Alcohols Alcohols as Acids Alcohols as Acids Alcohols








- Slides: 33

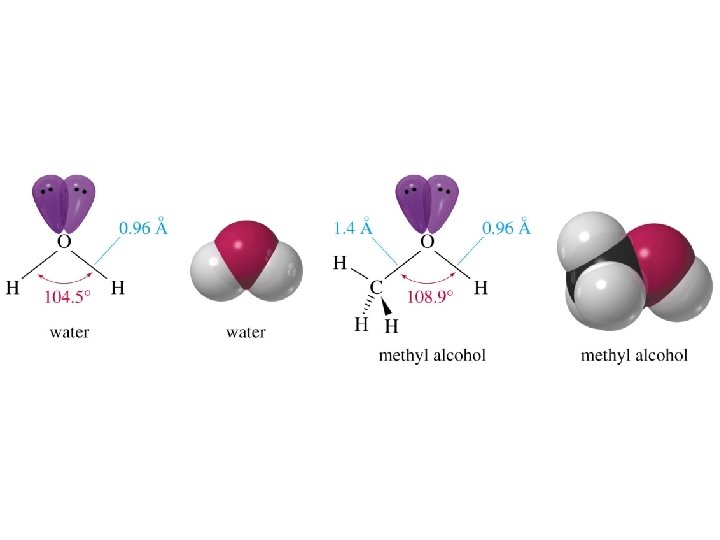
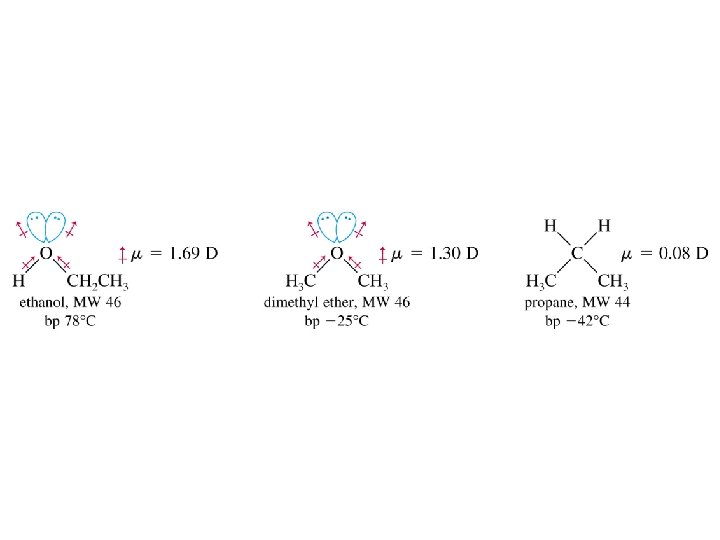
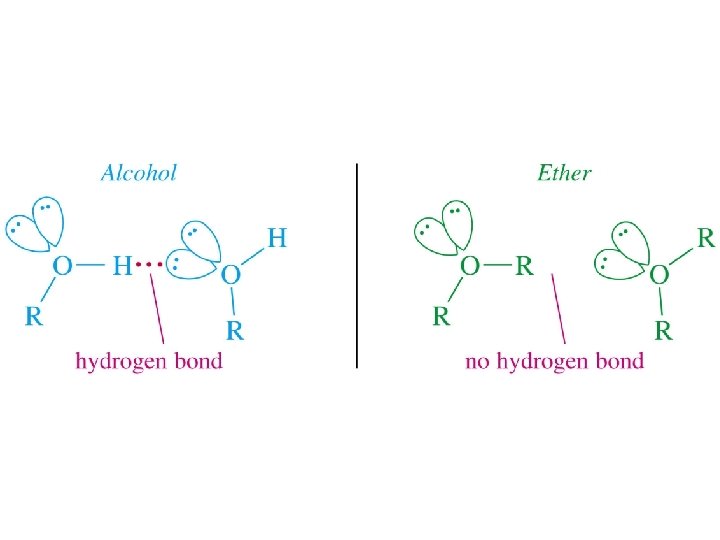
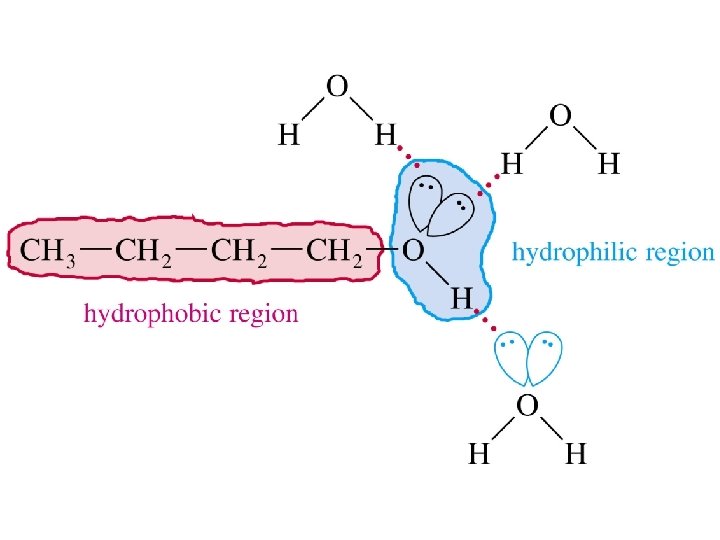
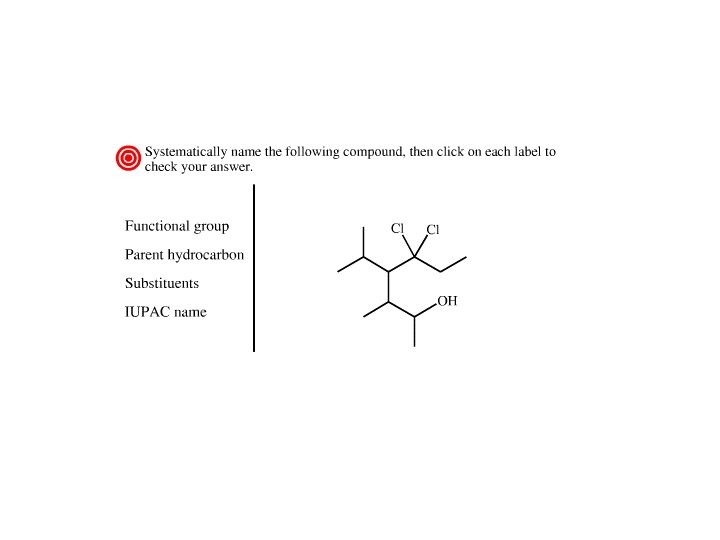
Alcohols





