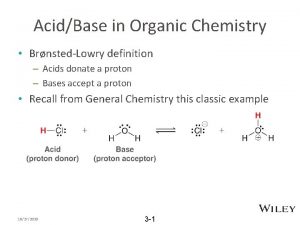
Organic Chemistry Chapter 24 Organic Chemistry Organic chemistry

























- Slides: 92

Organic Chemistry Chapter 24

Organic Chemistry • Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a few important chemical reactions.

The Bonding of Carbon • Because carbon has four valence electrons, it can form four covalent bonds. Carbon forms single, double, and triple bonds to achieve a filled octet. A unique feature of carbon is its ability to bond with other carbons to form long chains or rings of various length.

Why is Carbon Unique? • 1. Forms four covalent bonds • 2. Bonds covalently to: H, O, N, P, S, and all other nonmetals (except noble gases) • 3. Carbon atoms join to form: – a. Chains and b. Rings

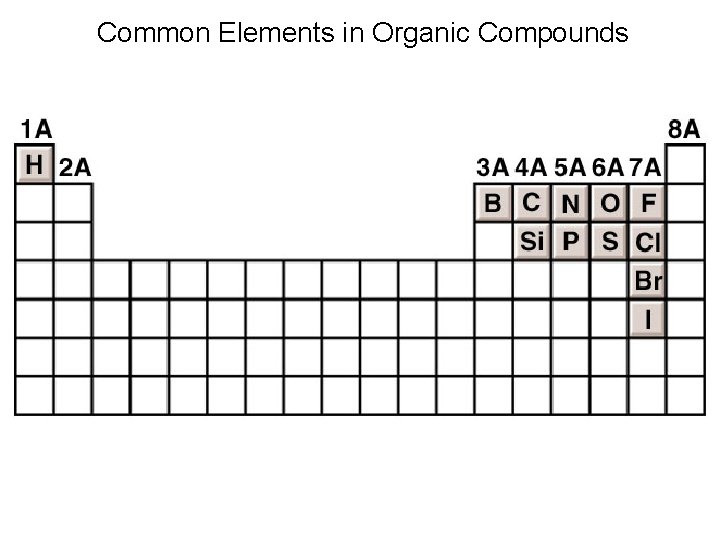
Common Elements in Organic Compounds

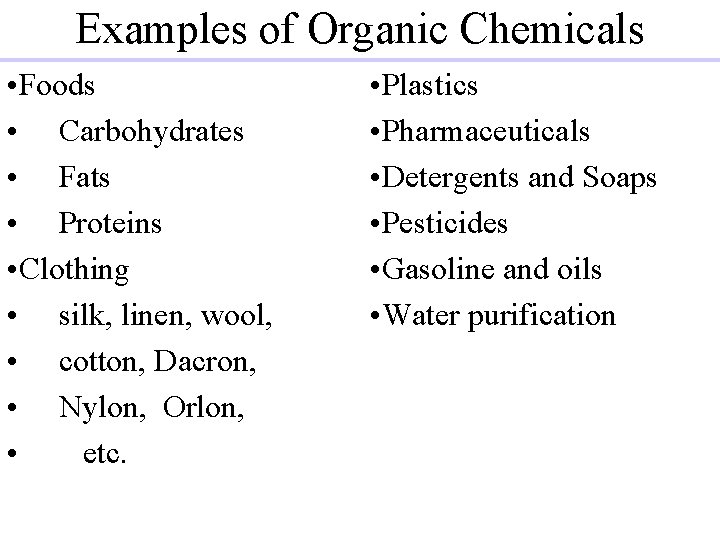
Examples of Organic Chemicals • Foods • Carbohydrates • Fats • Proteins • Clothing • silk, linen, wool, • cotton, Dacron, • Nylon, Orlon, • etc. • Plastics • Pharmaceuticals • Detergents and Soaps • Pesticides • Gasoline and oils • Water purification

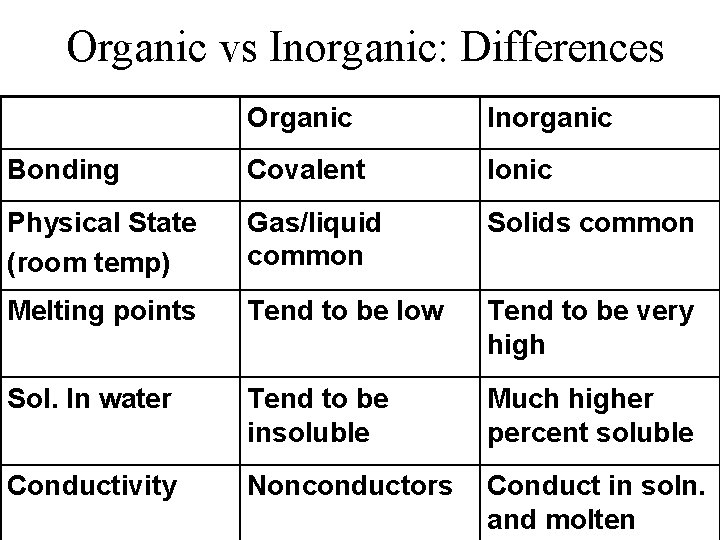
Organic vs Inorganic: Differences Organic Inorganic Bonding Covalent Ionic Physical State (room temp) Gas/liquid common Solids common Melting points Tend to be low Tend to be very high Sol. In water Tend to be insoluble Much higher percent soluble Conductivity Nonconductors Conduct in soln. and molten

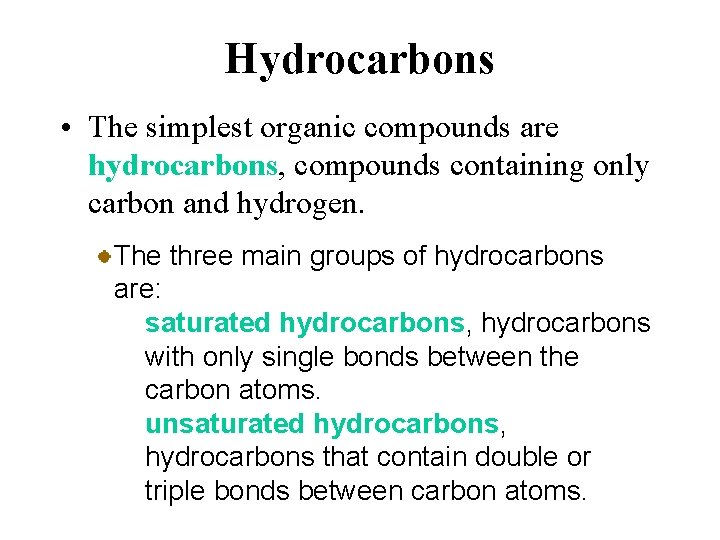
Hydrocarbons • The simplest organic compounds are hydrocarbons, compounds containing only carbon and hydrogen. The three main groups of hydrocarbons are: saturated hydrocarbons, hydrocarbons with only single bonds between the carbon atoms. unsaturated hydrocarbons, hydrocarbons that contain double or triple bonds between carbon atoms.

Hydrocarbons • The simplest organic compounds are hydrocarbons, compounds containing only carbon and hydrogen. The three main groups of hydrocarbons are: aromatic hydrocarbons, hydrocarbons that contain a benzene ring (a sixmembered ring of carbon atoms with alternating single and double carbon bonds described by resonance formulas).

Hydrocarbons • The simplest organic compounds are hydrocarbons, compounds containing only carbon and hydrogen. The saturated and unsaturated hydrocarbons are often referred to as the aliphatic hydrocarbons.

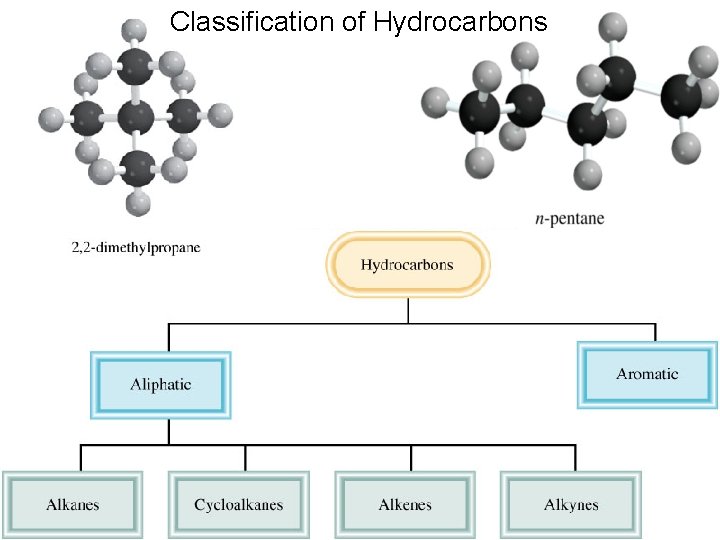
Classification of Hydrocarbons

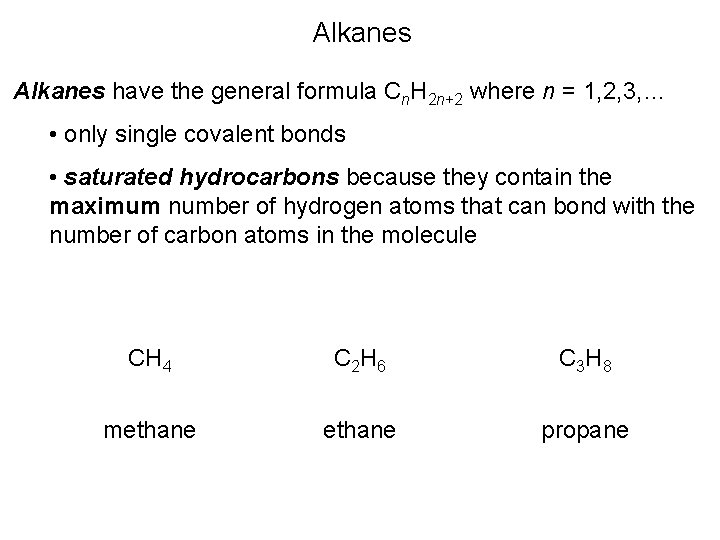
Alkanes have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … • only single covalent bonds • saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule CH 4 C 2 H 6 C 3 H 8 methane propane

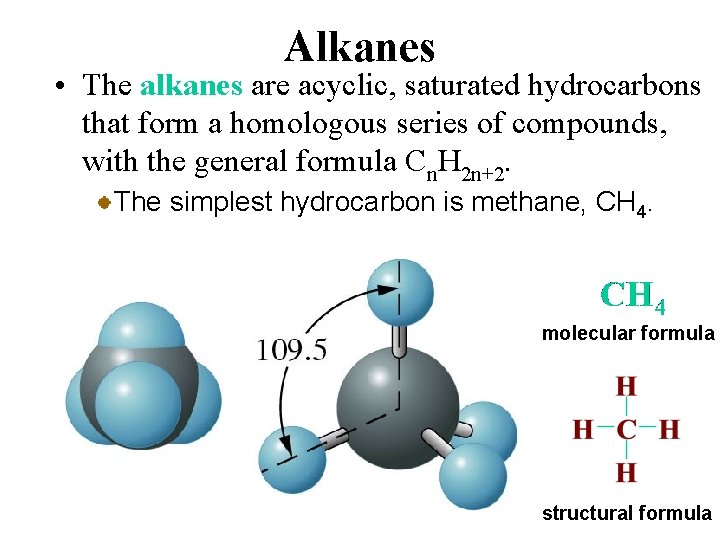
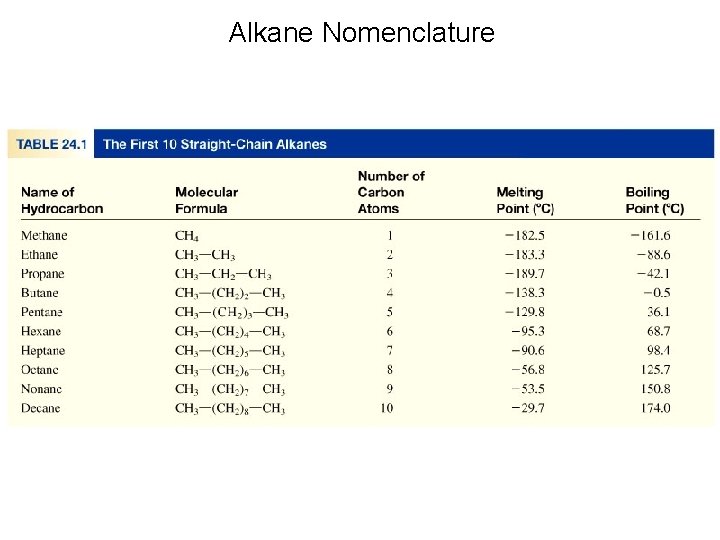
Alkanes • The alkanes are acyclic, saturated hydrocarbons that form a homologous series of compounds, with the general formula Cn. H 2 n+2. The simplest hydrocarbon is methane, CH 4. molecular formula structural formula

