Chemical Equations Reactions Chemistry A Chemical Reactions You



- Slides: 41

Chemical Equations & Reactions Chemistry A

Chemical Reactions You should be able to ØClassify reactions by type. ØWrite a balanced molecular equation, complete ionic equation, and a net ionic equation. ØPredict products of reactions given the chemical names of the reactants.

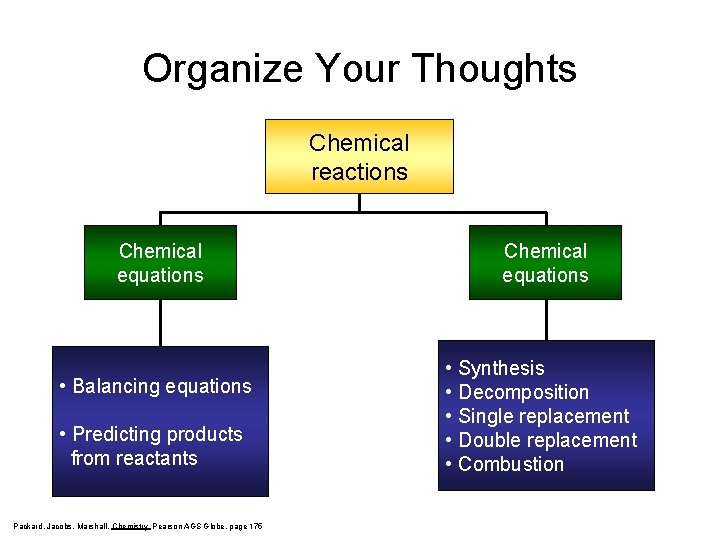
Organize Your Thoughts Chemical reactions Chemical equations • Balancing equations • Predicting products from reactants Packard, Jacobs, Marshall, Chemistry Pearson AGS Globe, page 175 Chemical equations • Synthesis • Decomposition • Single replacement • Double replacement • Combustion

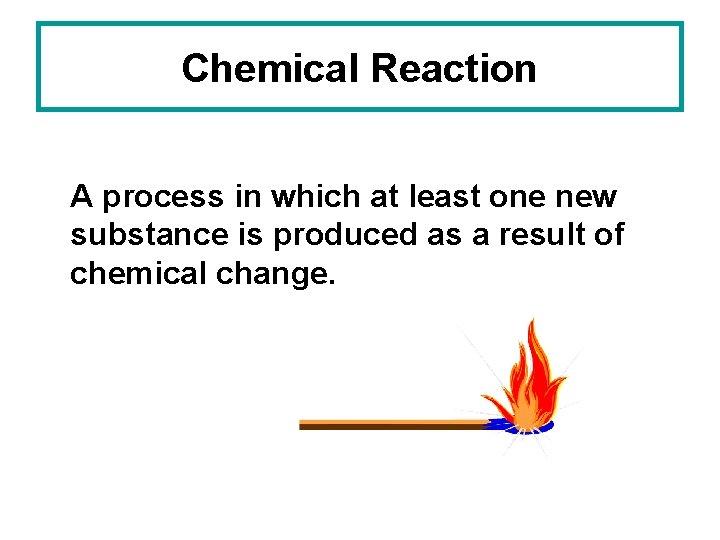
Chemical Reaction A process in which at least one new substance is produced as a result of chemical change.

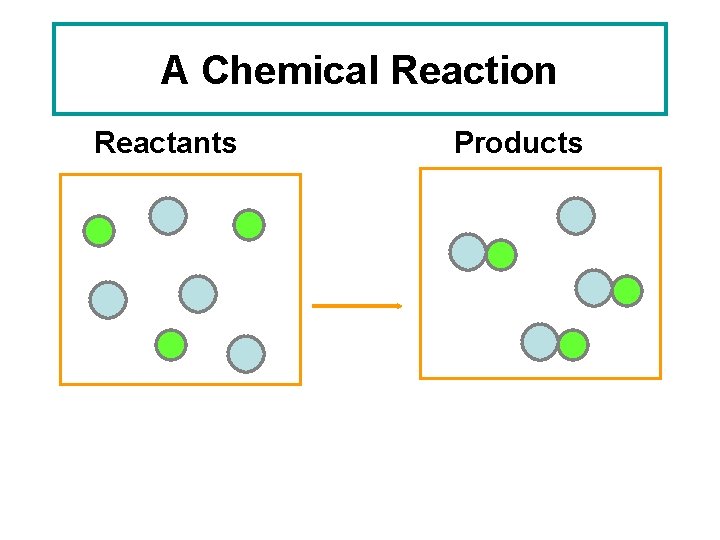
A Chemical Reaction Reactants Products

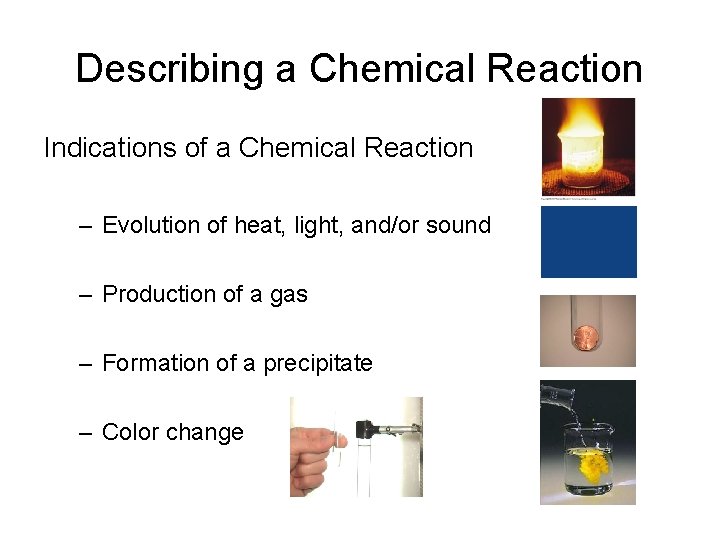
Describing a Chemical Reaction Indications of a Chemical Reaction – Evolution of heat, light, and/or sound – Production of a gas – Formation of a precipitate – Color change

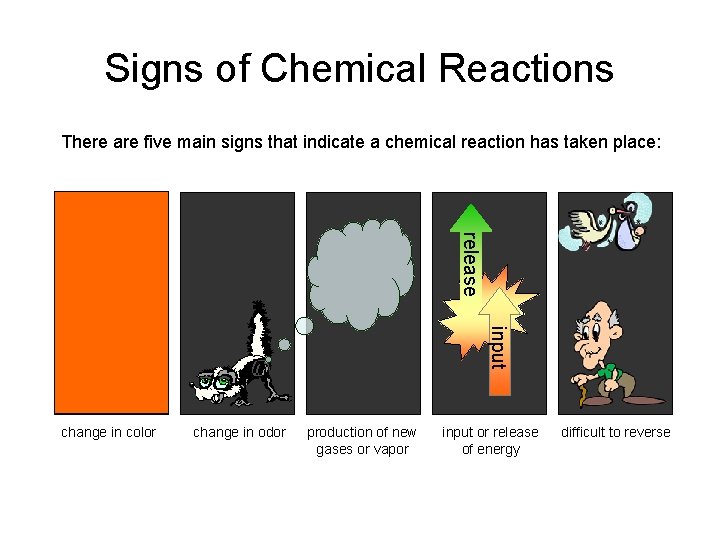
Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse