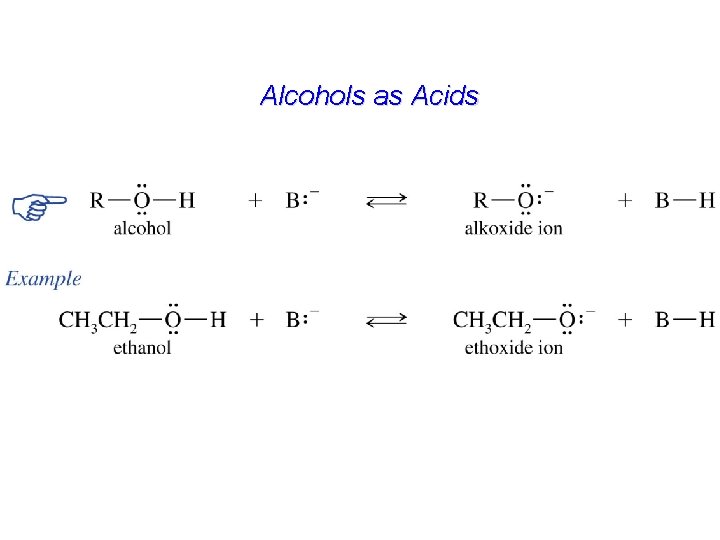
Alcohols as Acids

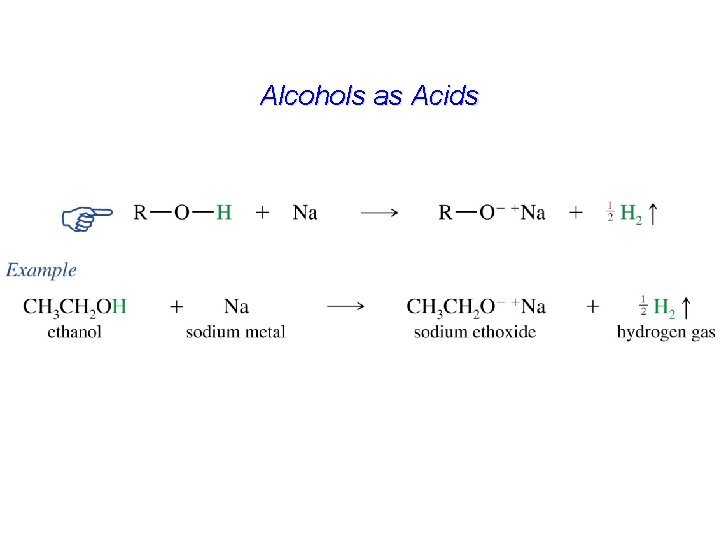
Alcohols as Acids

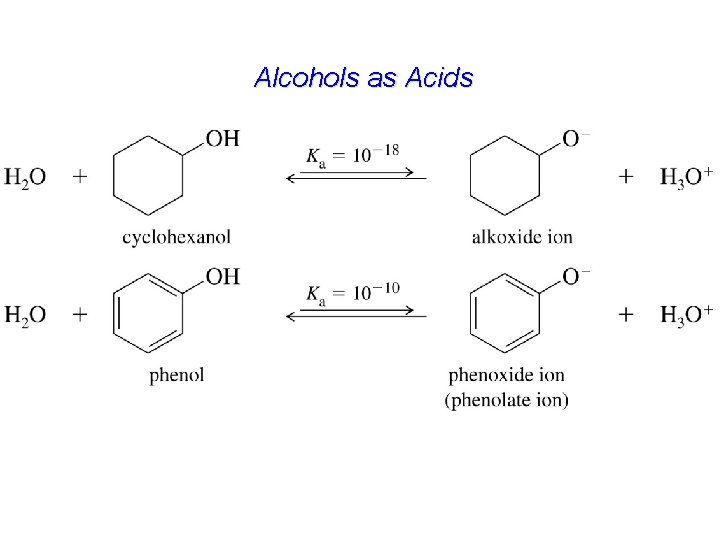
Alcohols as Acids

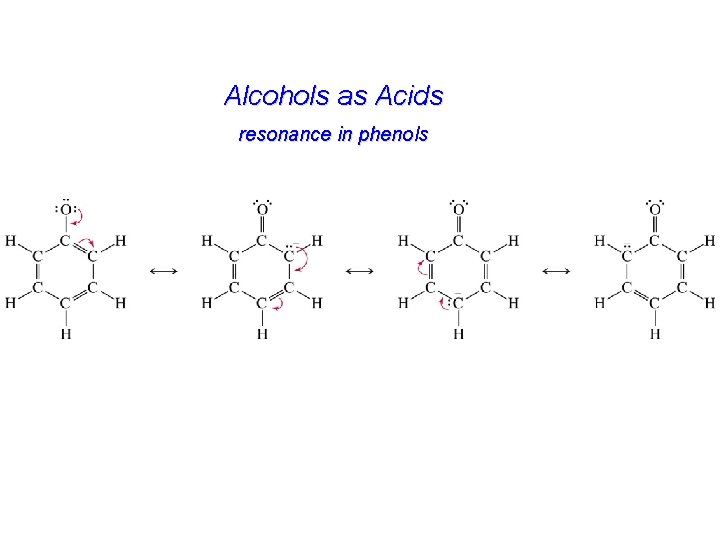
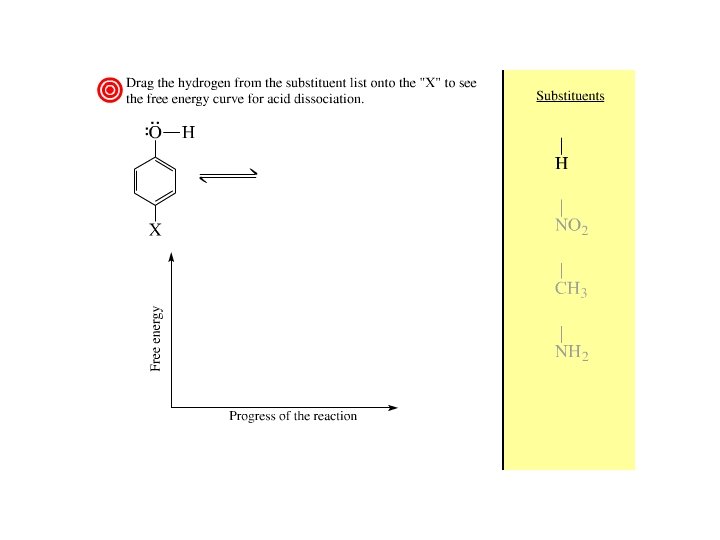
Alcohols as Acids resonance in phenols


Alcohols Methanol and ethanol are industrial chemicals: solvents, antifreeze, fuel, antiseptic, social / recreational Isopropyl alcohol is prepared by hydration of propene. All alcohols with four carbons or fewer are readily available. Most alcohols with five or six carbons are readily available.