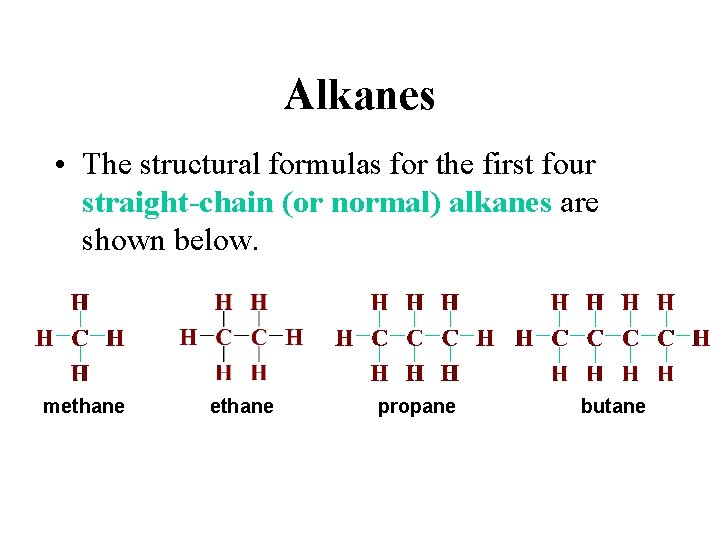
Alkanes • The structural formulas for the first four straight-chain (or normal) alkanes are shown below. methane propane butane

Alkanes • Chemists often use condensed structural formulas, where the bonds around each carbon atom are not explicitly written. methane propane butane


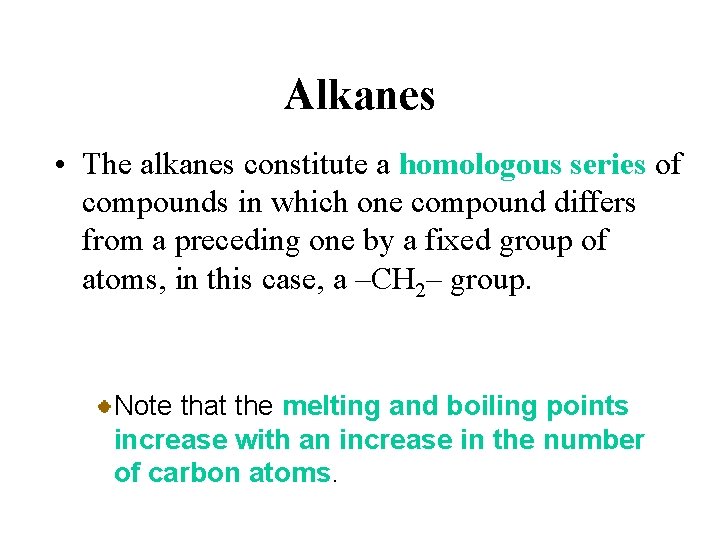
Alkanes • The alkanes constitute a homologous series of compounds in which one compound differs from a preceding one by a fixed group of atoms, in this case, a –CH 2– group. Members of a homologous series have similar chemical properties, and their physical properties change throughout the series in a regular way.

Hydrocarbons: They are nonpolar molecules and consequently are not soluble in water but are soluble in typical nonpolar organic solvents like toluene or pentane.

Alkanes • The alkanes constitute a homologous series of compounds in which one compound differs from a preceding one by a fixed group of atoms, in this case, a –CH 2– group. Note that the melting and boiling points increase with an increase in the number of carbon atoms.

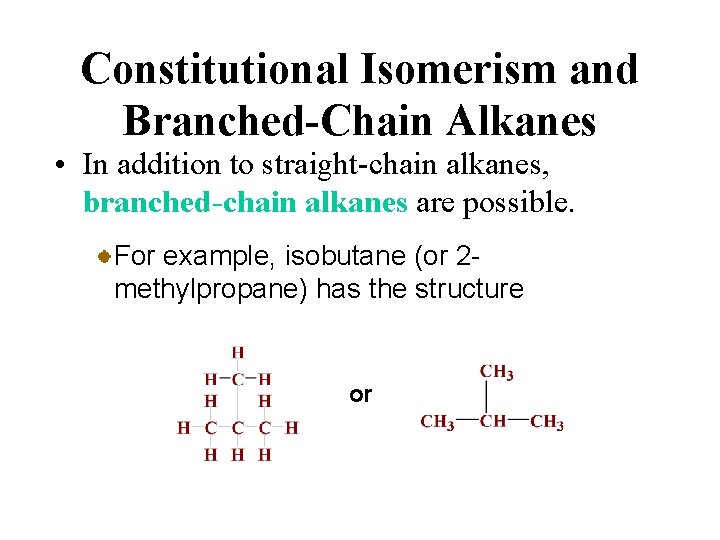
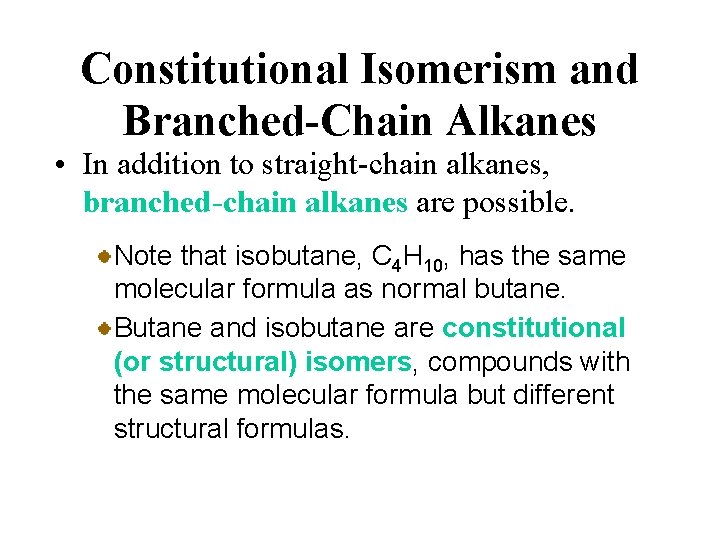
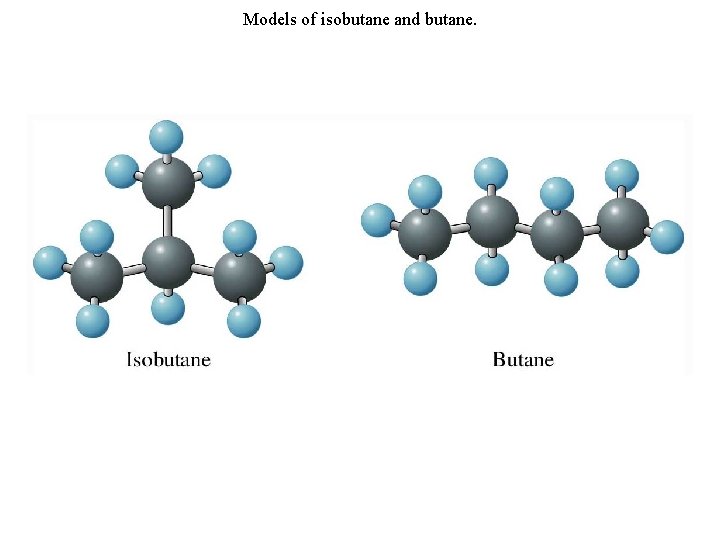
Constitutional Isomerism and Branched-Chain Alkanes • In addition to straight-chain alkanes, branched-chain alkanes are possible. For example, isobutane (or 2 methylpropane) has the structure or

Constitutional Isomerism and Branched-Chain Alkanes • In addition to straight-chain alkanes, branched-chain alkanes are possible. Note that isobutane, C 4 H 10, has the same molecular formula as normal butane. Butane and isobutane are constitutional (or structural) isomers, compounds with the same molecular formula but different structural formulas.

Models of isobutane and butane.

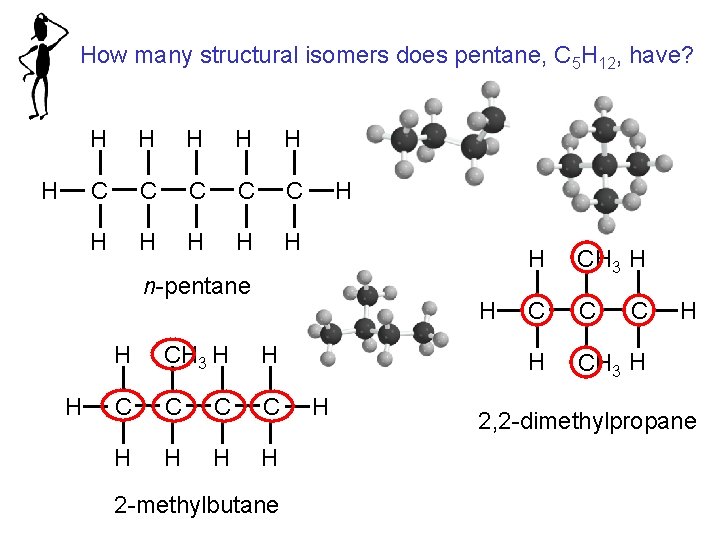
Structural isomers are molecules that have the same molecular formula but different structures

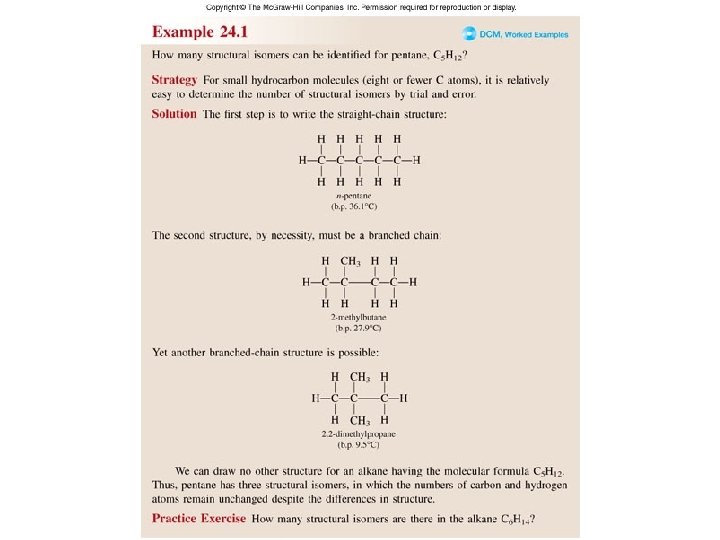
How many structural isomers does pentane, C 5 H 12, have? H H H C C C H H H n-pentane H H H CH 3 H H C C H H 2 -methylbutane H H CH 3 H C C H CH 3 H C H 2, 2 -dimethylpropane

