balancing redox reactions
Balancing redox reactions Redox equation
Redox equation How to write reduction half reactions
How to write reduction half reactions Oxidation–reduction reactions
Oxidation–reduction reactions Oxidation–reduction reactions
Oxidation–reduction reactions What is redox reaction
What is redox reaction How to write a balanced redox reaction
How to write a balanced redox reaction Oxide thickness color chart
Oxide thickness color chart Electrochemistry equations
Electrochemistry equations Balanceo por redox
Balanceo por redox How to find oxidizing and reducing agents
How to find oxidizing and reducing agents What isof
What isof Which equation represents an oxidation-reduction reaction
Which equation represents an oxidation-reduction reaction Oxidation reduction quiz
Oxidation reduction quiz Which equation represents an oxidation-reduction reaction
Which equation represents an oxidation-reduction reaction Leo goes ger
Leo goes ger Curvature permanent wrap
Curvature permanent wrap Oxidation reduction leo ger
Oxidation reduction leo ger Oxidative corrosion
Oxidative corrosion Leo and ger
Leo and ger Reactions19
Reactions19 Electrochemistry ap chemistry
Electrochemistry ap chemistry Oxidation reduction
Oxidation reduction