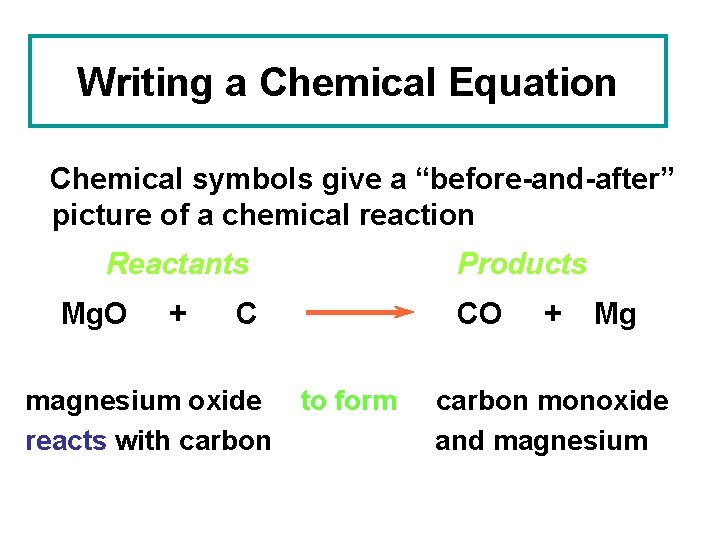
Writing a Chemical Equation Chemical symbols give a “before-and-after” picture of a chemical reaction Reactants Mg. O + Products C magnesium oxide reacts with carbon CO to form + Mg carbon monoxide and magnesium

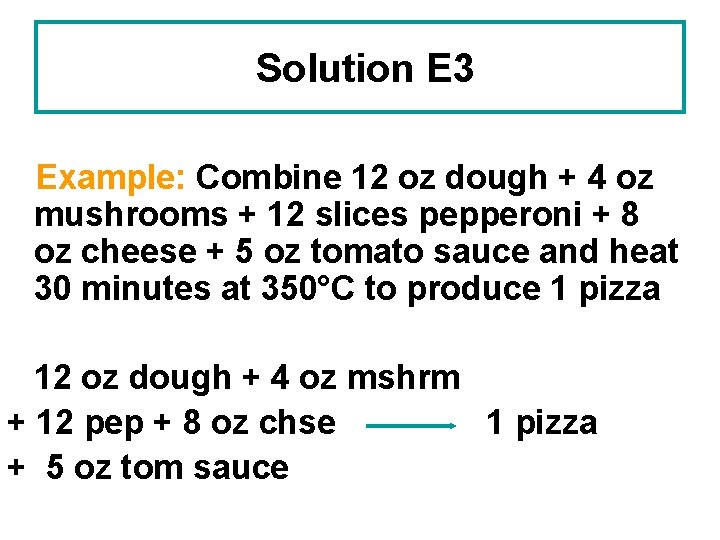
Learning Check E 3 12 oz of dough, 4 oz mushrooms, 12 slices pepperoni, 8 oz cheese and 5 oz tomato sauce are used to make a pizza. Write a recipe in words for putting together a pizza. How would you write the recipe as an equation?

Solution E 3 Example: Combine 12 oz dough + 4 oz mushrooms + 12 slices pepperoni + 8 oz cheese + 5 oz tomato sauce and heat 30 minutes at 350°C to produce 1 pizza 12 oz dough + 4 oz mshrm + 12 pep + 8 oz chse 1 pizza + 5 oz tom sauce

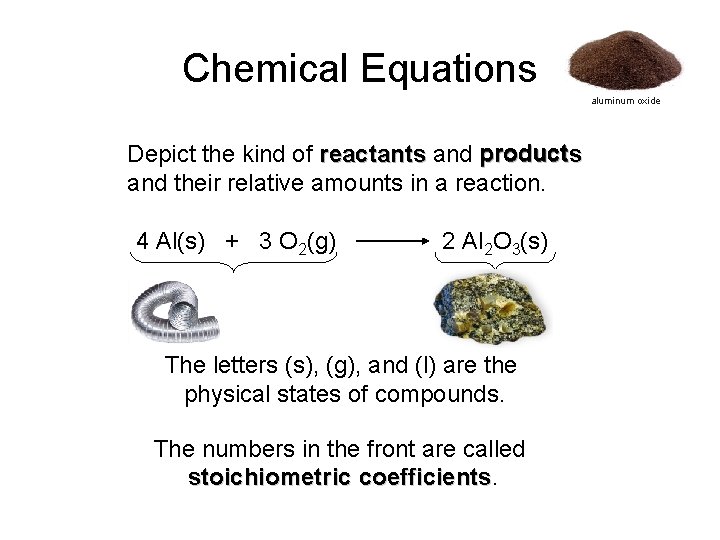
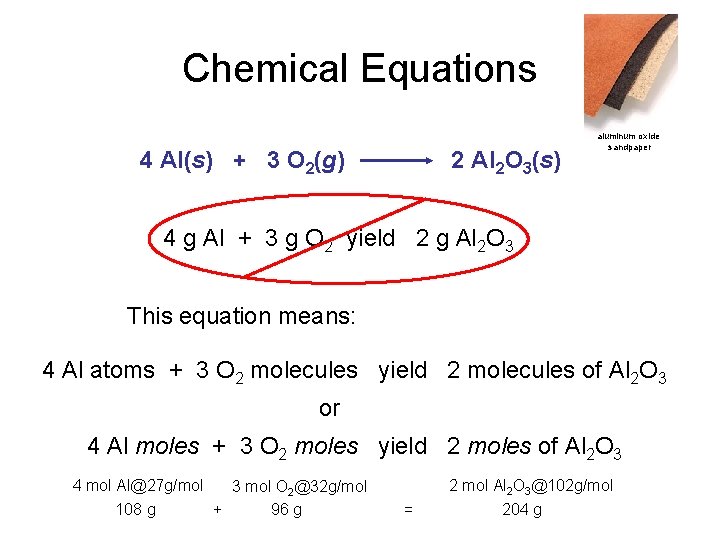
Chemical Equations aluminum oxide Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) The letters (s), (g), and (l) are the physical states of compounds. The numbers in the front are called stoichiometric coefficients

Chemical Equations 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) aluminum oxide sandpaper 4 g Al + 3 g O 2 yield 2 g Al 2 O 3 This equation means: 4 Al atoms + 3 O 2 molecules yield 2 molecules of Al 2 O 3 or 4 Al moles + 3 O 2 moles yield 2 moles of Al 2 O 3 4 mol Al@27 g/mol 3 mol O 2@32 g/mol 108 g + 96 g 2 mol Al 2 O 3@102 g/mol = 204 g

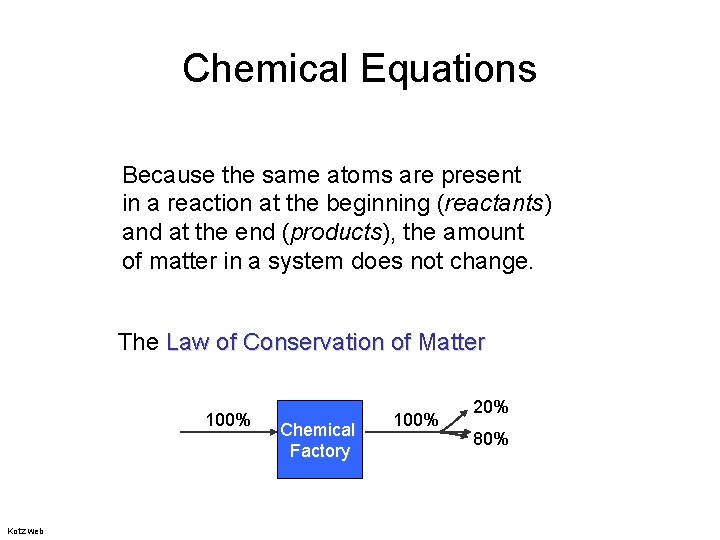
Chemical Equations Because the same atoms are present in a reaction at the beginning (reactants) and at the end (products), the amount of matter in a system does not change. The Law of Conservation of Matter 100% Kotz web Chemical Factory 100% 20% 80%