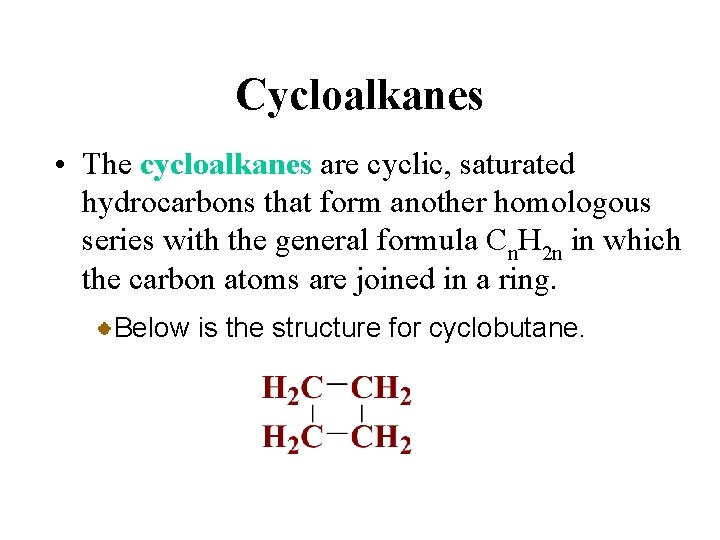
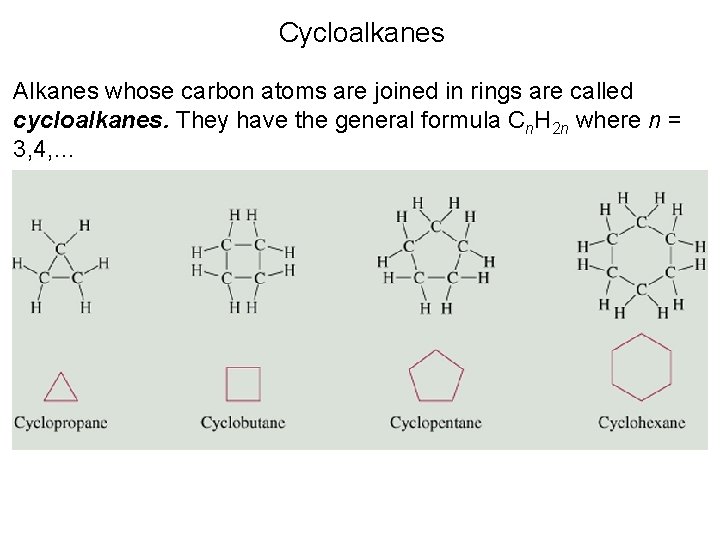
Cycloalkanes • The cycloalkanes are cyclic, saturated hydrocarbons that form another homologous series with the general formula Cn. H 2 n in which the carbon atoms are joined in a ring. Below is the structure for cyclobutane.

Cycloalkanes • The cycloalkanes are cyclic, saturated hydrocarbons that form another homologous series with the general formula Cn. H 2 n in which the carbon atoms are joined in a ring.

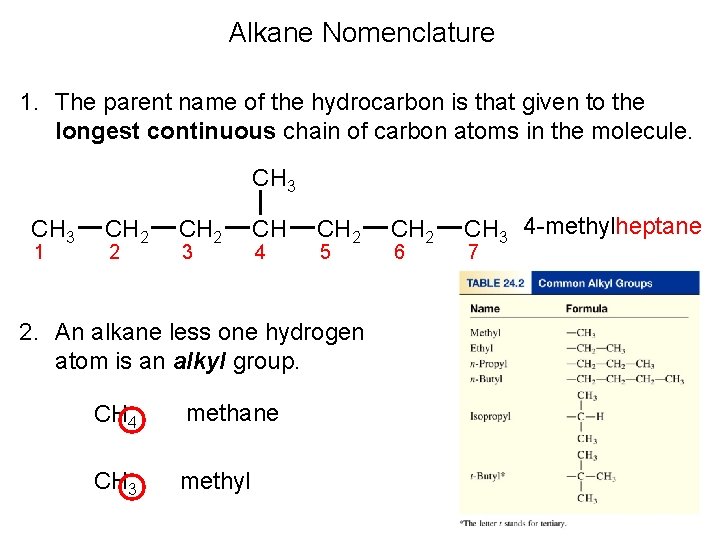
Alkane Nomenclature 1. The parent name of the hydrocarbon is that given to the longest continuous chain of carbon atoms in the molecule. CH 3 1 CH 2 2 CH 2 3 CH 4 CH 2 5 2. An alkane less one hydrogen atom is an alkyl group. CH 4 methane CH 3 methyl CH 2 6 CH 3 4 -methylheptane 7

Alkane Nomenclature

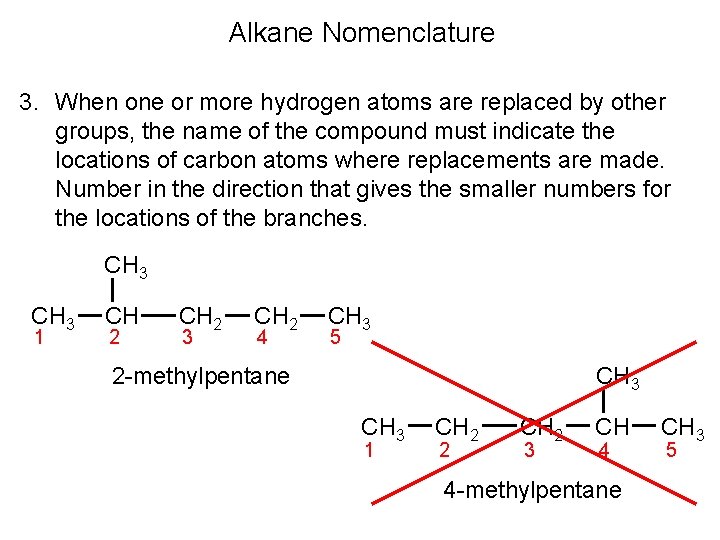
Alkane Nomenclature 3. When one or more hydrogen atoms are replaced by other groups, the name of the compound must indicate the locations of carbon atoms where replacements are made. Number in the direction that gives the smaller numbers for the locations of the branches. CH 3 1 CH 2 3 CH 2 4 CH 3 5 CH 3 2 -methylpentane CH 3 1 CH 2 2 CH 2 3 CH 4 4 -methylpentane CH 3 5

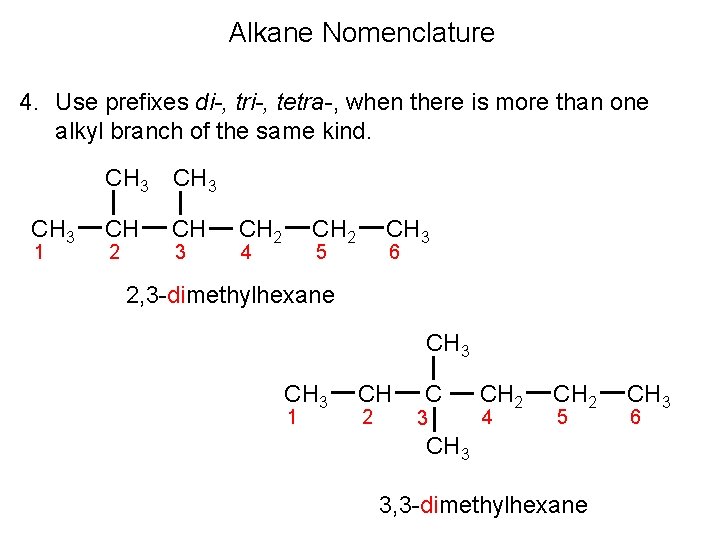
Alkane Nomenclature 4. Use prefixes di-, tri-, tetra-, when there is more than one alkyl branch of the same kind. CH 3 1 CH 3 CH CH 2 3 CH 2 4 CH 3 5 6 2, 3 -dimethylhexane CH 3 1 CH 2 C 3 CH 2 4 CH 2 5 CH 3 3, 3 -dimethylhexane CH 3 6

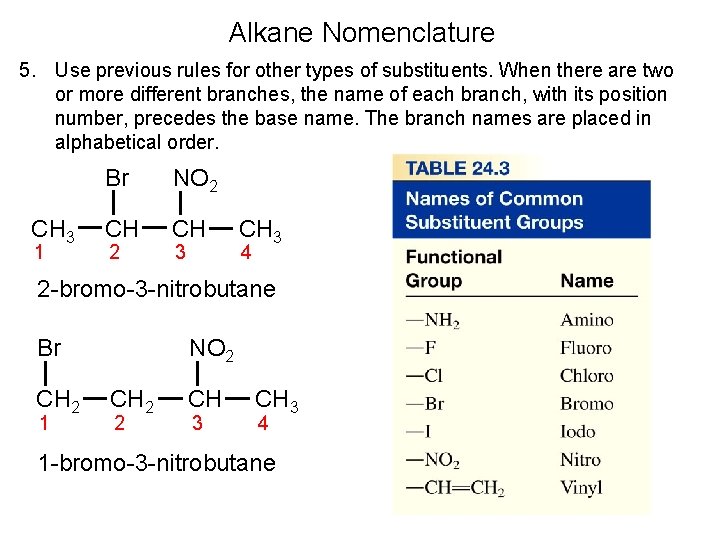
Alkane Nomenclature 5. Use previous rules for other types of substituents. When there are two or more different branches, the name of each branch, with its position number, precedes the base name. The branch names are placed in alphabetical order. CH 3 1 Br NO 2 CH CH 2 3 CH 3 4 2 -bromo-3 -nitrobutane Br CH 2 1 NO 2 CH 2 2 CH 3 4 1 -bromo-3 -nitrobutane

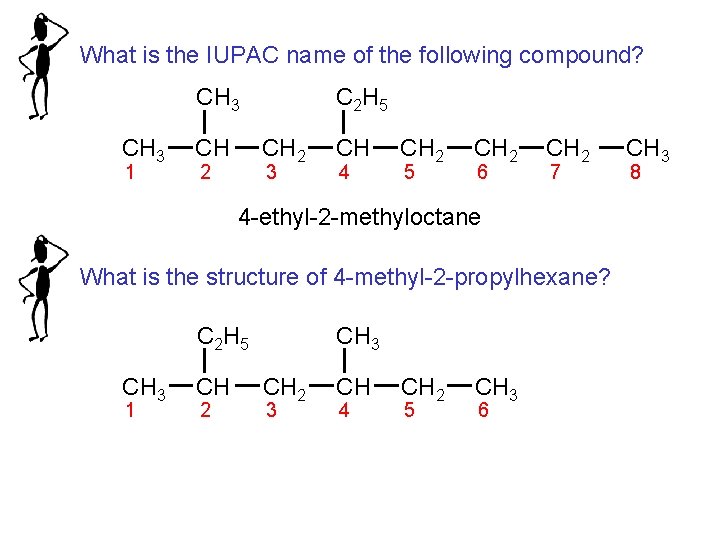
What is the IUPAC name of the following compound? CH 3 1 CH C 2 H 5 CH 2 2 3 CH 4 CH 2 5 CH 2 6 CH 2 7 4 -ethyl-2 -methyloctane What is the structure of 4 -methyl-2 -propylhexane? C 2 H 5 CH 3 1 CH 2 CH 3 CH 2 3 CH 4 CH 2 5 CH 3 6 CH 3 8

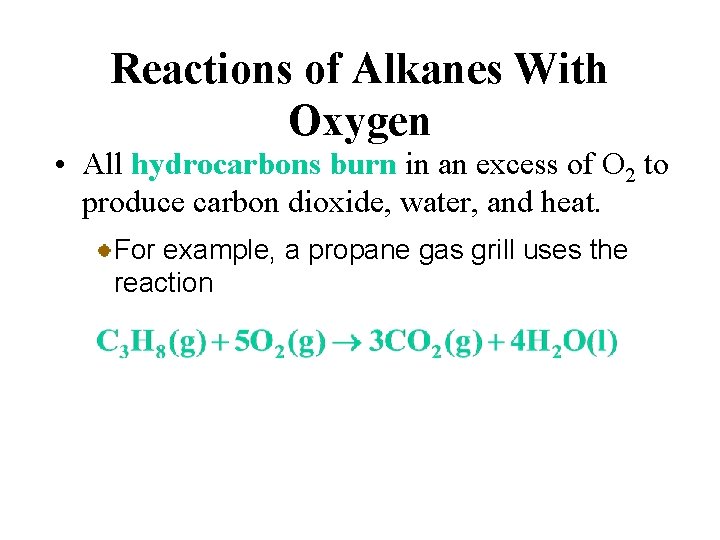
Reactions of Alkanes With Oxygen • All hydrocarbons burn in an excess of O 2 to produce carbon dioxide, water, and heat. For example, a propane gas grill uses the reaction