Sources of alcohols Reactions discussed in earlier chapters Hydration of alkenes Hydroboration-oxidation of alkenes Hydrolysis of alkyl halides

Sources of alcohols New methods: Reduction of aldehydes and ketones Reduction of carboxylic acids Reduction of esters Reaction of Grignard reagents

Preparation of Alcohols by Reduction of Aldehydes and Ketones

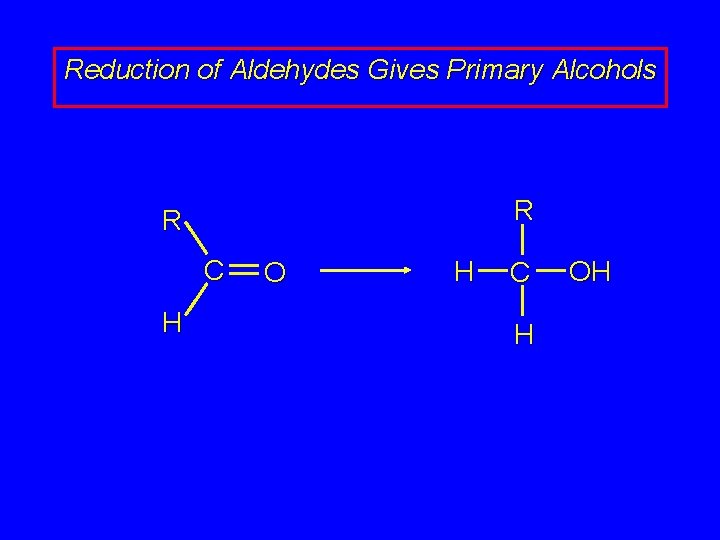
Reduction of Aldehydes Gives Primary Alcohols R R C H O H C H OH

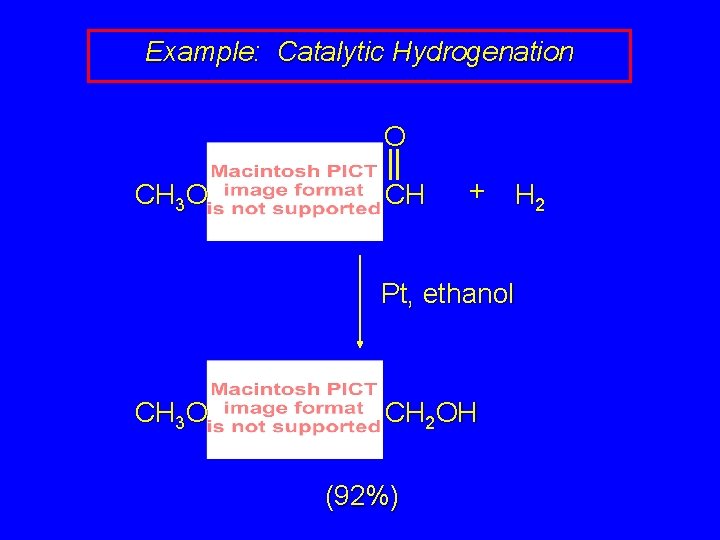
Example: Catalytic Hydrogenation O CH 3 O CH + H 2 Pt, ethanol CH 3 O CH 2 OH (92%)