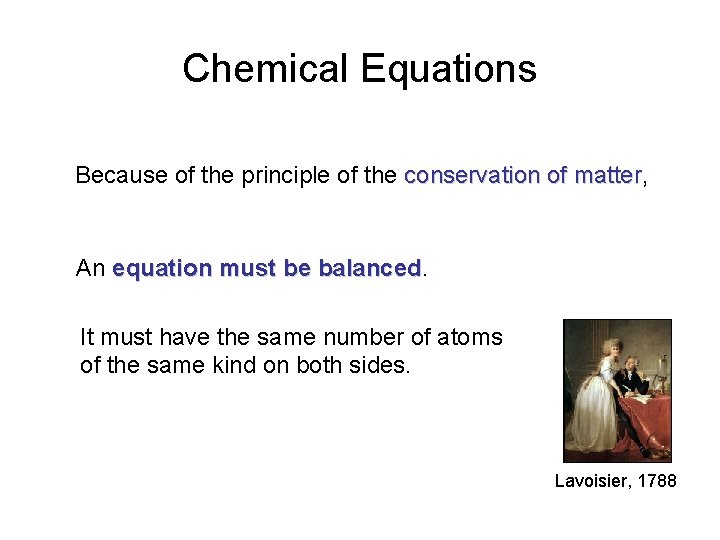
Chemical Equations Because of the principle of the conservation of matter, matter An equation must be balanced It must have the same number of atoms of the same kind on both sides. Lavoisier, 1788

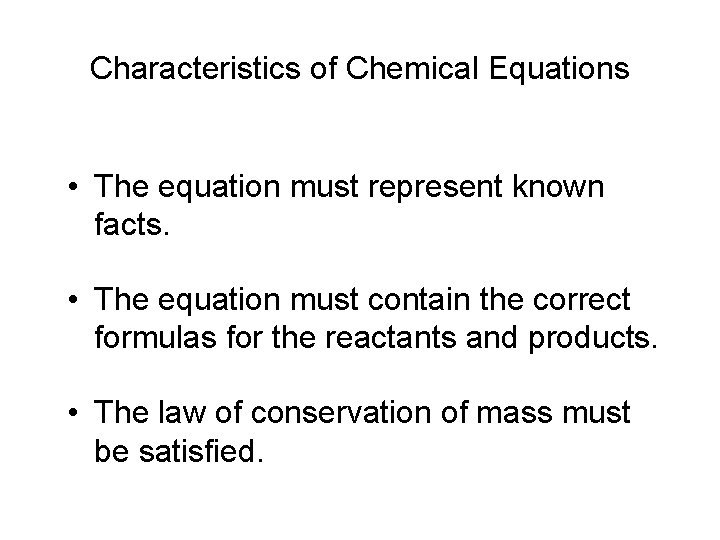
Characteristics of Chemical Equations • The equation must represent known facts. • The equation must contain the correct formulas for the reactants and products. • The law of conservation of mass must be satisfied.

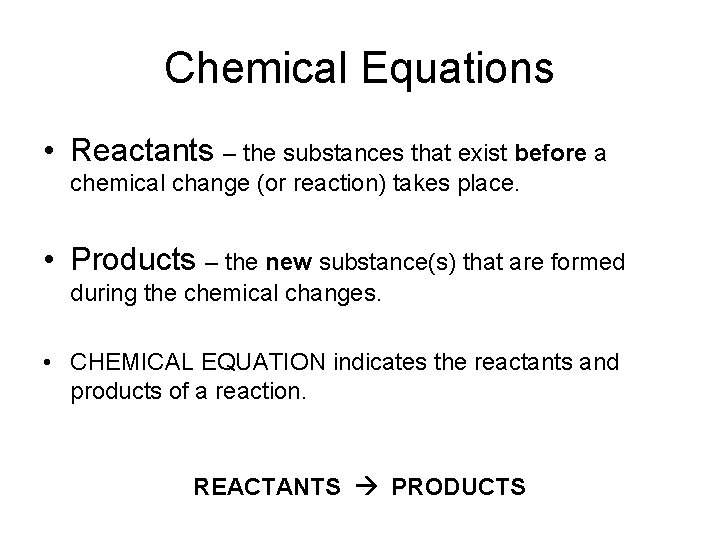
Chemical Equations • Reactants – the substances that exist before a chemical change (or reaction) takes place. • Products – the new substance(s) that are formed during the chemical changes. • CHEMICAL EQUATION indicates the reactants and products of a reaction. REACTANTS PRODUCTS

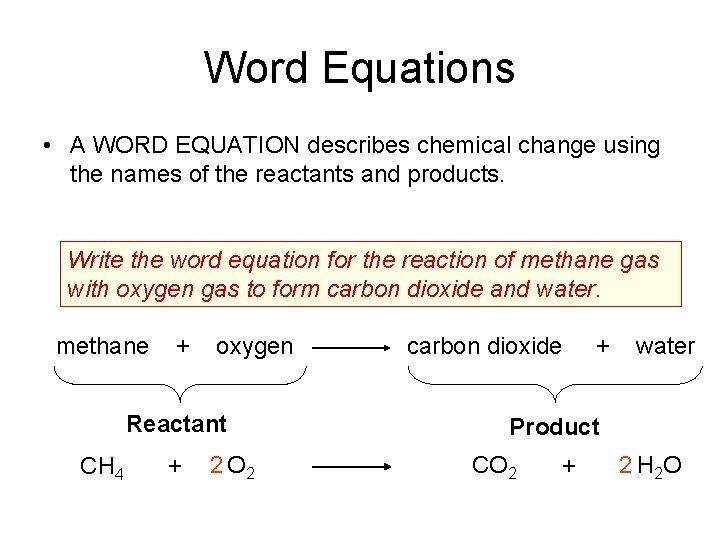
Word Equations • A WORD EQUATION describes chemical change using the names of the reactants and products. Write the word equation for the reaction of methane gas with oxygen gas to form carbon dioxide and water. methane + oxygen Reactant CH 4 + 2 O 2 carbon dioxide + water Product CO 2 + 2 H 2 O