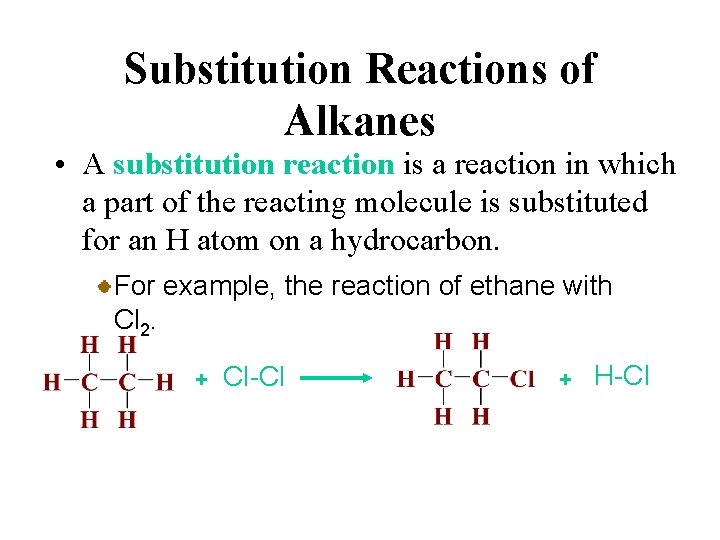
Substitution Reactions of Alkanes • A substitution reaction is a reaction in which a part of the reacting molecule is substituted for an H atom on a hydrocarbon. For example, the reaction of ethane with Cl 2. + Cl-Cl + H-Cl

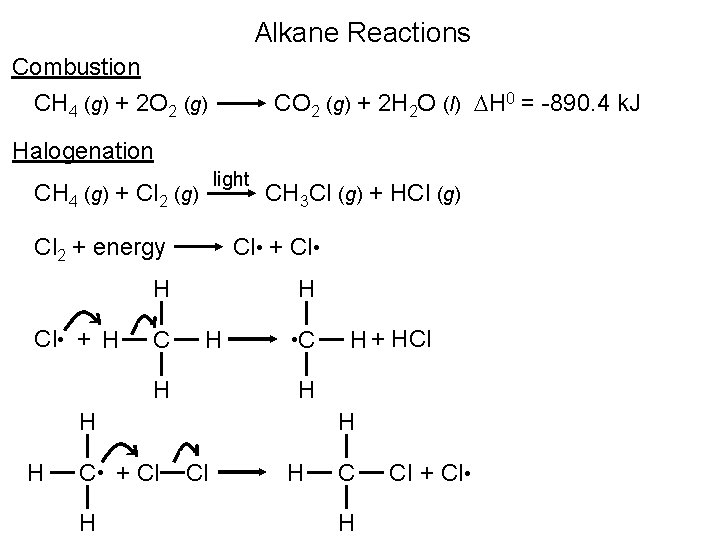
Alkane Reactions Combustion CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (l) DH 0 = -890. 4 k. J Halogenation light CH 4 (g) + Cl 2 (g) CH 3 Cl (g) + HCl (g) Cl 2 + energy Cl • + Cl • H H Cl • + H C H H • C H H H C • + Cl H H + HCl H C H Cl + Cl •

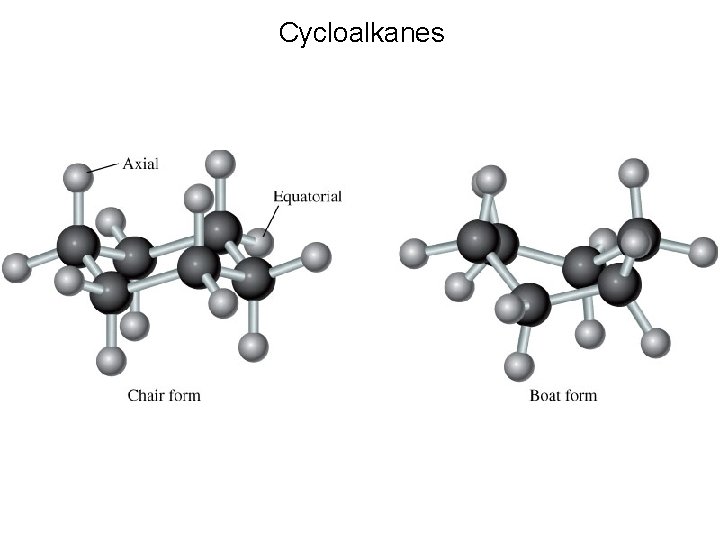
Cycloalkanes Alkanes whose carbon atoms are joined in rings are called cycloalkanes. They have the general formula Cn. H 2 n where n = 3, 4, …

Cycloalkanes

Alkenes and Alkynes • The alkenes and alkynes are unsaturated hydrocarbons (cyclic or acyclic) that contain carbon-carbon double or triple bonds. Under proper conditions, molecular hydrogen can be added to an alkene or an alkyne to produce a saturated compound in a process called catalytic hydrogenation.

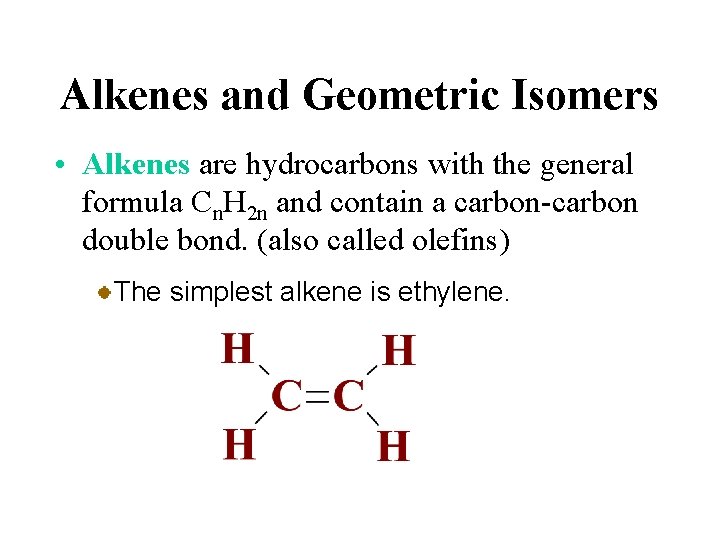
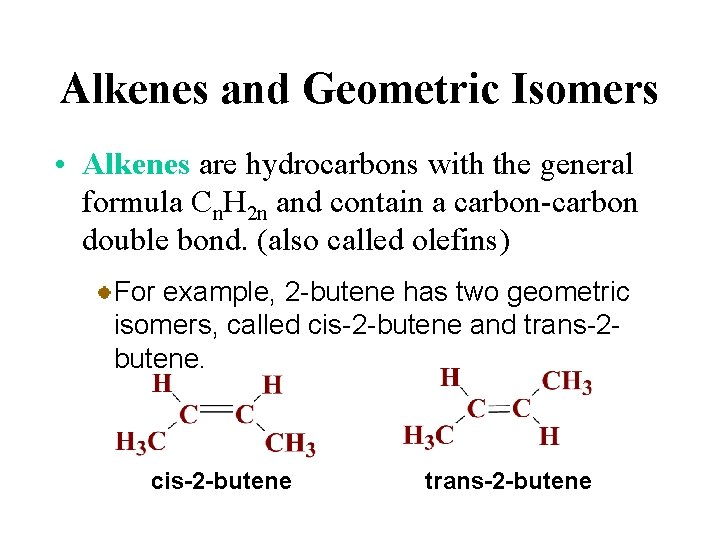
Alkenes and Geometric Isomers • Alkenes are hydrocarbons with the general formula Cn. H 2 n and contain a carbon-carbon double bond. (also called olefins) The simplest alkene is ethylene.

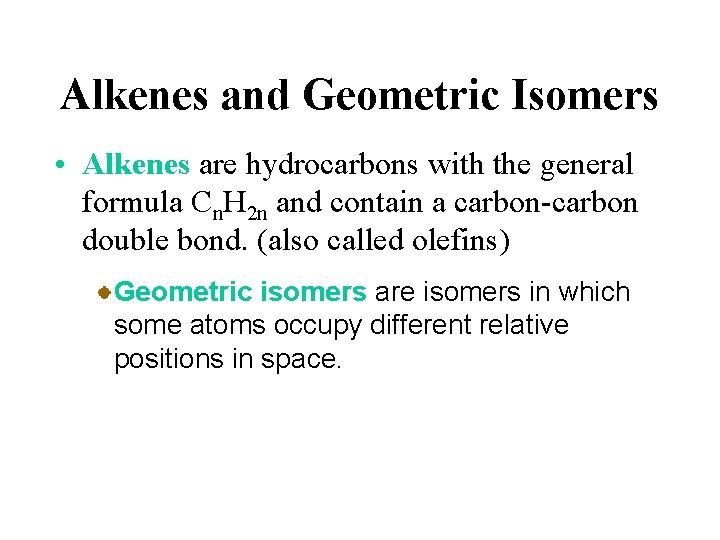
Alkenes and Geometric Isomers • Alkenes are hydrocarbons with the general formula Cn. H 2 n and contain a carbon-carbon double bond. (also called olefins) Geometric isomers are isomers in which some atoms occupy different relative positions in space.

achiral

Alkenes and Geometric Isomers • Alkenes are hydrocarbons with the general formula Cn. H 2 n and contain a carbon-carbon double bond. (also called olefins) For example, 2 -butene has two geometric isomers, called cis-2 -butene and trans-2 butene. cis-2 -butene trans-2 -butene

Alkenes have the general formula Cn. H 2 n where n = 2, 3, … • contain at least one carbon-carbon double bond • also called olefins CH CH 2 CH 3 CH 1 -butene Cl Cl C H Cl H C cis-dichloroethylene CH 3 2 -butene C H CH H C Cl trans-dichloroethylene

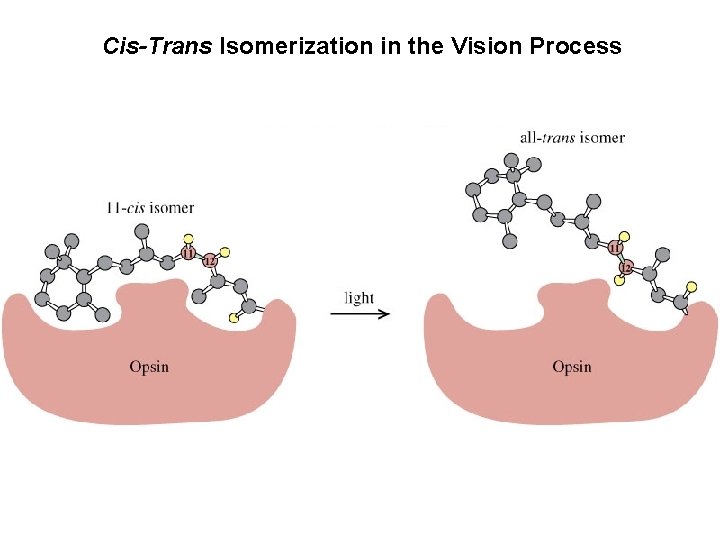
Cis-Trans Isomerization in the Vision Process

Oxidation Reactions of Alkenes • Because alkenes are hydrocarbons, they undergo complete combustion reactions with oxygen. Unsaturated hydrocarbons can also be partially oxidized under relatively mild conditions. For example, when aqueous potassium permanganate is added to an alkene (or alkyne), the purple color of KMn. O 4 fades as a brown precipitate of Mn. O 2 forms.

Test for unsaturation using KMn. O 4(aq) solution.