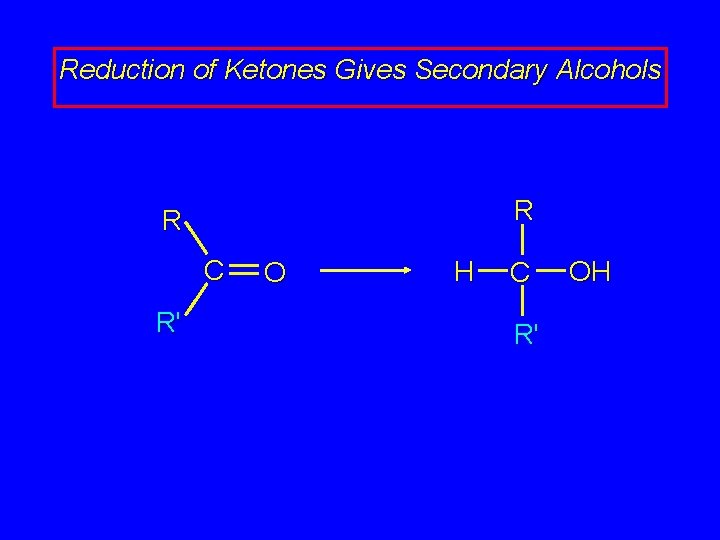
Reduction of Ketones Gives Secondary Alcohols R R C R' O H C R' OH

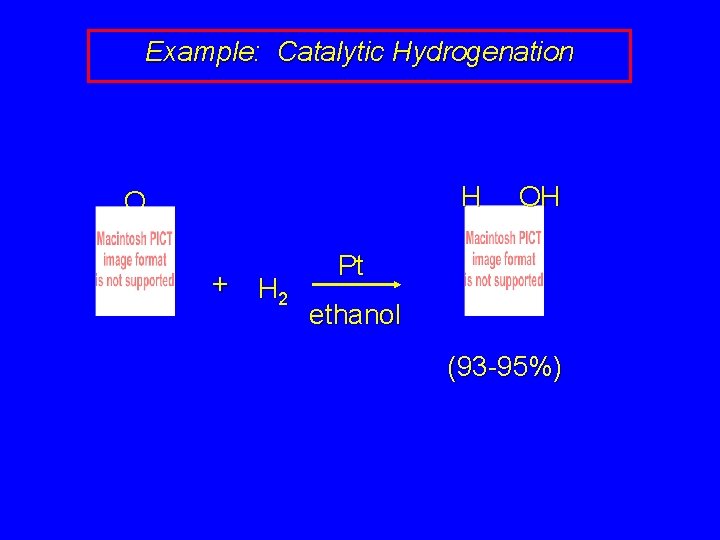
Example: Catalytic Hydrogenation H O + H 2 OH Pt ethanol (93 -95%)

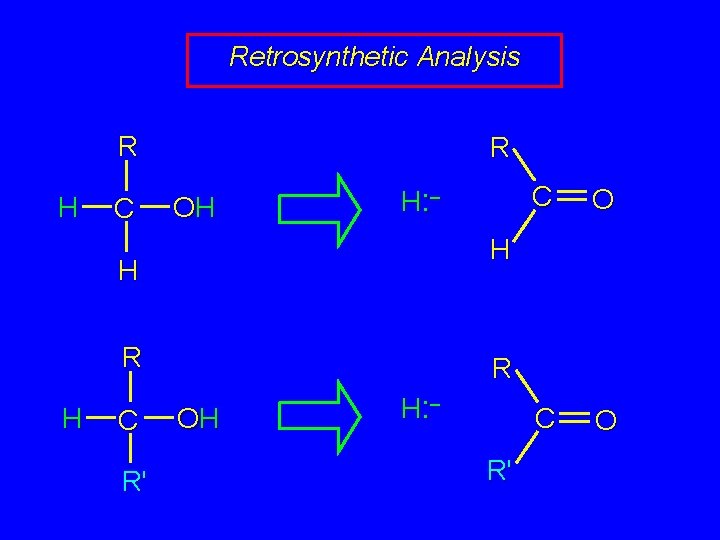
Retrosynthetic Analysis R H C R OH H: – R C R' O C O H H H C R OH H: – R'