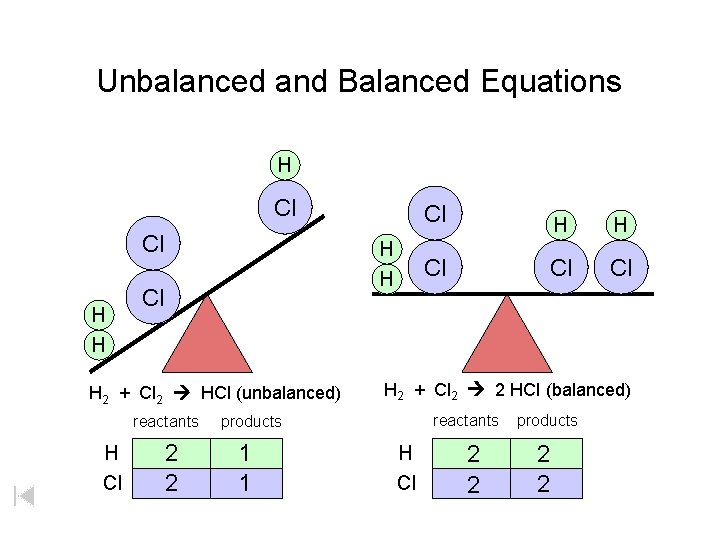
Unbalanced and Balanced Equations H Cl Cl H H Cl H 2 + Cl 2 HCl (unbalanced) reactants H Cl 2 2 H H Cl Cl Cl H 2 + Cl 2 2 HCl (balanced) reactants products 1 1 Cl H Cl 2 2 products 2 2

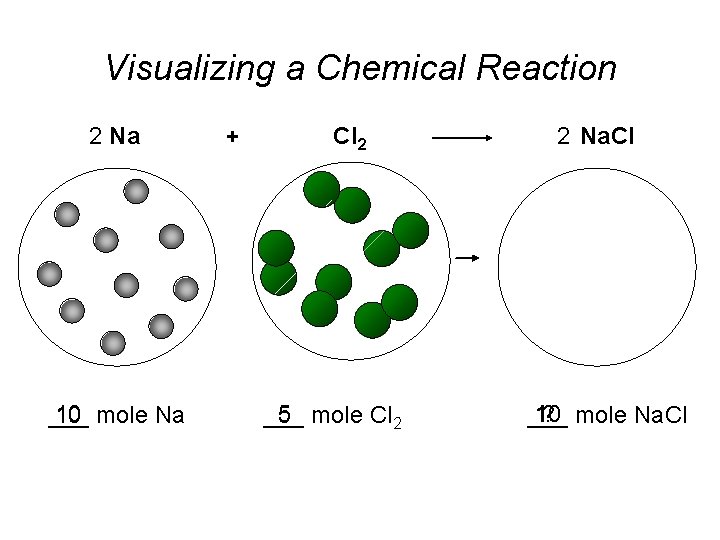
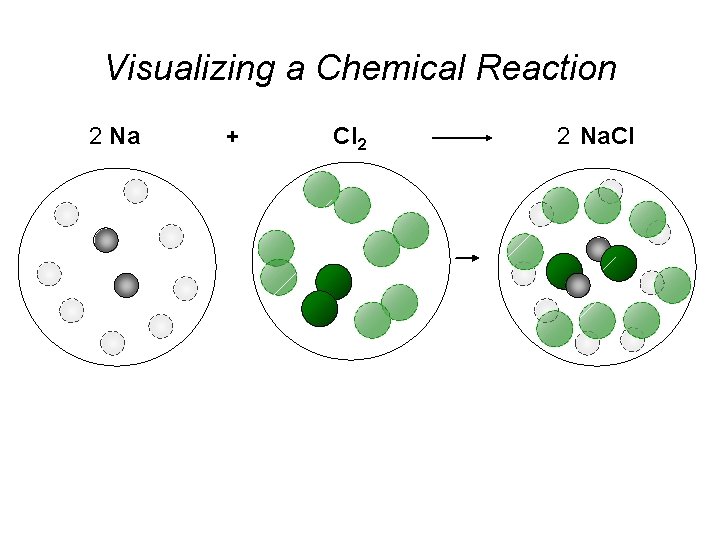
Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

Visualizing a Chemical Reaction 2 Na + Cl 2 2 Na. Cl

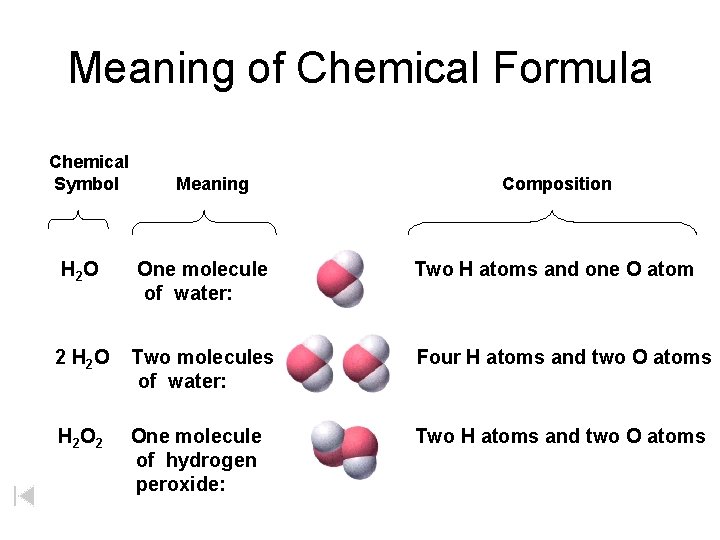
Meaning of Chemical Formula Chemical Symbol Meaning Composition H 2 O One molecule of water: Two H atoms and one O atom 2 H 2 O Two molecules of water: Four H atoms and two O atoms H 2 One molecule of hydrogen peroxide: Two H atoms and two O atoms

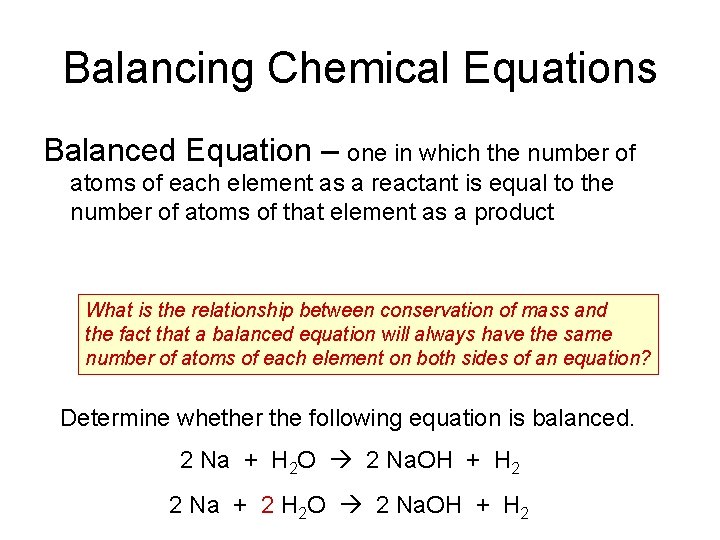
Balancing Chemical Equations Balanced Equation – one in which the number of atoms of each element as a reactant is equal to the number of atoms of that element as a product What is the relationship between conservation of mass and the fact that a balanced equation will always have the same number of atoms of each element on both sides of an equation? Determine whether the following equation is balanced. 2 Na + H 2 O 2 Na. OH + H 2 2 Na + 2 H 2 O 2 Na. OH + H 2

Balancing Chemical Equations • Write a word equation for the reaction. • Write the correct formulas for all reactants and products. • Determine the coefficients that make the equation balance.