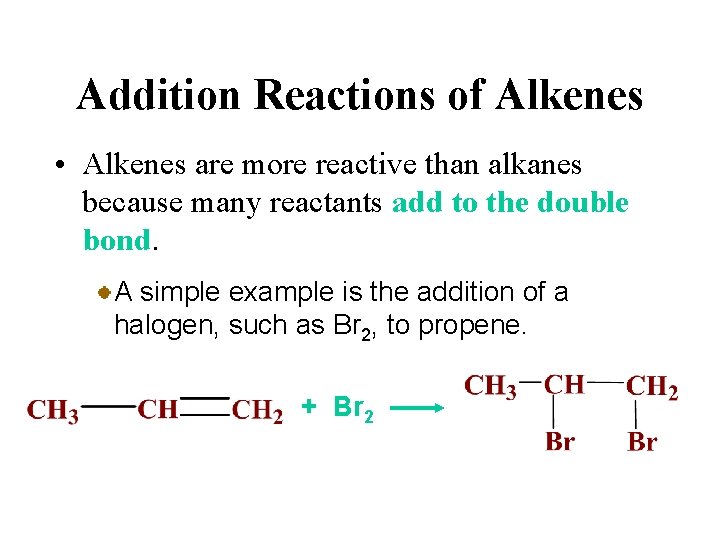
Addition Reactions of Alkenes • Alkenes are more reactive than alkanes because many reactants add to the double bond. An addition reaction is a reaction in which parts of a reactant are added to each carbon atom of a carbon-carbon double bond which converts to a carbon-carbon single bond.

Addition Reactions of Alkenes • Alkenes are more reactive than alkanes because many reactants add to the double bond. A simple example is the addition of a halogen, such as Br 2, to propene. + Br 2

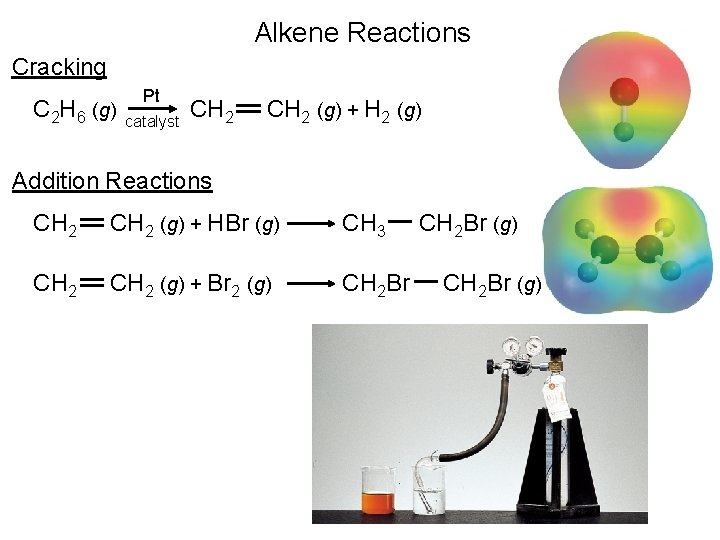
Alkene Reactions Cracking C 2 H 6 (g) Pt catalyst CH 2 (g) + H 2 (g) Addition Reactions CH 2 (g) + HBr (g) CH 3 CH 2 (g) + Br 2 (g) CH 2 Br (g)

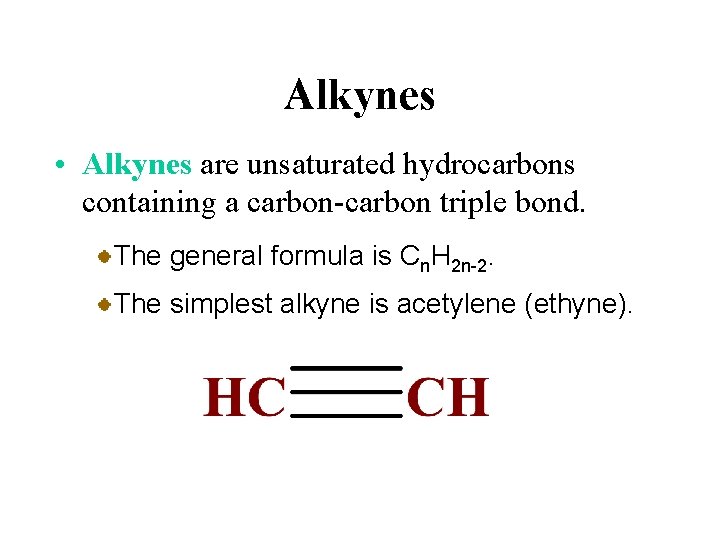
Alkynes • Alkynes are unsaturated hydrocarbons containing a carbon-carbon triple bond. The general formula is Cn. H 2 n-2. The simplest alkyne is acetylene (ethyne).

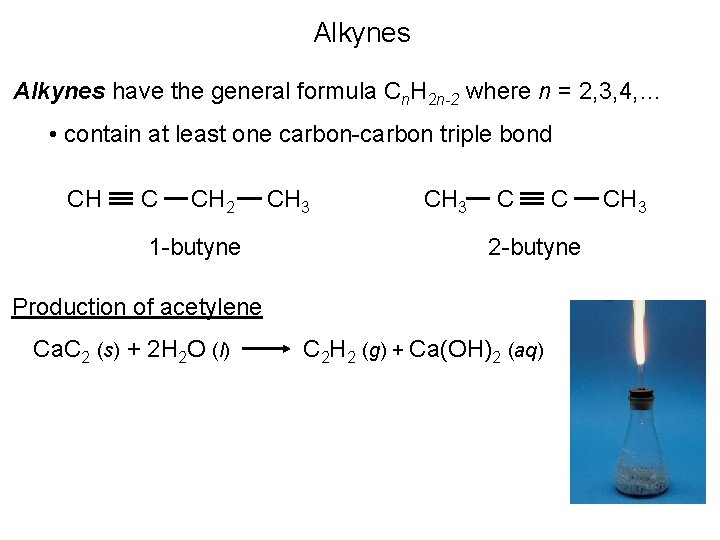
Alkynes have the general formula Cn. H 2 n-2 where n = 2, 3, 4, … • contain at least one carbon-carbon triple bond CH C CH 2 1 -butyne CH 3 C 2 -butyne Production of acetylene Ca. C 2 (s) + 2 H 2 O (l) C C 2 H 2 (g) + Ca(OH)2 (aq) CH 3

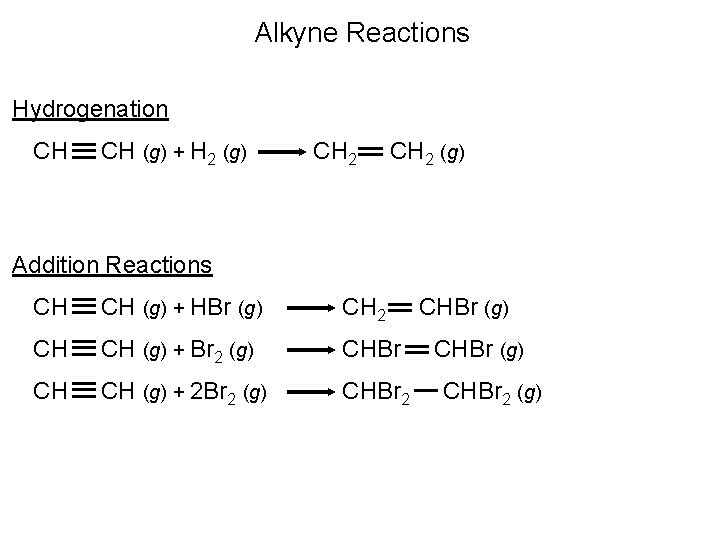
Alkyne Reactions Hydrogenation CH CH (g) + H 2 (g) CH 2 (g) Addition Reactions CH CH (g) + HBr (g) CH 2 CH CH (g) + Br 2 (g) CHBr CH CH (g) + 2 Br 2 (g) CHBr 2 CHBr (g) CHBr 2 (g)

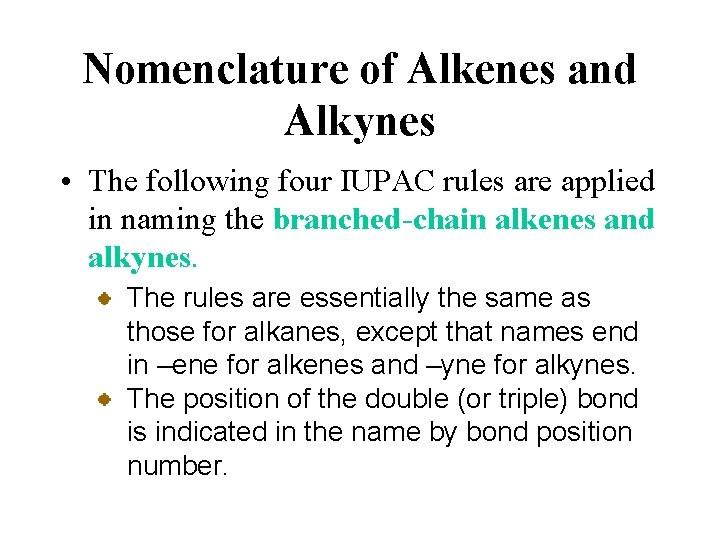
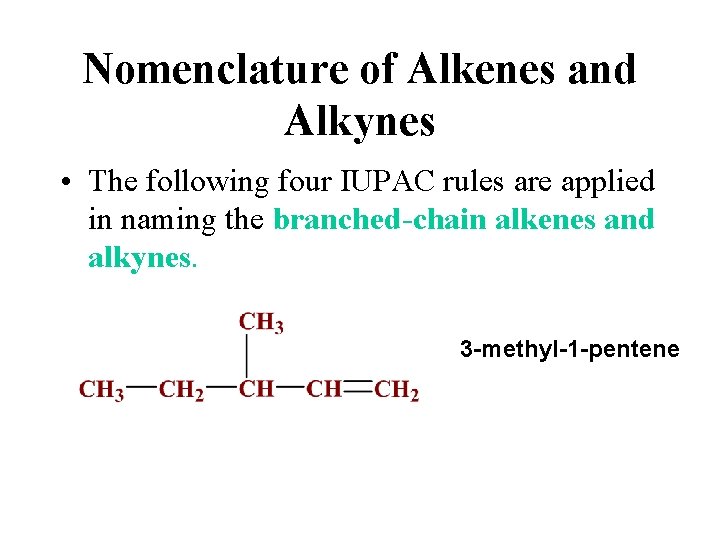
Nomenclature of Alkenes and Alkynes • The following four IUPAC rules are applied in naming the branched-chain alkenes and alkynes. The rules are essentially the same as those for alkanes, except that names end in –ene for alkenes and –yne for alkynes. The position of the double (or triple) bond is indicated in the name by bond position number.

Nomenclature of Alkenes and Alkynes • The following four IUPAC rules are applied in naming the branched-chain alkenes and alkynes. 3 -methyl-1 -pentene

Nomenclature of Alkenes and Alkynes • The following four IUPAC rules are applied in naming the branched-chain alkenes and alkynes. Recall that alkenes also exhibit cis and trans isomerism and so either cis or trans must be included in the name.

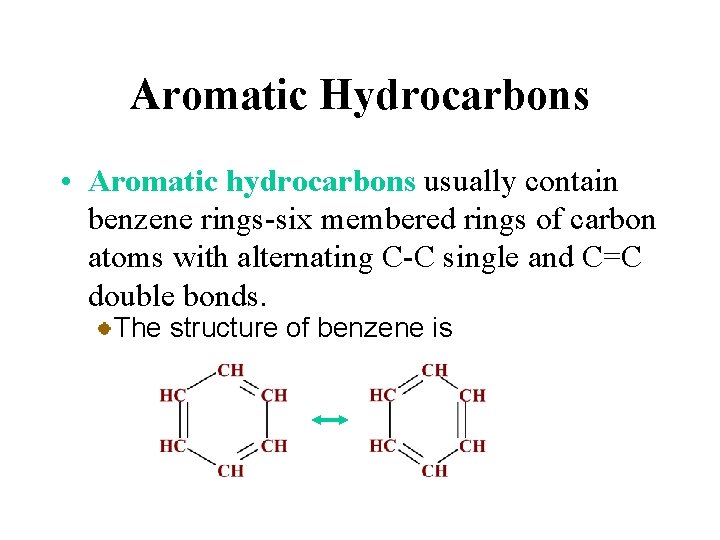
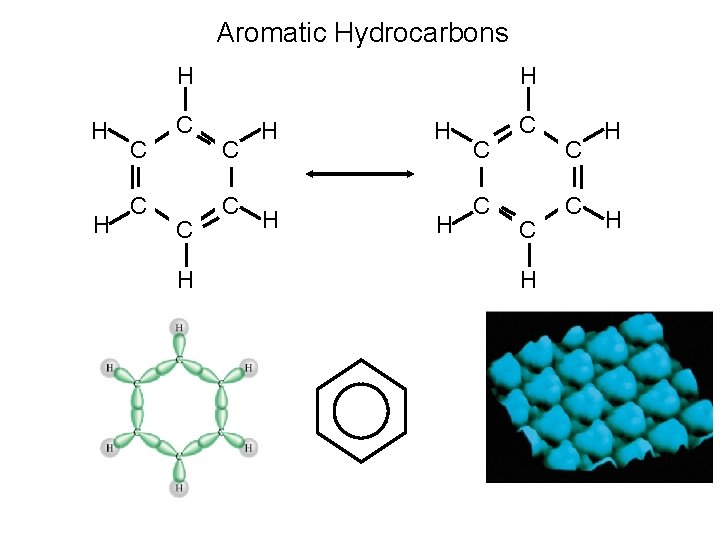
Aromatic Hydrocarbons • Aromatic hydrocarbons usually contain benzene rings-six membered rings of carbon atoms with alternating C-C single and C=C double bonds. The structure of benzene is

Aromatic Hydrocarbons • Aromatic hydrocarbons usually contain benzene rings-six membered rings of carbon atoms with alternating C-C single and C=C double bonds. . Aromatic compounds are found everywhere from pain relievers to flavoring agents.