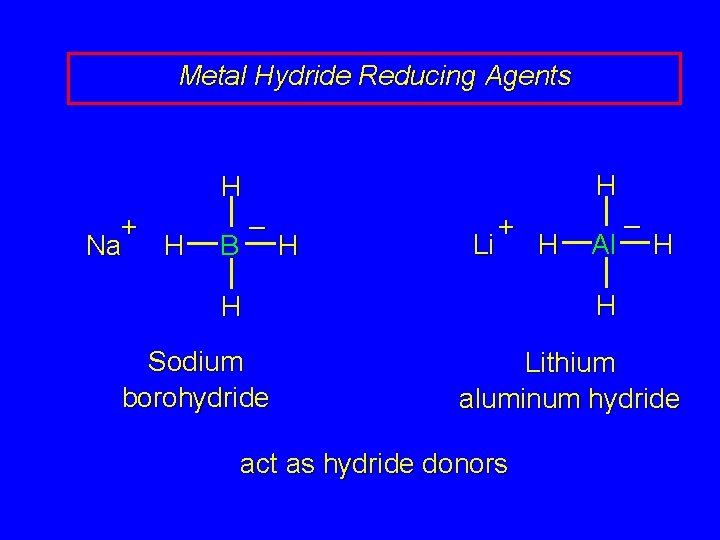
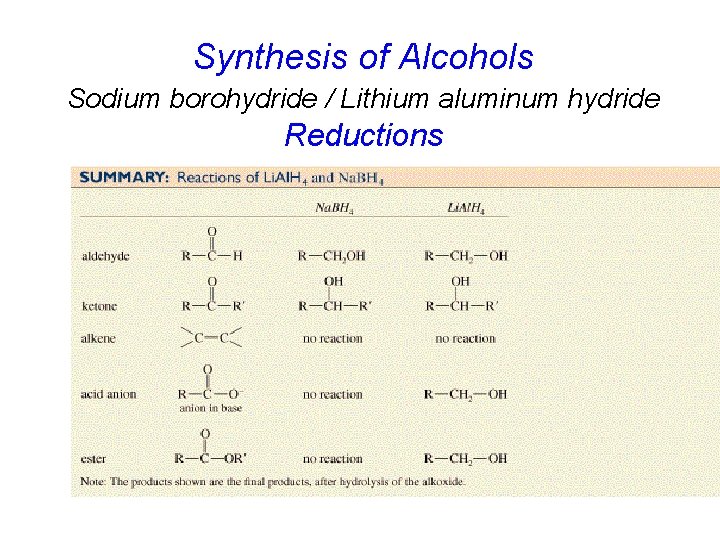
Metal Hydride Reducing Agents H H + Na H – B H Li + Al H H H Sodium borohydride H – Lithium aluminum hydride act as hydride donors

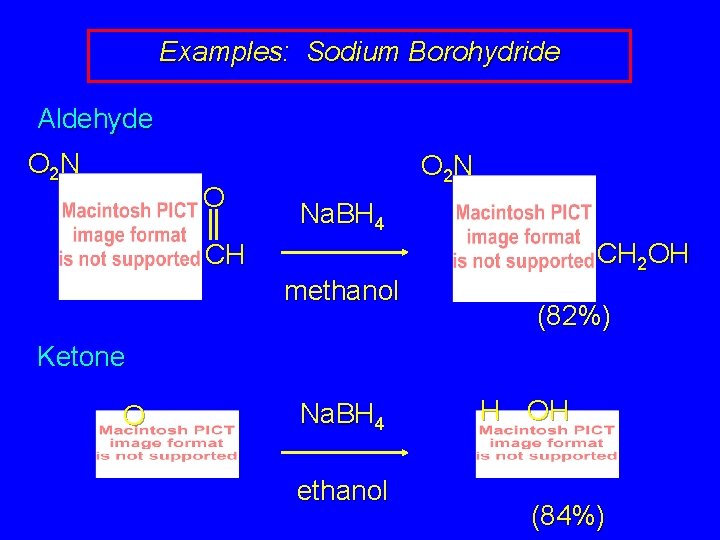
Examples: Sodium Borohydride Aldehyde O 2 N O O 2 N Na. BH 4 CH 2 OH CH methanol (82%) Ketone O Na. BH 4 ethanol H OH (84%)

Lithium aluminum hydride more reactive than sodium borohydride cannot use water, ethanol, methanol etc. as solvents diethyl ether is most commonly used solvent