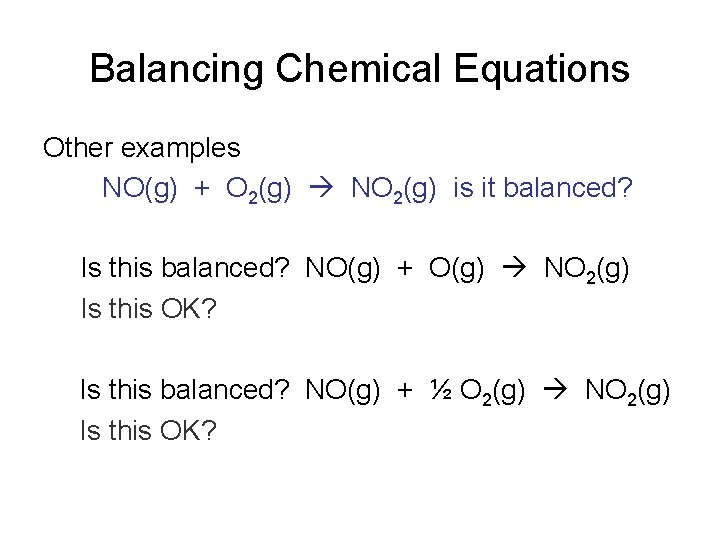
Balancing Chemical Equations Other examples NO(g) + O 2(g) NO 2(g) is it balanced? Is this balanced? NO(g) + O(g) NO 2(g) Is this OK? Is this balanced? NO(g) + ½ O 2(g) NO 2(g) Is this OK?

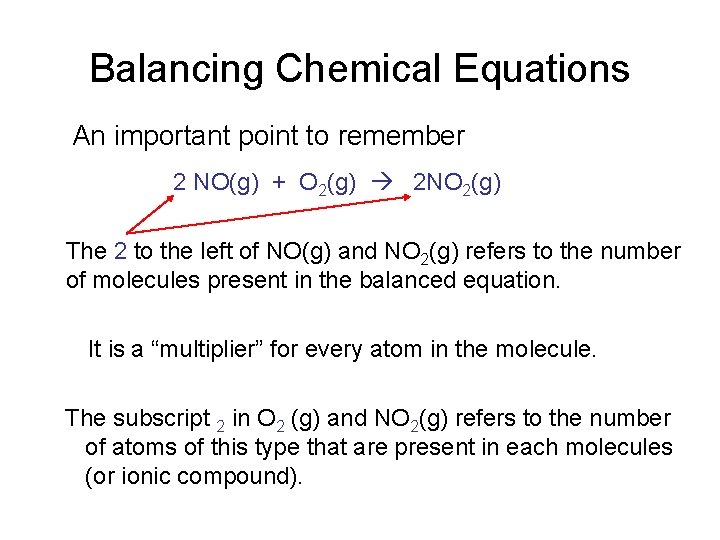
Balancing Chemical Equations An important point to remember 2 NO(g) + O 2(g) 2 NO 2(g) The 2 to the left of NO(g) and NO 2(g) refers to the number of molecules present in the balanced equation. It is a “multiplier” for every atom in the molecule. The subscript 2 in O 2 (g) and NO 2(g) refers to the number of atoms of this type that are present in each molecules (or ionic compound).

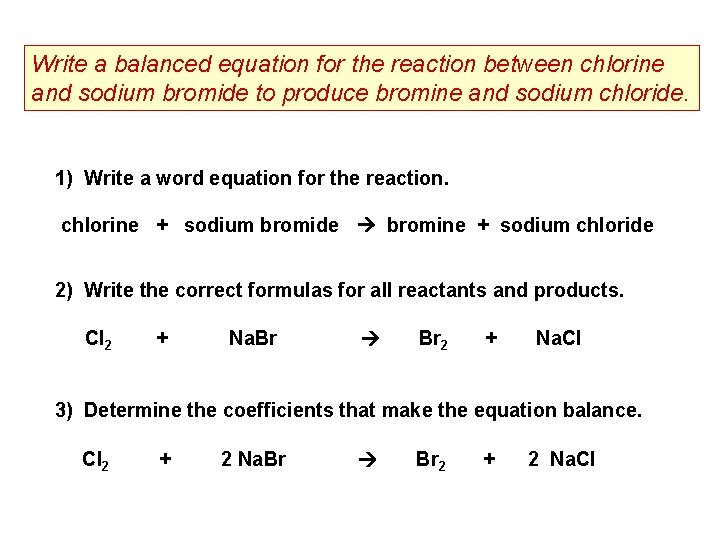
Write a balanced equation for the reaction between chlorine and sodium bromide to produce bromine and sodium chloride. 1) Write a word equation for the reaction. chlorine + sodium bromide bromine + sodium chloride 2) Write the correct formulas for all reactants and products. Cl 2 + Na. Br Br 2 + Na. Cl 3) Determine the coefficients that make the equation balance. Cl 2 + 2 Na. Br Br 2 + 2 Na. Cl

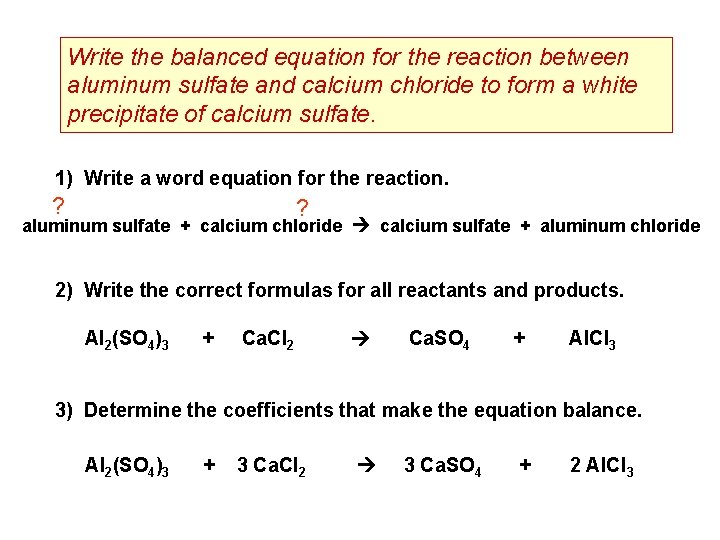
Write the balanced equation for the reaction between aluminum sulfate and calcium chloride to form a white precipitate of calcium sulfate. 1) Write a word equation for the reaction. ? ? aluminum sulfate + calcium chloride calcium sulfate + aluminum chloride 2) Write the correct formulas for all reactants and products. Al 2(SO 4)3 + Ca. Cl 2 Ca. SO 4 + Al. Cl 3 3) Determine the coefficients that make the equation balance. Al 2(SO 4)3 + 3 Ca. Cl 2 3 Ca. SO 4 + 2 Al. Cl 3

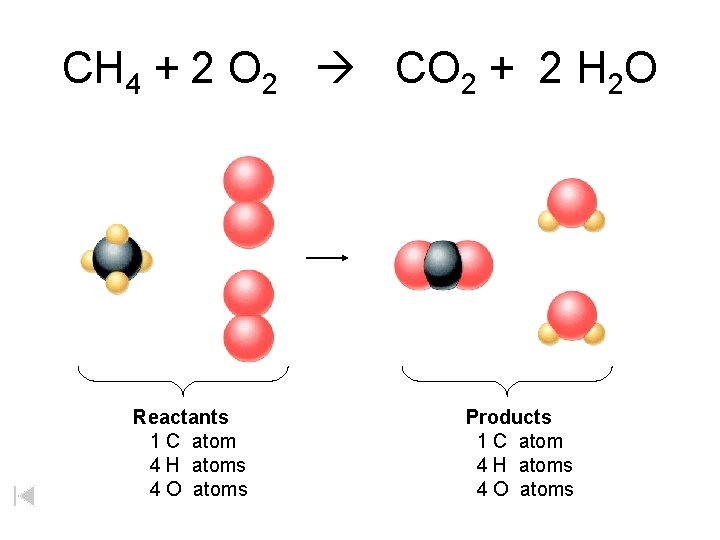
CH 4 + 2 O 2 CO 2 + 2 H 2 O Reactants 1 C atom 4 H atoms 4 O atoms Products 1 C atom 4 H atoms 4 O atoms