Aromatic Hydrocarbons H H H H C C C C H H

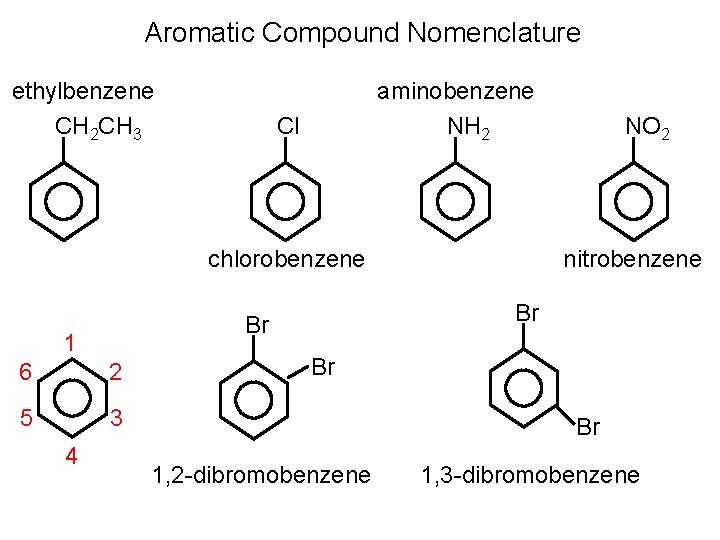
Aromatic Compound Nomenclature ethylbenzene CH 2 CH 3 aminobenzene NH 2 Cl chlorobenzene 6 2 5 3 4 nitrobenzene Br Br 1 NO 2 Br Br 1, 2 -dibromobenzene 1, 3 -dibromobenzene

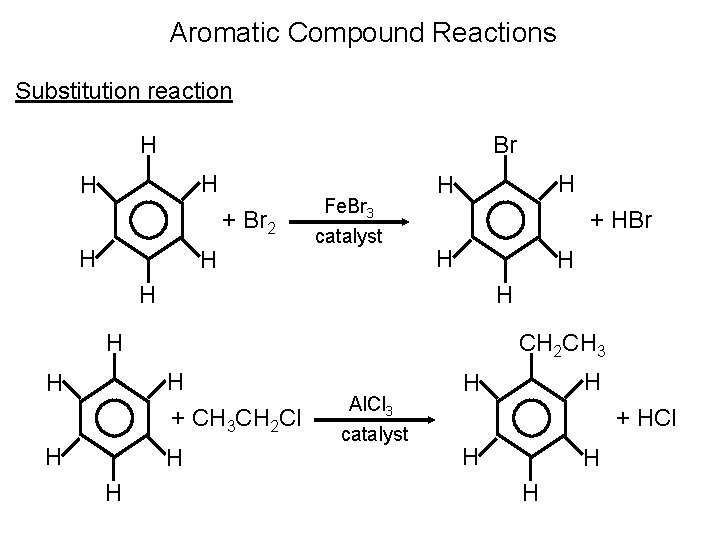
Aromatic Compound Reactions Substitution reaction H Br H H + Br 2 H H Fe. Br 3 catalyst H H + HBr H H H CH 2 CH 3 H + CH 3 CH 2 Cl H H Al. Cl 3 catalyst H H + HCl H H H

Polycyclic Aromatic Hydrocarbons

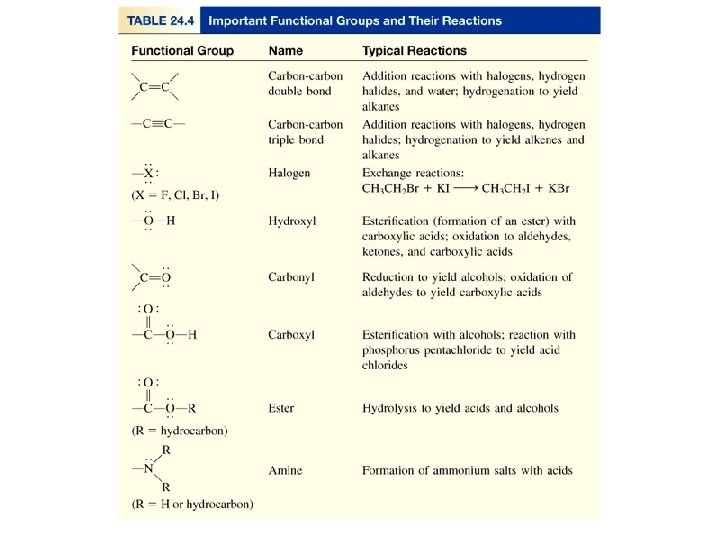
Derivatives of Hydrocarbons • A functional group is a reactive portion of a molecule that undergoes predictable reactions. In the previous sections we discussed the hydrocarbons and their reactions. All other organic compounds can be considered to be derivatives of hydrocarbons.

Organic Compounds Containing Oxygen • Many of the important functional groups in organic compounds contain oxygen. Examples are alcohols ethers aldehydes ketones carboxylic acids esters

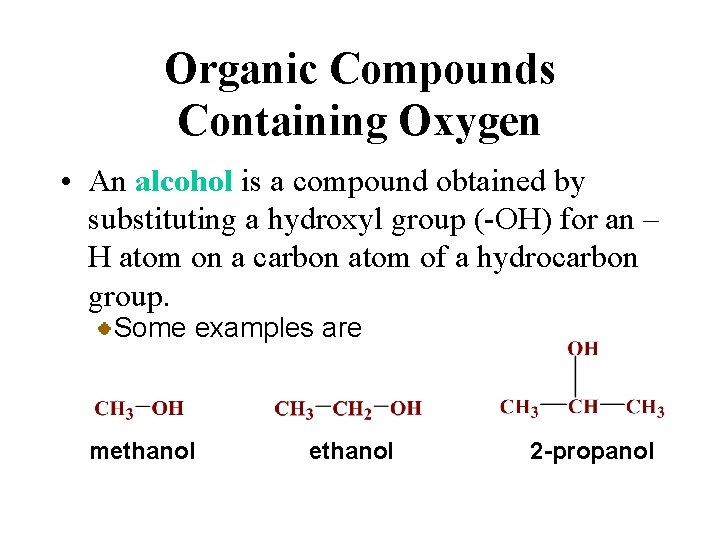
Organic Compounds Containing Oxygen • An alcohol is a compound obtained by substituting a hydroxyl group (-OH) for an – H atom on a carbon atom of a hydrocarbon group. Some examples are methanol 2 -propanol

Functional Group Chemistry Alcohols contain the hydroxyl functional group and have the general formula R-OH.

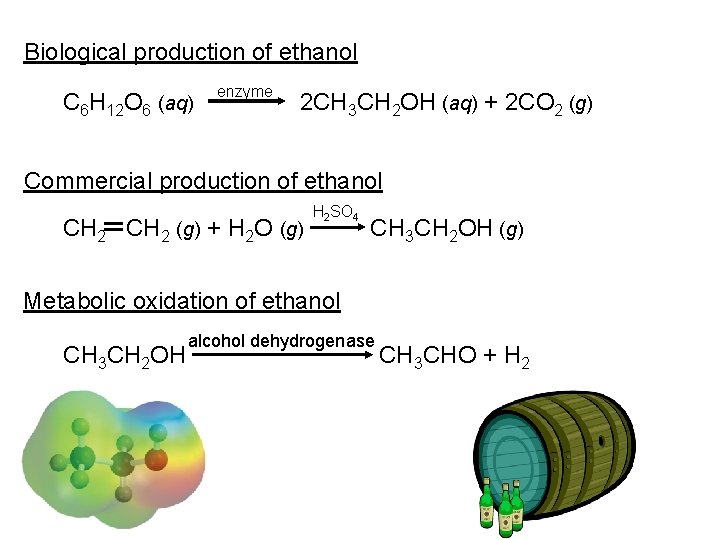
Biological production of ethanol C 6 H 12 O 6 (aq) enzyme 2 CH 3 CH 2 OH (aq) + 2 CO 2 (g) Commercial production of ethanol CH 2 (g) + H 2 O (g) H 2 SO 4 CH 3 CH 2 OH (g) Metabolic oxidation of ethanol CH 3 CH 2 OH alcohol dehydrogenase CH 3 CHO + H 2

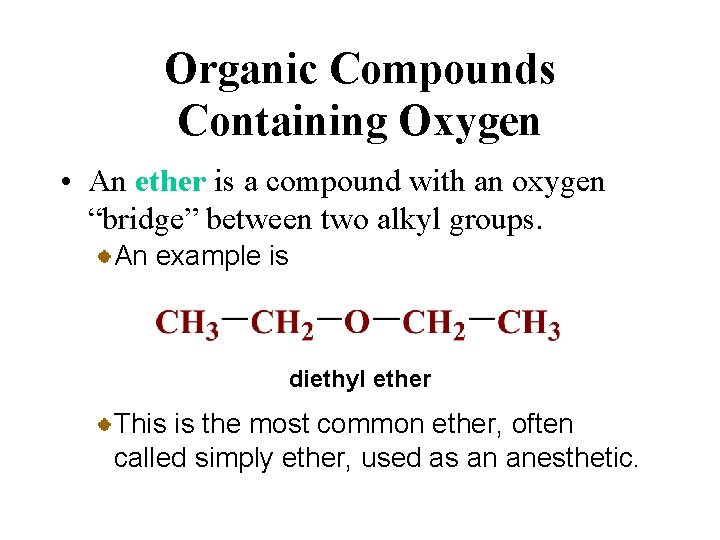
Organic Compounds Containing Oxygen • An ether is a compound with an oxygen “bridge” between two alkyl groups. An example is diethyl ether This is the most common ether, often called simply ether, used as an anesthetic.

