Redox reactions Redox reactions of primary alcohols l









- Slides: 16

Redox reactions

Redox reactions of primary alcohols l l l Primary alcohols react with oxidising agents such as potassium manganate(VII) or sodium dichromate(VI), forming the corresponding aldehyde For example, ethanol reacts forming ethanal Ethanal is also formed in the metabolism of ethanol in the human body

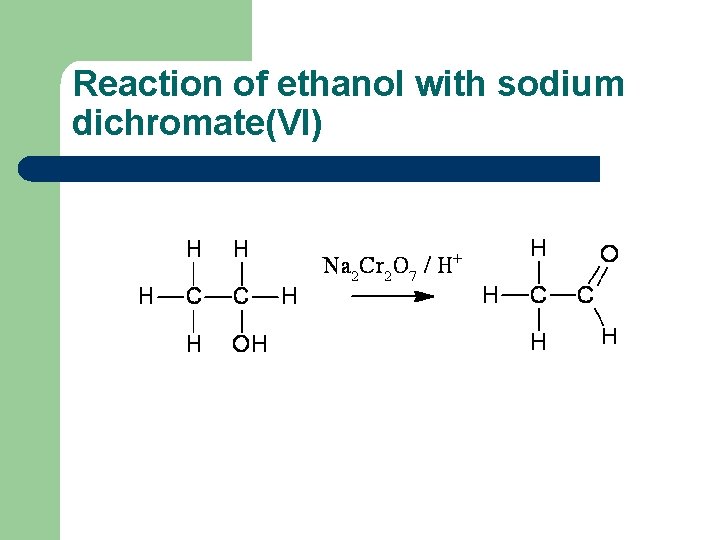
Reaction of ethanol with sodium dichromate(VI)

Reaction of ethanol with sodium dichromate(VI) l l l This reaction is used in the preparation of ethanal Reaction conditions: heat, excess ethanol, acidified sodium dichromate(VI) solution The aldehyde is distilled off as it is formed in order to prevent further oxidation to ethanoic acid

Preparation of ethanal

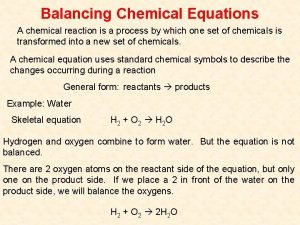
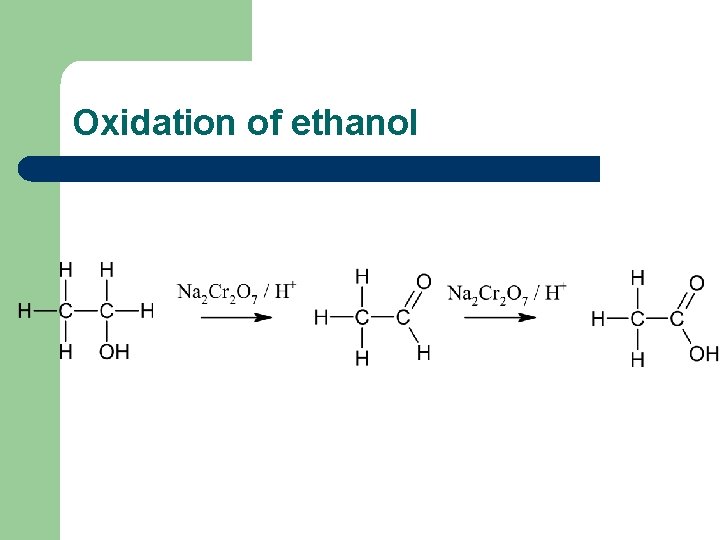
Oxidation of primary alcohols l Primary alcohols such as ethanol are oxidised to the corresponding aldehydes, which can be further oxidised to the corresponding carboxylic acids.