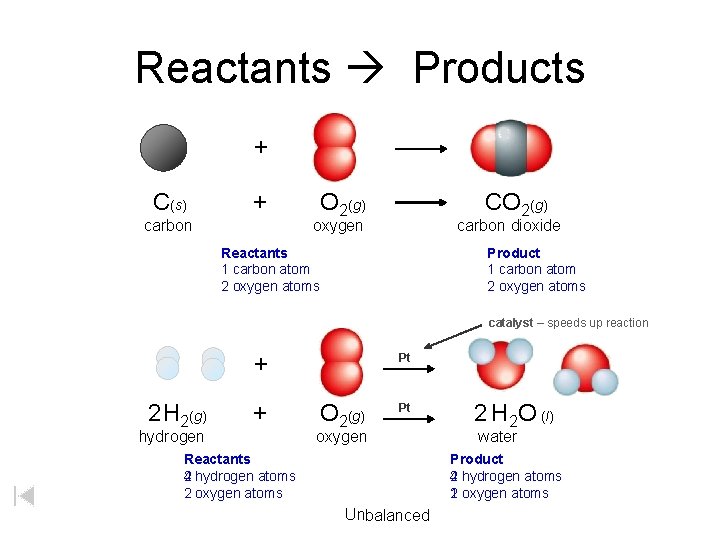
Reactants Products + C(s) + carbon O 2(g) CO 2(g) oxygen carbon dioxide Reactants 1 carbon atom 2 oxygen atoms Product 1 carbon atom 2 oxygen atoms catalyst – speeds up reaction + 2 H 2(g) + hydrogen Pt O 2(g) Pt oxygen Reactants 2 hydrogen atoms 4 2 oxygen atoms 2 H 2 O (l) water Product 2 hydrogen atoms 4 1 oxygen atoms 2 Unbalanced

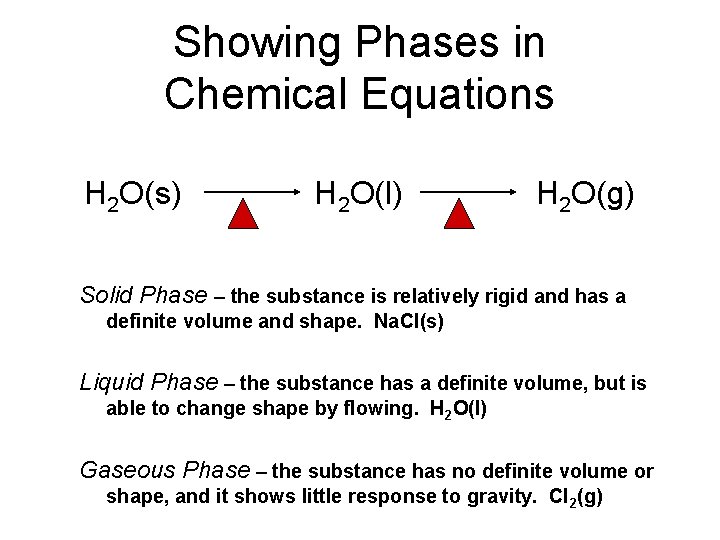
Showing Phases in Chemical Equations H 2 O(s) H 2 O(l) H 2 O(g) Solid Phase – the substance is relatively rigid and has a definite volume and shape. Na. Cl(s) Liquid Phase – the substance has a definite volume, but is able to change shape by flowing. H 2 O(l) Gaseous Phase – the substance has no definite volume or shape, and it shows little response to gravity. Cl 2(g)

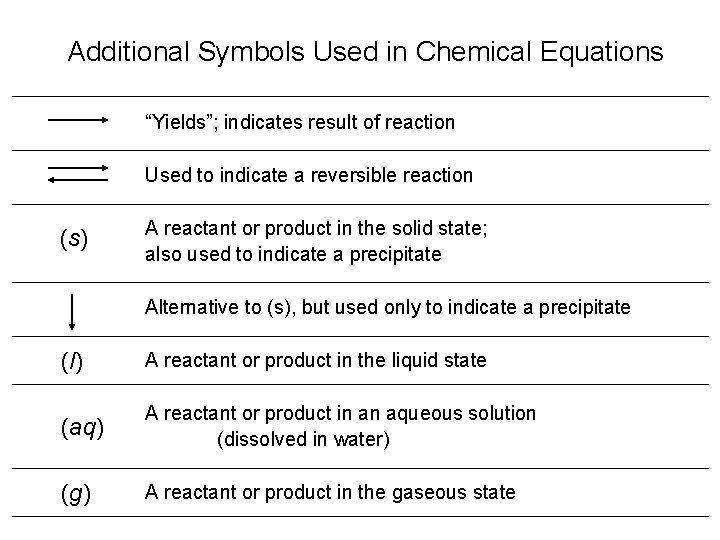
Additional Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction (s) A reactant or product in the solid state; also used to indicate a precipitate Alternative to (s), but used only to indicate a precipitate (l) A reactant or product in the liquid state (aq) A reactant or product in an aqueous solution (dissolved in water) (g) A reactant or product in the gaseous state

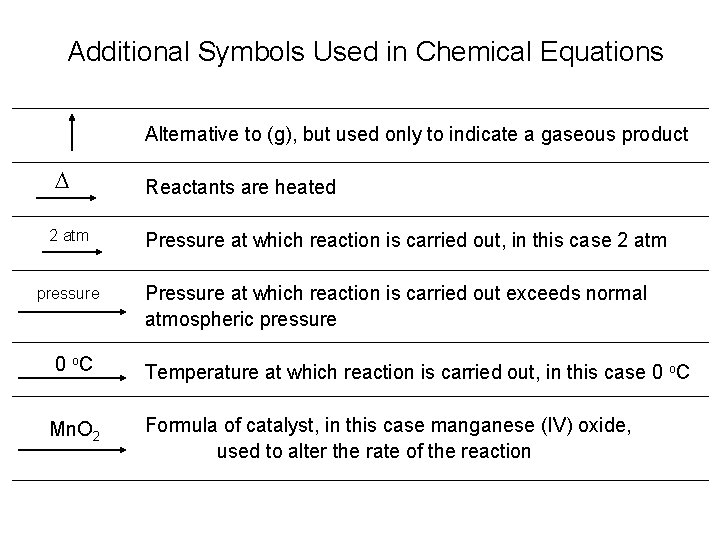
Additional Symbols Used in Chemical Equations Alternative to (g), but used only to indicate a gaseous product D 2 atm pressure Reactants are heated Pressure at which reaction is carried out, in this case 2 atm Pressure at which reaction is carried out exceeds normal atmospheric pressure 0 o. C Temperature at which reaction is carried out, in this case 0 o. C Mn. O 2 Formula of catalyst, in this case manganese (IV) oxide, used to alter the rate of the reaction