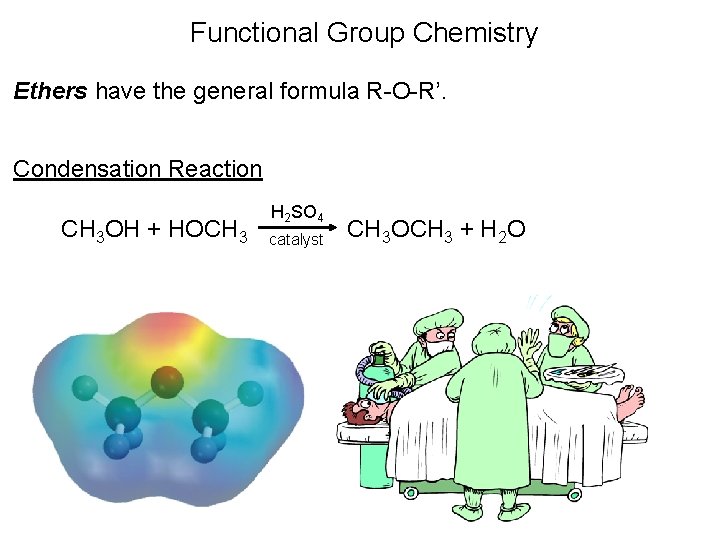
Functional Group Chemistry Ethers have the general formula R-O-R’. Condensation Reaction CH 3 OH + HOCH 3 H 2 SO 4 catalyst CH 3 OCH 3 + H 2 O

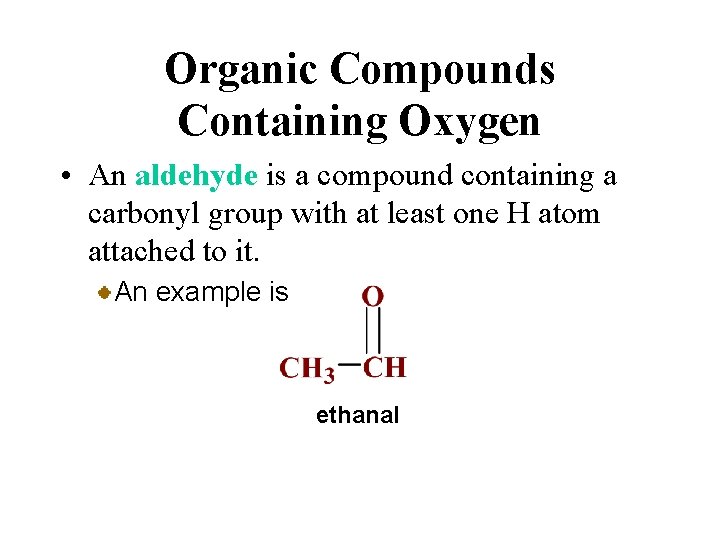
Organic Compounds Containing Oxygen • An aldehyde is a compound containing a carbonyl group with at least one H atom attached to it. An example is ethanal

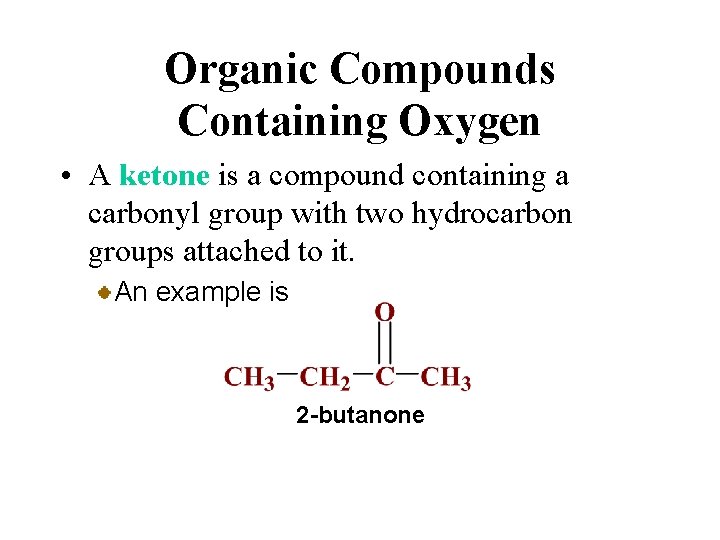
Organic Compounds Containing Oxygen • A ketone is a compound containing a carbonyl group with two hydrocarbon groups attached to it. An example is 2 -butanone

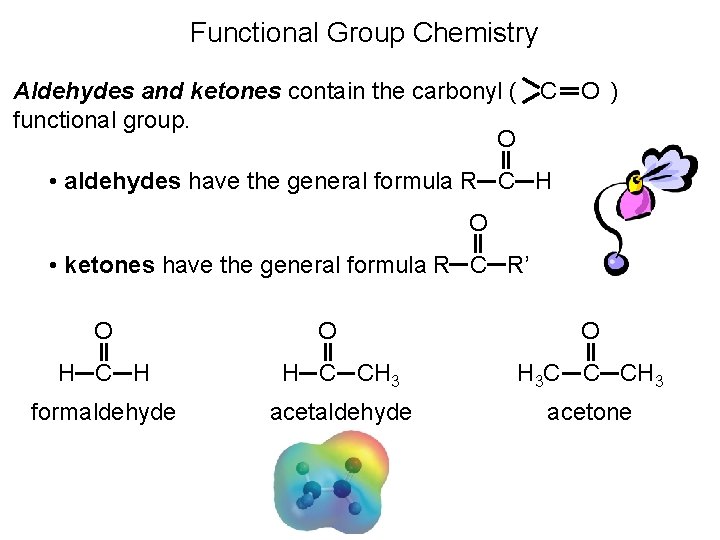
Functional Group Chemistry O Aldehydes and ketones contain the carbonyl ( C functional group. O ) • aldehydes have the general formula R C H O • ketones have the general formula R C R’ O O O H C H H C CH 3 H 3 C C CH 3 formaldehyde acetone

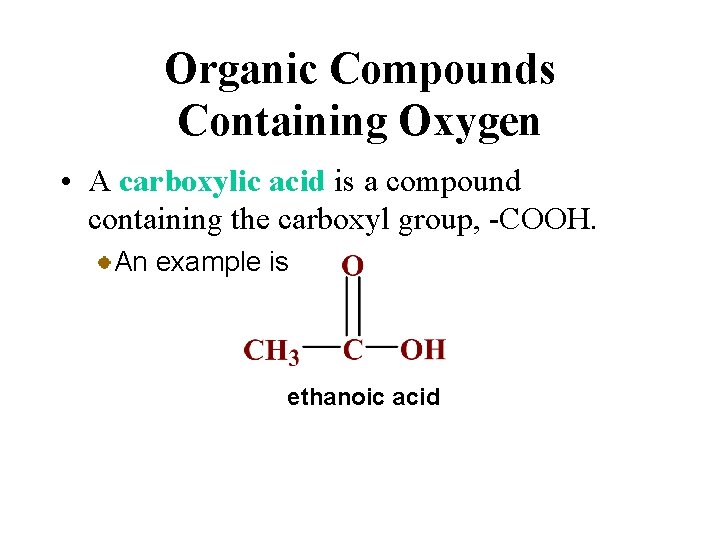
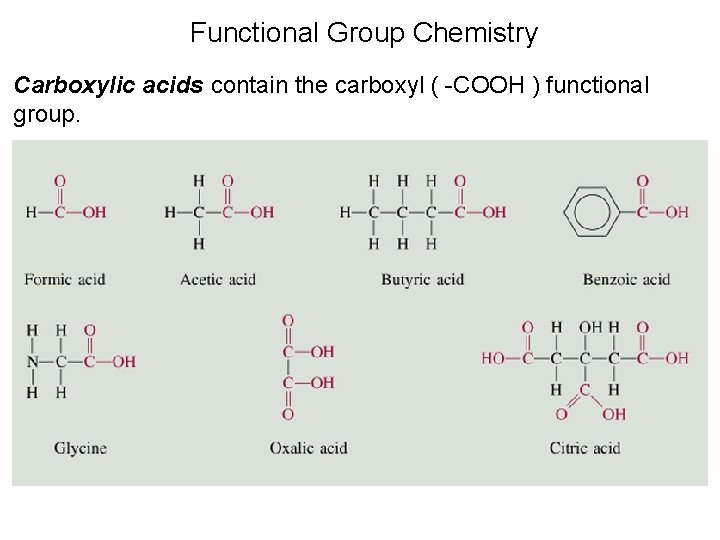
Organic Compounds Containing Oxygen • A carboxylic acid is a compound containing the carboxyl group, -COOH. An example is ethanoic acid

Functional Group Chemistry Carboxylic acids contain the carboxyl ( -COOH ) functional group.

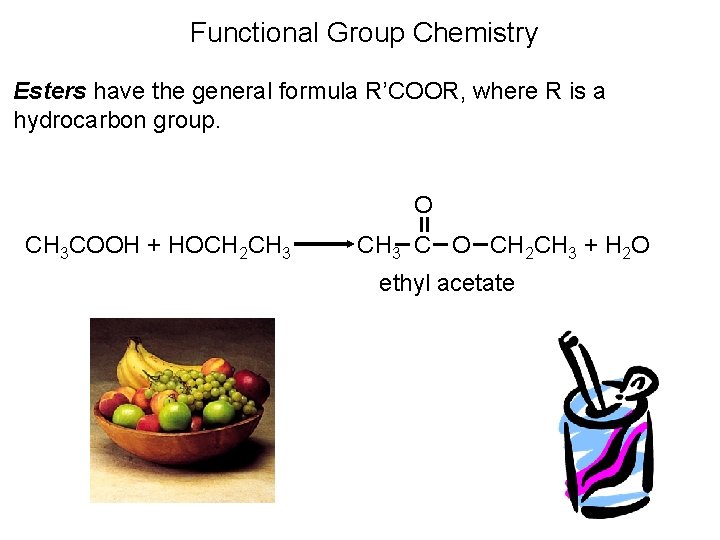
Organic Compounds Containing Oxygen • An ester is a compound formed from a carboxylic acid, RCOOH, and an alcohol, R’OH. The general structure is

Functional Group Chemistry Esters have the general formula R’COOR, where R is a hydrocarbon group. O CH 3 COOH + HOCH 2 CH 3 C O CH 2 CH 3 + H 2 O ethyl acetate

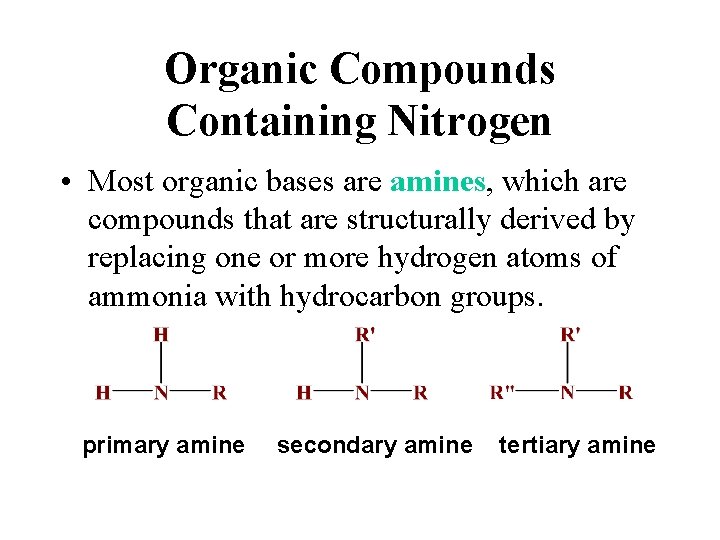
Organic Compounds Containing Nitrogen • Most organic bases are amines, which are compounds that are structurally derived by replacing one or more hydrogen atoms of ammonia with hydrocarbon groups. primary amine secondary amine tertiary amine

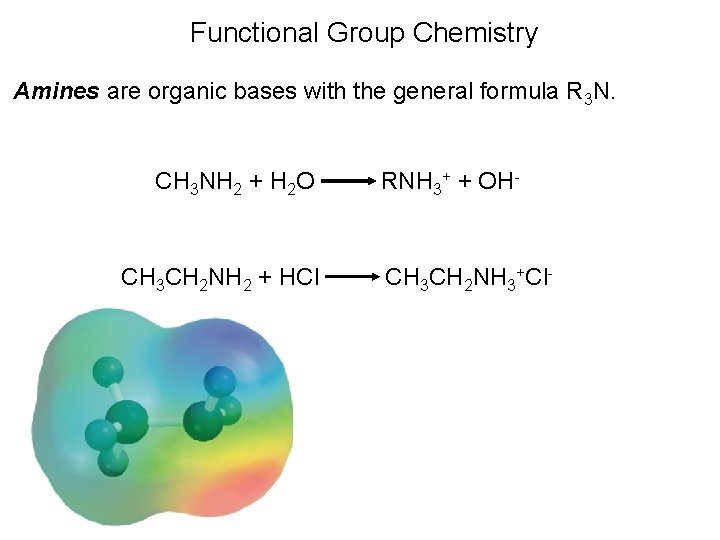
Functional Group Chemistry Amines are organic bases with the general formula R 3 N. CH 3 NH 2 + H 2 O CH 3 CH 2 NH 2 + HCl RNH 3+ + OH- CH 3 CH 2 NH 3+Cl-

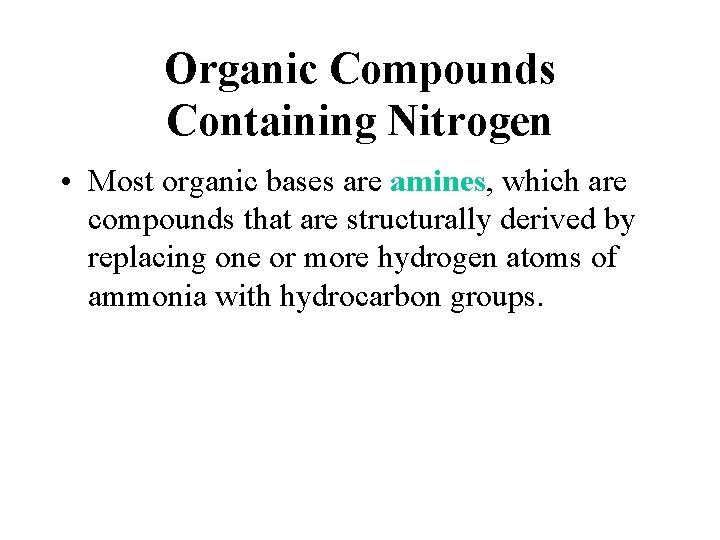
Organic Compounds Containing Nitrogen • Most organic bases are amines, which are compounds that are structurally derived by replacing one or more hydrogen atoms of ammonia with hydrocarbon groups.

