CHAPTER 4 FOOD CHEMISTRY Food Science I De










- Slides: 16

CHAPTER 4: FOOD CHEMISTRY Food Science I De. Crescentis

PRE-TEST 1. Is food Matter? 2. In order to be matter what 2 characteristics does it need? 3. What is an atom? 4. What are the parts of an atom? 5. What is an element?

6. Name an element most commonly found in food? 7. Give an example of a physical change in food? 8. Give an example of a chemical change in food? 9. What are the phases or states of matter? 10. What is a compound?

WHY IS CHEMISTRY IMPORTANT? Processing food Packaging food Preserving food Adjusting for recipes –altitude

WHAT IS CHEMISTRY? Chemistry is the study of the make-up, structure, and properties of substances and the changes that occur to them. Matter is anything that occupies space and has mass.

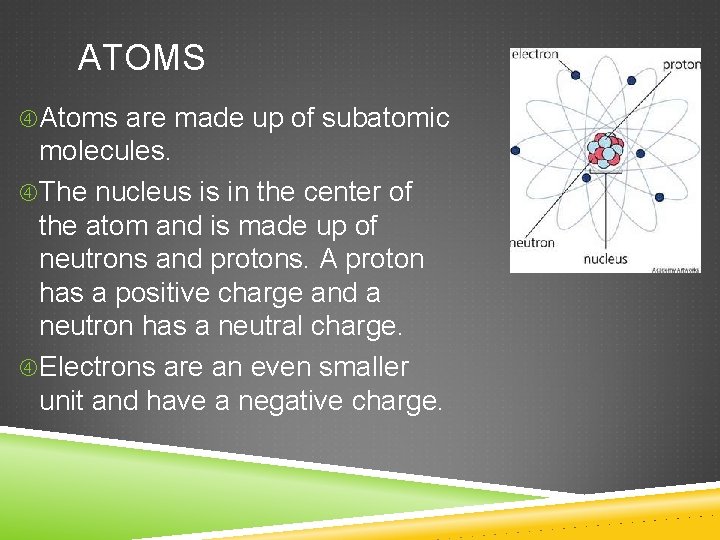
THE BASIC NATURE OF MATTER Everything you encounter is made up of atoms. An atom is the smallest unit of a substance. We cannot see atoms, even with powerful microscopes.

ATOMS Atoms are made up of subatomic molecules. The nucleus is in the center of the atom and is made up of neutrons and protons. A proton has a positive charge and a neutron has a neutral charge. Electrons are an even smaller unit and have a negative charge.

THE CLASSIFICATION OF MATTER Pure Substances: Can be grouped by elements and compounds All basic units are the same. Example: Baking Soda Mixtures: Put together, but not chemically combined Example: Milk contains calcium and salt There are 2 types of mixtures: Heterogeneous and Homogeneous.

MIXTURES Heterogeneous mixture: Non-uniform Examples: salad, vegetable soup. (You can see all ingredients) If you puree soup or salad in a blender it would become homogeneous. Homogeneous mixture: Uniform distribution of particles throughout You cannot tell one part of the mixture from another Examples: alfredo sauce, milk, mayonnaise

MIXTURES CONT. Is hot chocolate a homogenous mixture or a heterogeneous mixture? Solution: One material is dissolved into another. The material dissolved is called the solute.

PHYSICAL AND CHEMICAL CHANGES Physical Change: Change the temperature, shape, size, or physical state of an object without changing the chemical makeup of the substance. Example: freezing water or melting ice Chemical Change: Color, odor, or flavor change. Example: baking bread or fermenting grapes.

ELEMENTS There are 90 naturally occurring elements An element is a substance that only contains one type of atom The number of protons in the nucleus determines which element the atom is.

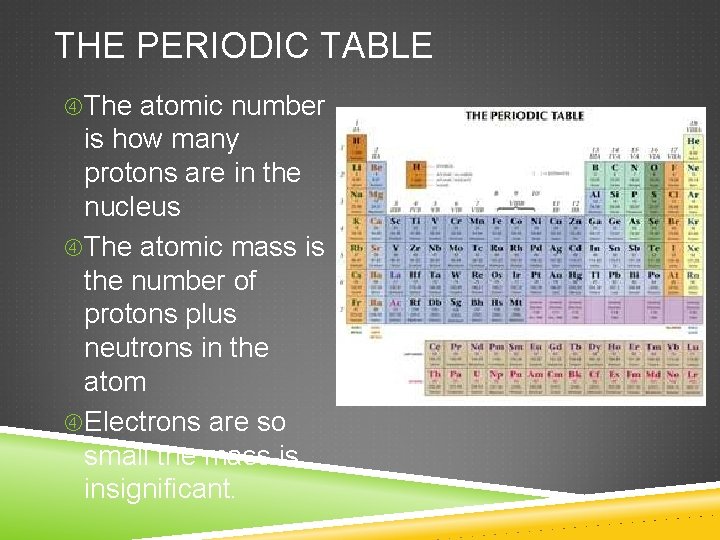
THE PERIODIC TABLE The atomic number is how many protons are in the nucleus The atomic mass is the number of protons plus neutrons in the atom Electrons are so small the mass is insignificant.