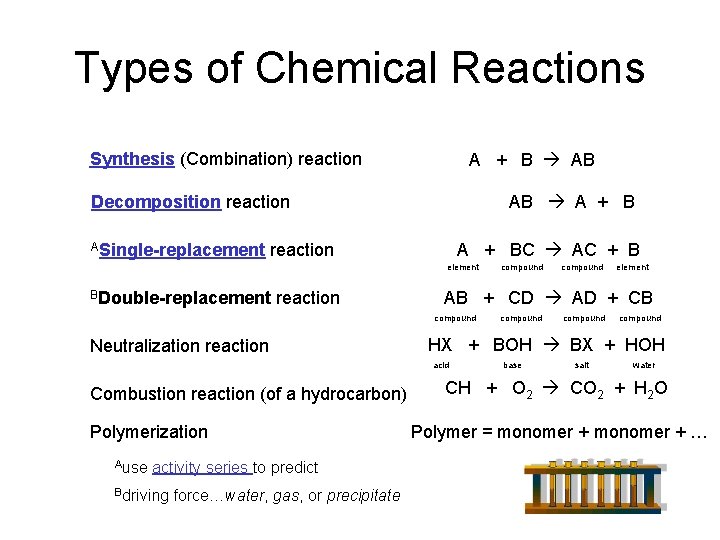
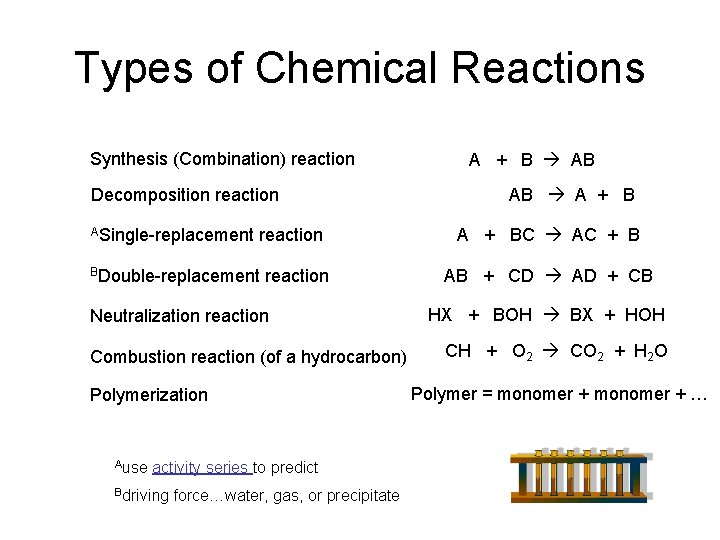
Types of Chemical Reactions Synthesis (Combination) reaction A + B AB AB A + B Decomposition reaction ASingle-replacement A + BC AC + B reaction element BDouble-replacement reaction Polymerization Ause activity series to predict Bdriving force…water, gas, or precipitate element compound HX + BOH BX + HOH acid Combustion reaction (of a hydrocarbon) compound AB + CD AD + CB compound Neutralization reaction compound base salt water CH + O 2 CO 2 + H 2 O Polymer = monomer + …

Types of Chemical Reactions Synthesis (Combination) reaction Decomposition reaction ASingle-replacement reaction BDouble-replacement reaction Neutralization reaction Combustion reaction (of a hydrocarbon) Polymerization Ause activity series to predict Bdriving force…water, gas, or precipitate A + B AB AB A + BC AC + B AB + CD AD + CB HX + BOH BX + HOH CH + O 2 CO 2 + H 2 O Polymer = monomer + …

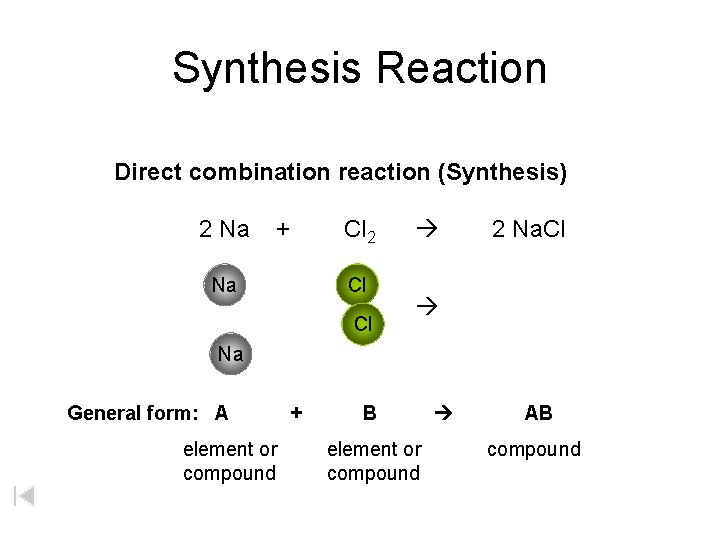
Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Na Cl 2 Cl Cl 2 Na. Cl Na General form: A element or compound + B element or compound AB compound

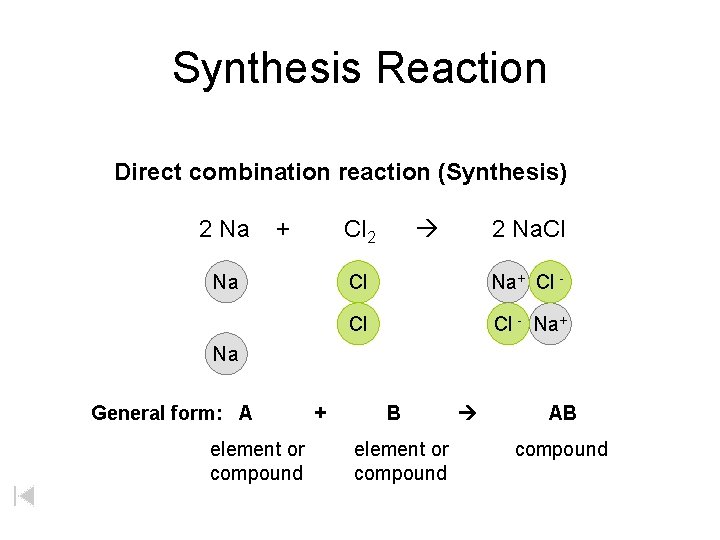
Synthesis Reaction Direct combination reaction (Synthesis) 2 Na + Cl 2 Na. Cl Cl Na+ Cl - Cl Cl - Na+ Na General form: A element or compound + B element or compound AB compound

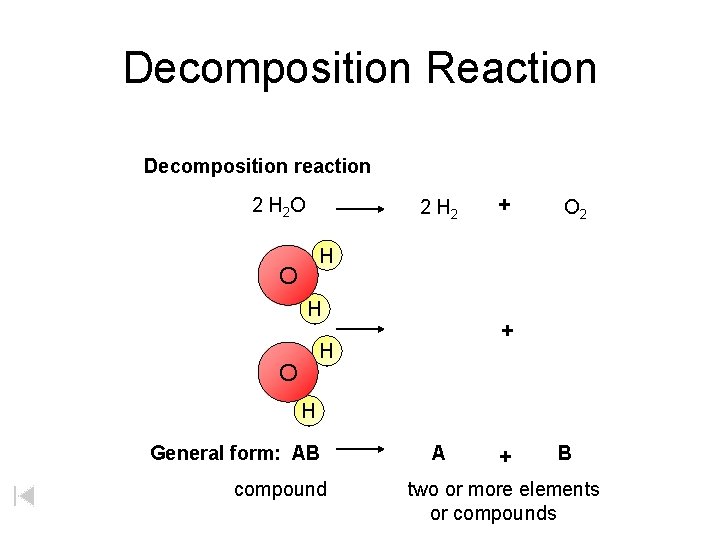
Decomposition Reaction Decomposition reaction 2 H 2 O 2 H 2 + O 2 H O H + H O H General form: AB compound A + B two or more elements or compounds