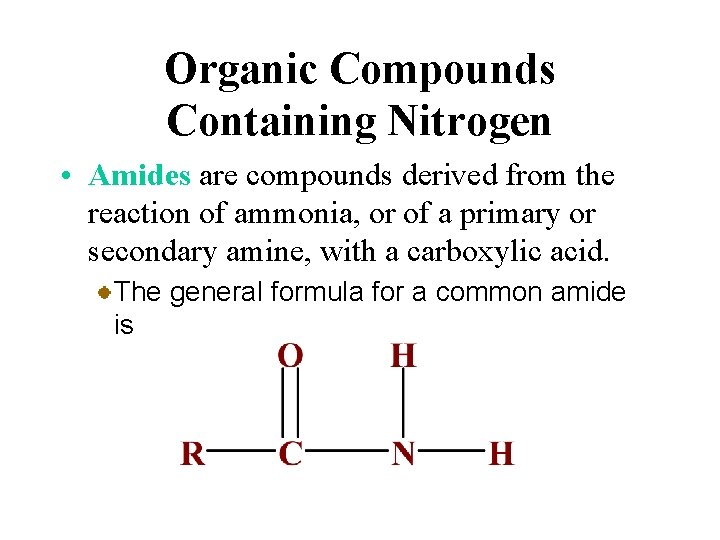
Organic Compounds Containing Nitrogen • Amides are compounds derived from the reaction of ammonia, or of a primary or secondary amine, with a carboxylic acid. The general formula for a common amide is


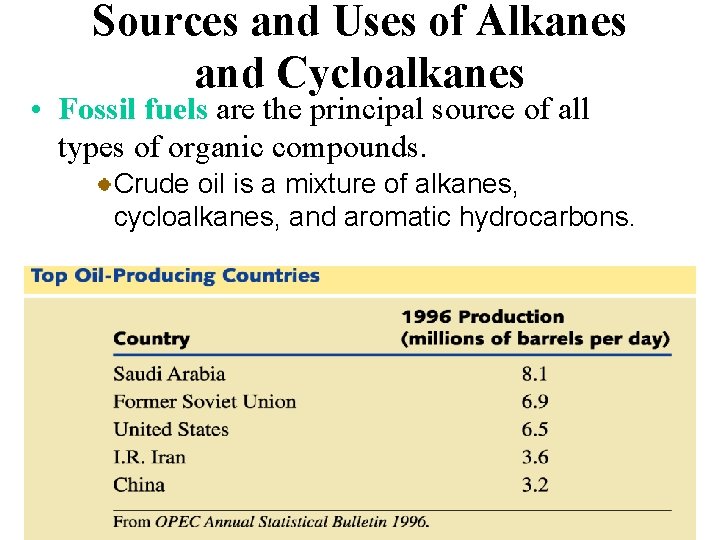
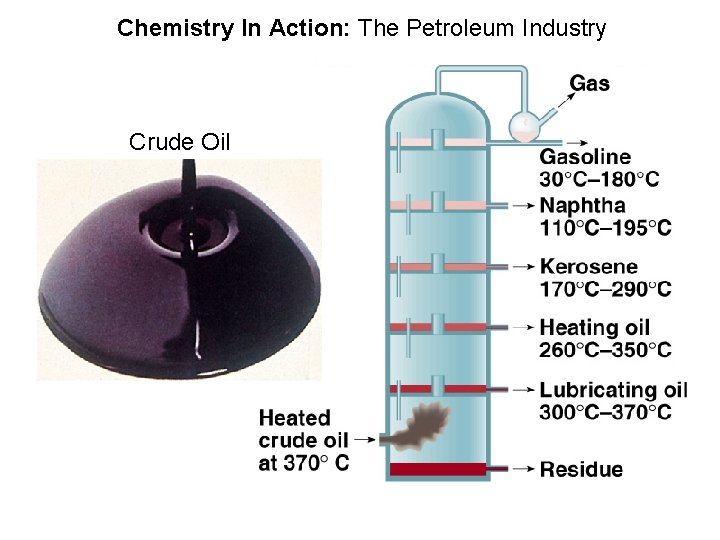
Sources and Uses of Alkanes and Cycloalkanes • Fossil fuels are the principal source of all types of organic compounds. Crude oil is a mixture of alkanes, cycloalkanes, and aromatic hydrocarbons.

Sources and Uses of Alkanes and Cycloalkanes • Fossil fuels are the principal source of all types of organic compounds. Because fossil fuels are mixtures of hydrocarbons, it is usually necessary to separate these mixtures by distillation.

Chemistry In Action: The Petroleum Industry Crude Oil

Sources and Uses of Alkanes and Cycloalkanes The alkanes serve as the starting point for most plastics and pharmaceuticals.


Class Exercises • • Organic Functional Groups Chart Organic Nomenclature II Drawing organic structures

WORKED EXAMPLES

Worked Example 24. 1


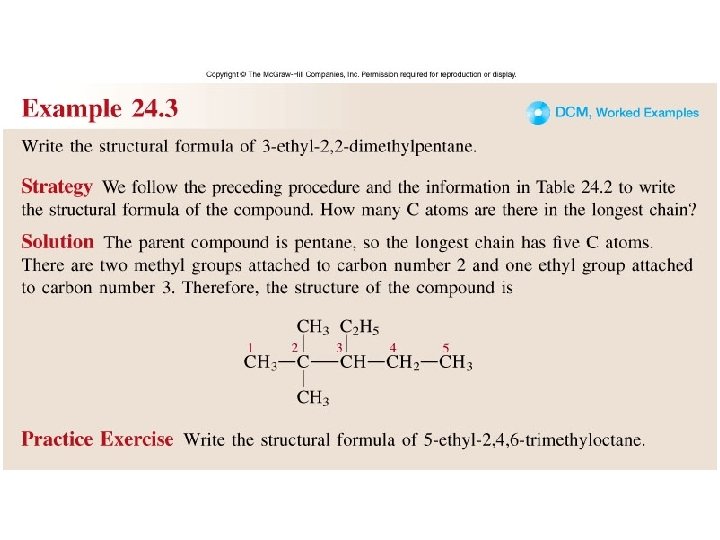
Worked Example 24. 3


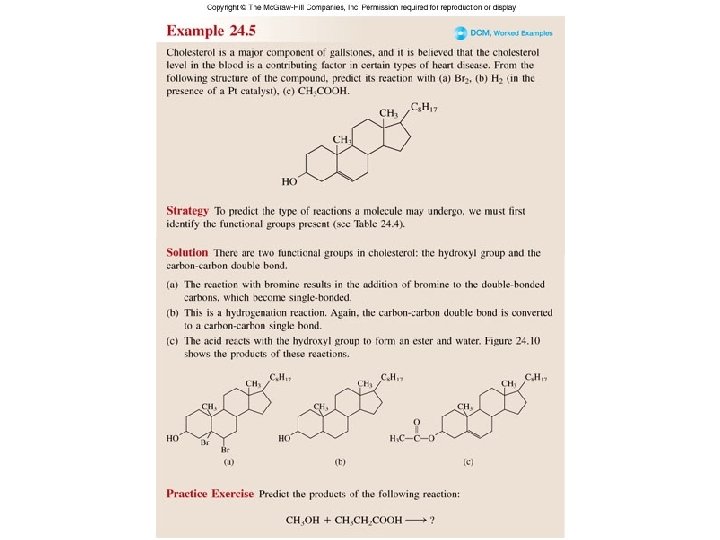
Worked Example 24. 5
 Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Pericyclic
Pericyclic What is organic chemistry
What is organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Nonene
Nonene Analytical chemistry chapters
Analytical chemistry chapters Halohydrin
Halohydrin Cycloalkanes
Cycloalkanes Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry Homologous series formula
Homologous series formula Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Is alkane an organic compound
Is alkane an organic compound Leveling effect organic chemistry
Leveling effect organic chemistry Nomenclature of organic compounds
Nomenclature of organic compounds Organic chemistry lab report format
Organic chemistry lab report format Britannica.com
Britannica.com Grade 10 organic chemistry
Grade 10 organic chemistry Cyclo organic chemistry
Cyclo organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis Meth eth prop table
Meth eth prop table How is cracking done
How is cracking done Meth eth prop but
Meth eth prop but Organic chemistry myanmar
Organic chemistry myanmar Br2aq
Br2aq Gc organic chemistry
Gc organic chemistry Hono organic chemistry
Hono organic chemistry Leaving group ability
Leaving group ability Organic chemistry topic 11
Organic chemistry topic 11 Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry nomenclature
Organic chemistry nomenclature What is organic chemistry like
What is organic chemistry like Organic vs inorganic molecules
Organic vs inorganic molecules Organic chemistry vocabulary
Organic chemistry vocabulary Separation scheme of caffeine from vivarin tablets
Separation scheme of caffeine from vivarin tablets A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Rancidity meaning
Rancidity meaning Ario+
Ario+ How to calculate yield in chemistry
How to calculate yield in chemistry Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Radicals
Radicals Hammonds postulate
Hammonds postulate Klein
Klein Chemistry ethics case studies
Chemistry ethics case studies Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Octane lewis structure
Octane lewis structure Carbohydrates organic chemistry
Carbohydrates organic chemistry Resonance in benzyl carbocation
Resonance in benzyl carbocation Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Macromolecule cheat sheet
Macromolecule cheat sheet Chemistry mind map
Chemistry mind map Organic chemistry stuart warren
Organic chemistry stuart warren Bu organic chemistry
Bu organic chemistry The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms Conjugation organic chemistry
Conjugation organic chemistry Organic chemistry
Organic chemistry Cyclopentane condensed structural formula
Cyclopentane condensed structural formula Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? All structural isomers of hexane
All structural isomers of hexane Organic chemistry
Organic chemistry Wiley
Wiley Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Nbs reaction
Nbs reaction Organic chemistry
Organic chemistry Butan 2 on
Butan 2 on Chemistry organic
Chemistry organic Organic chemistry class 11 notes
Organic chemistry class 11 notes Hybridisation
Hybridisation Number of organic compounds
Number of organic compounds Danswer
Danswer Resonance hybrid
Resonance hybrid Iupac
Iupac Functional groups in organic chemistry
Functional groups in organic chemistry Organic chemistry
Organic chemistry Met et prop but
Met et prop but Silver nitrate test
Silver nitrate test Organic chemistry william h brown
Organic chemistry william h brown Acetoacetic ester synthesis mechanism
Acetoacetic ester synthesis mechanism Organic chemistry
Organic chemistry Brooklyn college organic chemistry
Brooklyn college organic chemistry