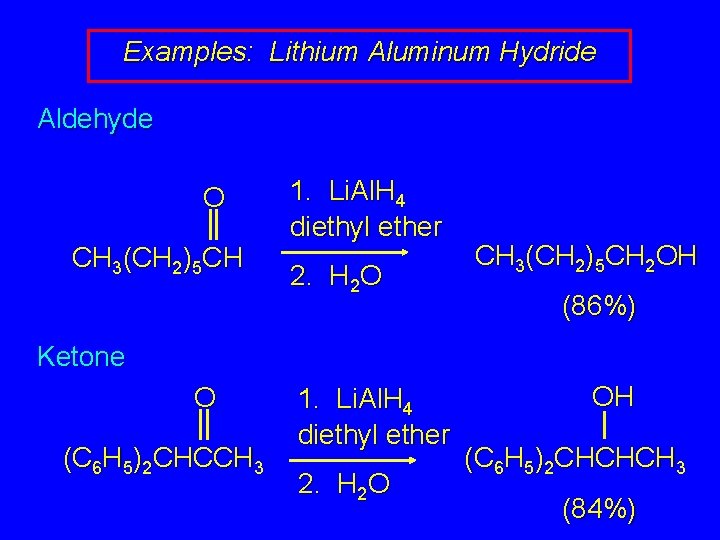
Examples: Lithium Aluminum Hydride Aldehyde O CH 3(CH 2)5 CH 1. Li. Al. H 4 diethyl ether 2. H 2 O CH 3(CH 2)5 CH 2 OH (86%) Ketone O (C 6 H 5)2 CHCCH 3 1. Li. Al. H 4 diethyl ether 2. H 2 O OH (C 6 H 5)2 CHCHCH 3 (84%)

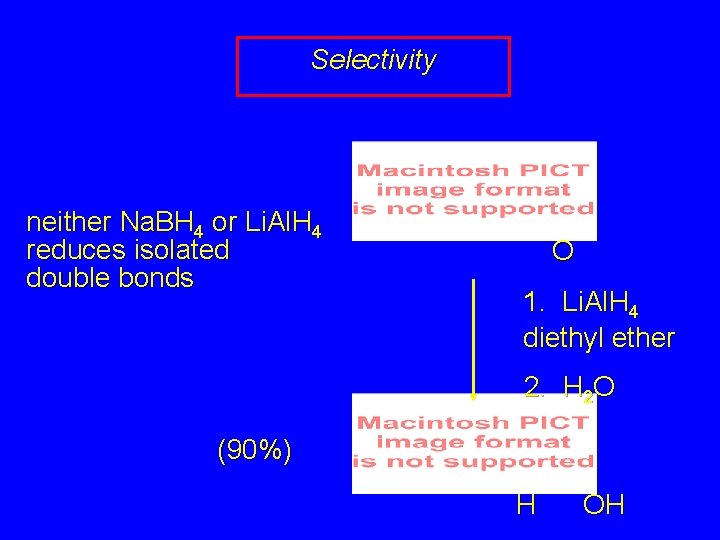
Selectivity neither Na. BH 4 or Li. Al. H 4 reduces isolated double bonds O 1. Li. Al. H 4 diethyl ether 2. H 2 O (90%) H OH

Preparation of Alcohols By Reduction of Carboxylic Acids and Esters

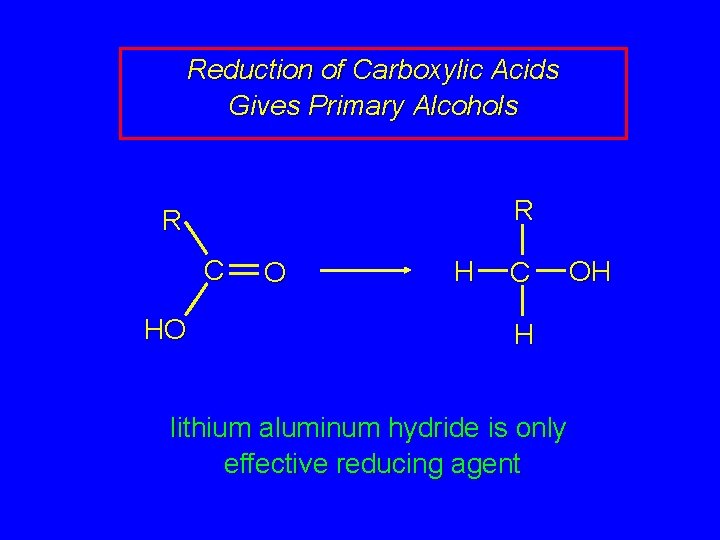
Reduction of Carboxylic Acids Gives Primary Alcohols R R C HO O H C H lithium aluminum hydride is only effective reducing agent OH