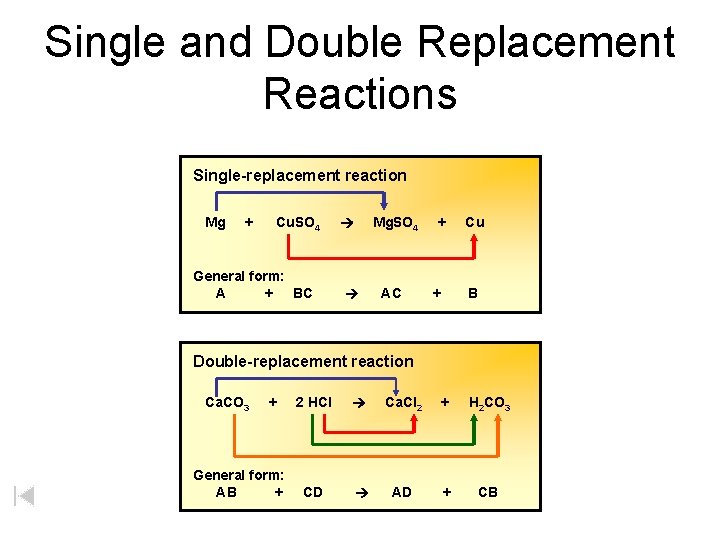
Single and Double Replacement Reactions Single-replacement reaction Mg + Cu. SO 4 General form: A + BC Mg. SO 4 AC + + Cu B Double-replacement reaction Ca. CO 3 + General form: AB + 2 HCl Ca. Cl 2 + H 2 CO 3 CD AD + CB

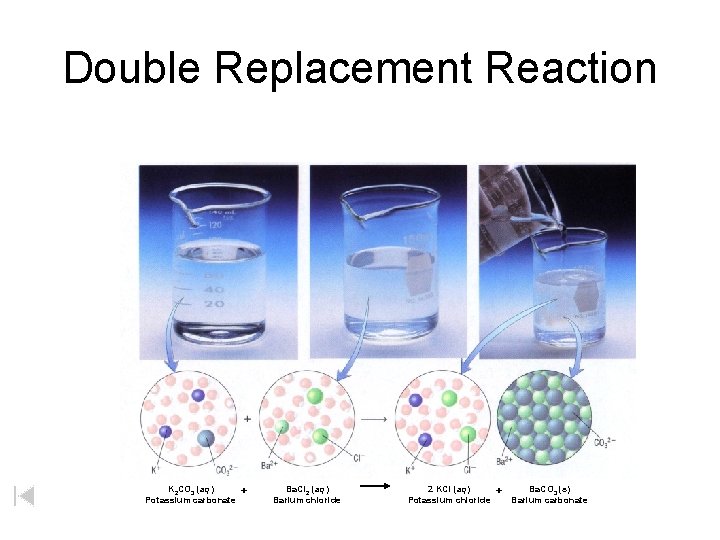
Double Replacement Reaction K 2 CO 3 (aq) Potassium carbonate + Ba. Cl 2 (aq) Barium chloride 2 KCl (aq) Potassium chloride + Ba. CO 3 (s) Barium carbonate

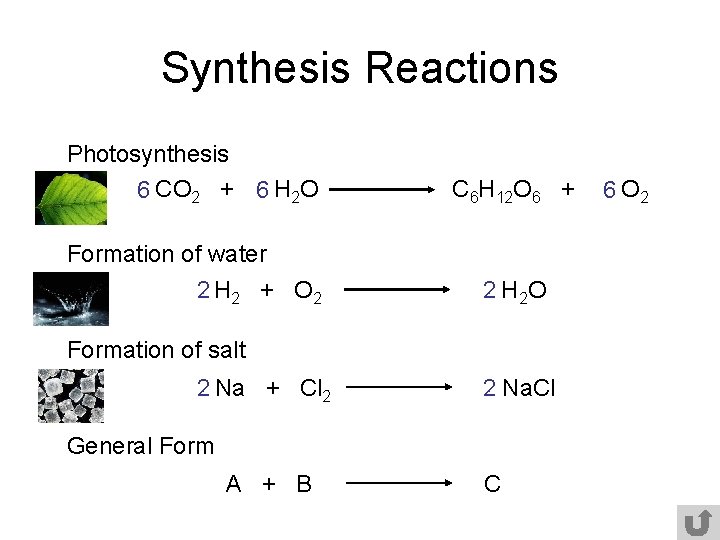
Synthesis Reactions Photosynthesis 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + Formation of water 2 H 2 + O 2 2 H 2 O Formation of salt 2 Na + Cl 2 2 Na. Cl General Form A + B C 6 O 2

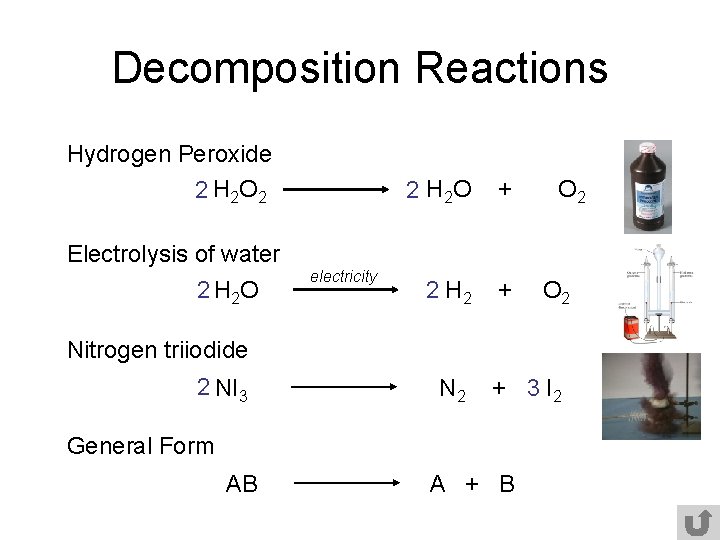
Decomposition Reactions Hydrogen Peroxide 2 H 2 O 2 2 H 2 O + 2 H 2 + O 2 Electrolysis of water 2 H 2 O electricity O 2 Nitrogen triiodide 2 NI 3 N 2 + 3 I 2 General Form AB A + B
 Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Types of reactions
Types of reactions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions I intro
I intro Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Translate word equations to chemical equations
Translate word equations to chemical equations Oxidation half reaction
Oxidation half reaction Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Synthesis reaction types
Synthesis reaction types Slidetodoc.com
Slidetodoc.com Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Phosphorous oxide formula
Phosphorous oxide formula Tips for balancing chemical equations
Tips for balancing chemical equations 50 examples of balanced chemical equations with answers
50 examples of balanced chemical equations with answers Stoichiometry mole island diagram
Stoichiometry mole island diagram Different types of redox reactions
Different types of redox reactions Identify types of reactions
Identify types of reactions Reaction type
Reaction type Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Principles of immuno chemical reactions
Principles of immuno chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products synthesis
Predicting products synthesis Combination reaction example
Combination reaction example Unit 11 chemical reactions
Unit 11 chemical reactions