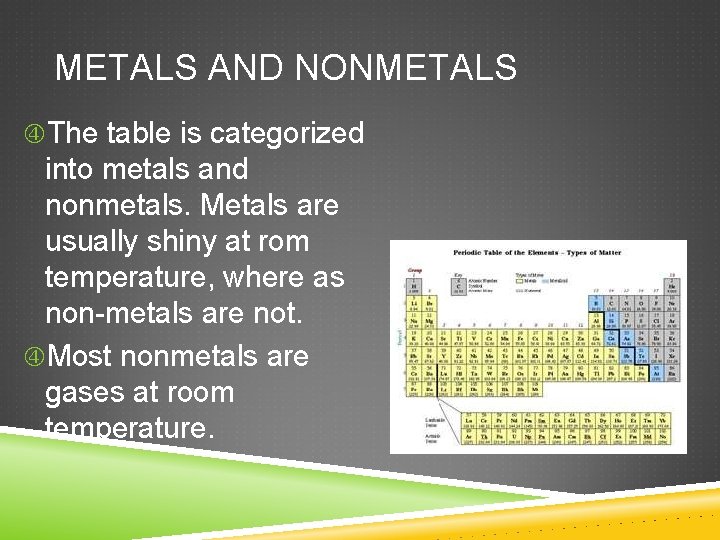
METALS AND NONMETALS The table is categorized into metals and nonmetals. Metals are usually shiny at rom temperature, where as non-metals are not. Most nonmetals are gases at room temperature.

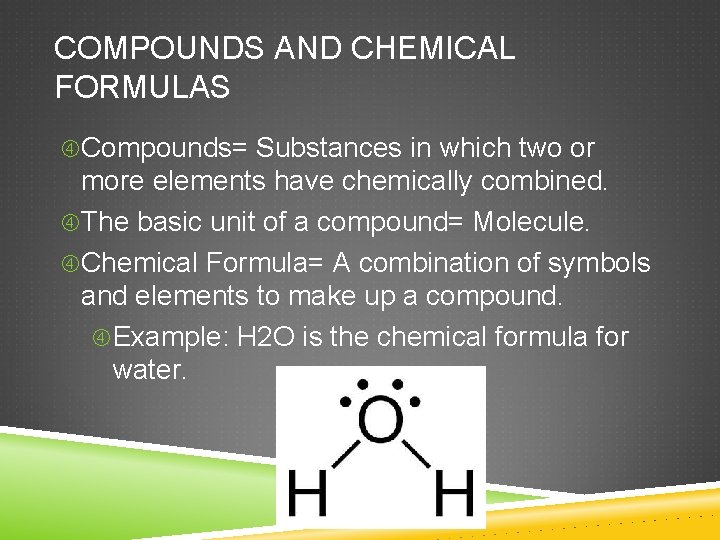
COMPOUNDS AND CHEMICAL FORMULAS Compounds= Substances in which two or more elements have chemically combined. The basic unit of a compound= Molecule. Chemical Formula= A combination of symbols and elements to make up a compound. Example: H 2 O is the chemical formula for water.

BONDS A chemical bond is a force that hold two atoms together. There are two types of chemical bond: Ionic and Covalent. Ionic= electrons are transferred from one atom to another Covalent= atoms share one or more pairs of electrons
 What is your favorite subject answer
What is your favorite subject answer Chemistry central science 14th edition
Chemistry central science 14th edition Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 1 food science an old but new subject
Chapter 1 food science an old but new subject Unit 2 food food food
Unit 2 food food food Food chain sequence
Food chain sequence Gz science chemistry
Gz science chemistry Environmental chemistry science olympiad
Environmental chemistry science olympiad Food adulteration investigatory project
Food adulteration investigatory project Chemistry food and drugs
Chemistry food and drugs Food chemistry and biochemistry
Food chemistry and biochemistry Social science vs natural science
Social science vs natural science Three branches of science
Three branches of science