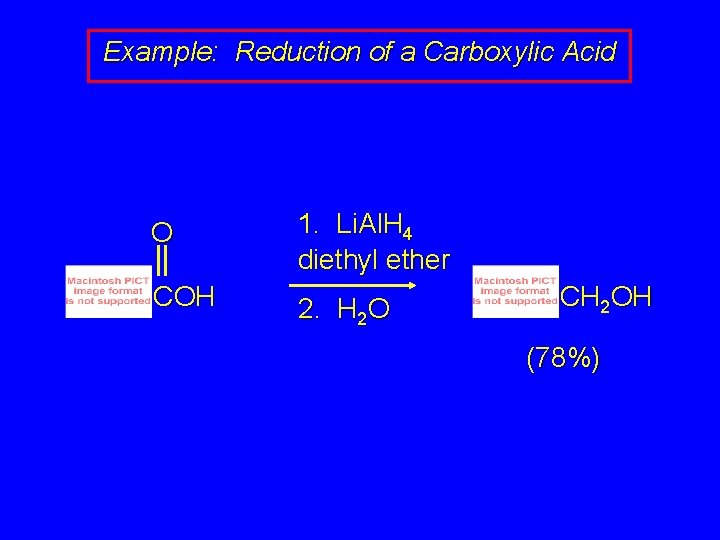
Example: Reduction of a Carboxylic Acid O 1. Li. Al. H 4 diethyl ether COH 2. H 2 O CH 2 OH (78%)

Reduction of Esters Gives Primary Alcohols Lithium aluminum hydride preferred for laboratory reductions Sodium borohydride reduction is too slow to be useful Catalytic hydrogenolysis used in industry but conditions difficult or dangerous to duplicate in the laboratory (special catalyst, high temperature, high pressure

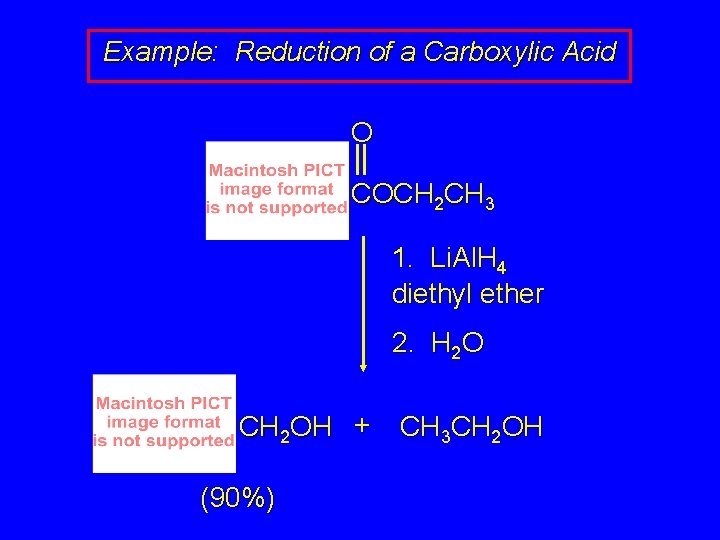
Example: Reduction of a Carboxylic Acid O COCH 2 CH 3 1. Li. Al. H 4 diethyl ether 2. H 2 O CH 2 OH + (90%) CH 3 CH 2 OH

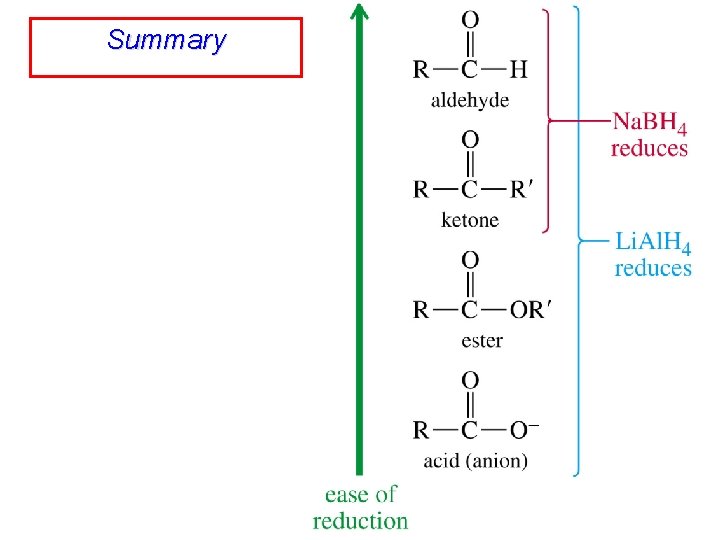
Summary

Synthesis of Alcohols Sodium borohydride / Lithium aluminum hydride Reductions