Oxidation of ethanol

Reaction of ethanol with sodium dichromate(VI) l l l This reaction is used in the preparation of ethanoic acid Reaction conditions: heat, excess acidified sodium dichromate(VI) solution The reaction mixture is refluxed in order to bring about oxidation to ethanoic acid

Preparation of ethanoic acid Reflux followed by Distillation

Oxidation of secondary alcohols l l Secondary alcohols such as propan-2 -ol are oxidised to the corresponding ketones, such as propanone Unlike aldehydes, ketones are not easily oxidised, and so no further oxidation takes place

Oxidation of propan-2 -ol

Combustion of organic compounds l l l Most organic compounds burn in air, forming carbon dioxide and water The structure of the compounds’ molecules is completely destroyed, with the carbon and hydrogen atoms in each molecule being oxidised Combustion is exothermic, and ethanol is used as a fuel where it can be produced cheaply

Non-flammable organic compounds l l l Fully halogenated alkanes such as bromochlorodifluoromethane are nonflammable Because of this they can be used in fire extinguishers and as flame retardants For environmental reasons, the use of many of these substances is being phased out

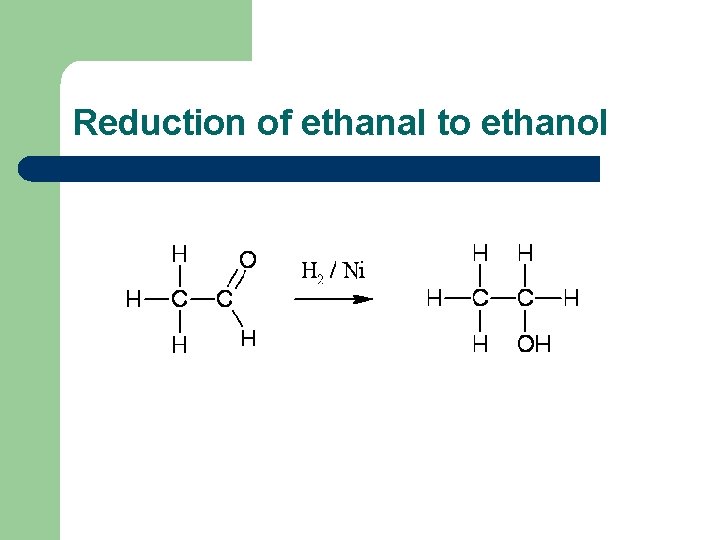
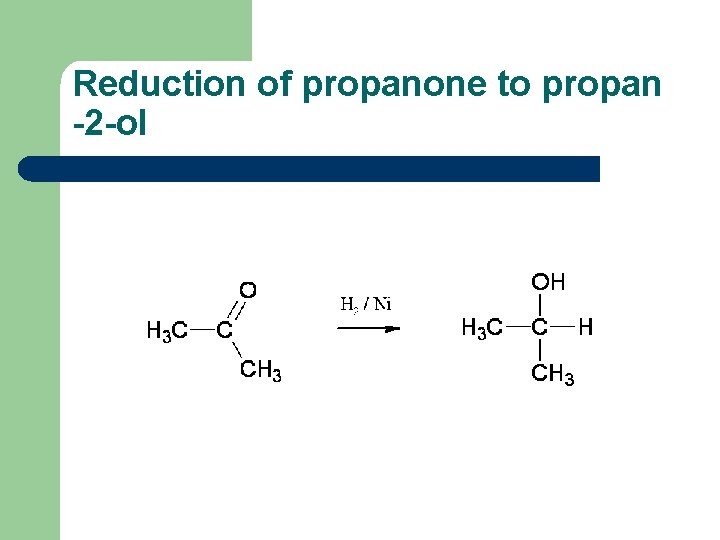
Reduction of aldehydes and ketones l l Aldehydes and ketones can be reduced to the corresponding alcohols, using hydrogen passed over the heated surface of a nickel catalyst For example, ethanal is reduced to ethanol

Reduction of ethanal to ethanol

Reduction of propanone to propan -2 -ol
 5 examples of redox reaction
5 examples of redox reaction Chemsheets reactions of alcohols 1 answers
Chemsheets reactions of alcohols 1 answers Primary alcohol oxidation
Primary alcohol oxidation Predicting redox reactions
Predicting redox reactions Oxidation meaning in chemistry
Oxidation meaning in chemistry Redox reactions ncea level 2
Redox reactions ncea level 2 Leo goes ger
Leo goes ger Different types of redox reactions
Different types of redox reactions Khan academy balancing equations
Khan academy balancing equations How to balance an equation step by step
How to balance an equation step by step Balancing redox reactions
Balancing redox reactions Spontaneity electrochemistry
Spontaneity electrochemistry Combining half equations chemsheets answers
Combining half equations chemsheets answers Balancing complex redox reactions
Balancing complex redox reactions Oilrig redox
Oilrig redox Balancing redox reactions
Balancing redox reactions How to write half reactions
How to write half reactions