Chemistry Topic 11 Organic Chemistry Chemistry Organic Chemistry











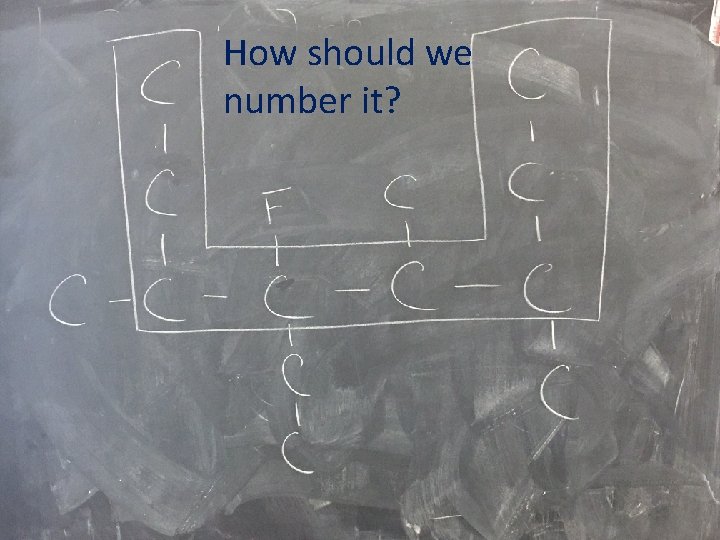




















- Slides: 61

Chemistry Topic 11 Organic Chemistry

• Chemistry • Organic Chemistry • Obj: SWBAT elucidate partial structures of organic compounds based on structure, formula, and names • Do Now: What comes to mind when you hear the word “organic”?

Bonding of Carbon Atoms • Organic Chemistry = study of carbon-based compounds • Carbon can form 4 covalent bonds (tetravalent) • Saturated = compound only has single bonds • Unsaturated = compound contains one or more double or triple bonds

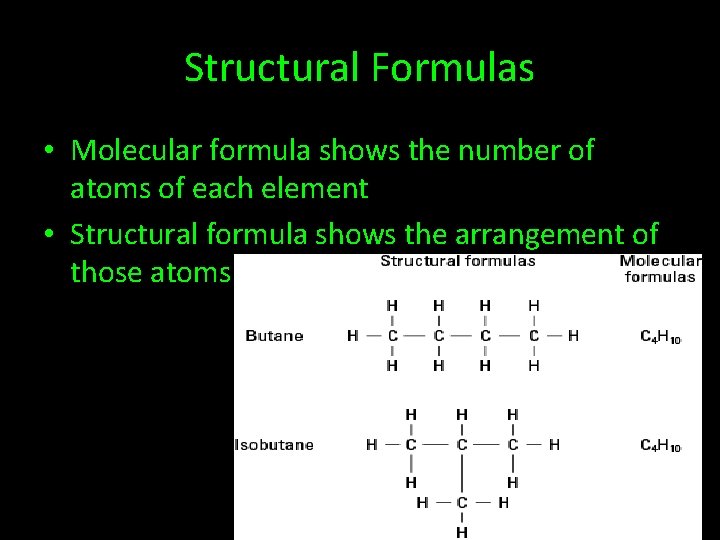
Structural Formulas • Molecular formula shows the number of atoms of each element • Structural formula shows the arrangement of those atoms

Hydrocarbons • Compounds that ONLY have HYDROGEN and CARBON • Split up into 3 homologous series alkanes, alkenes, and alkynes • Generally, the larger (heavier) the compound, the higher the boiling point. • Table Q


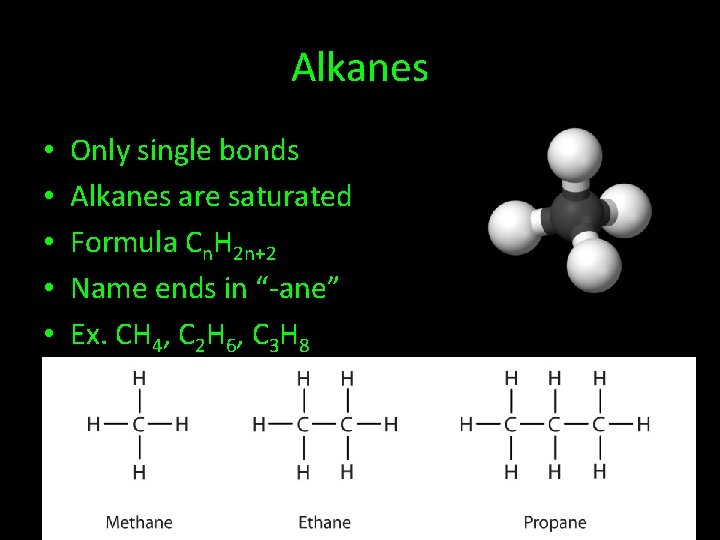
Alkanes • • • Only single bonds Alkanes are saturated Formula Cn. H 2 n+2 Name ends in “-ane” Ex. CH 4, C 2 H 6, C 3 H 8

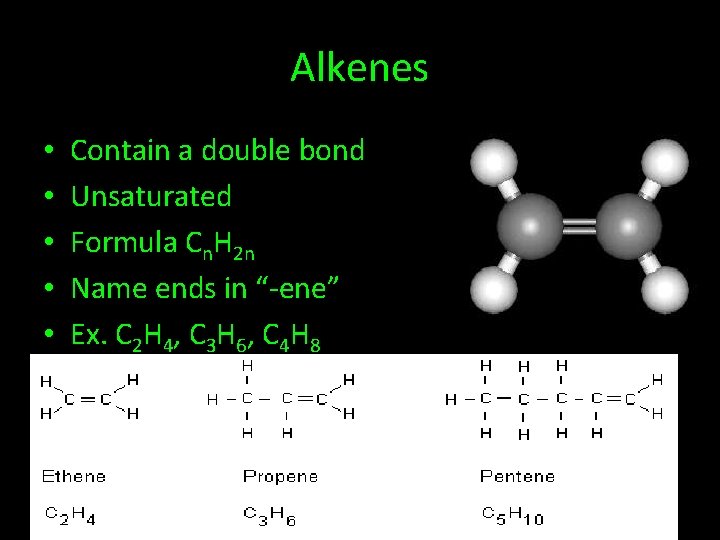
Alkenes • • • Contain a double bond Unsaturated Formula Cn. H 2 n Name ends in “-ene” Ex. C 2 H 4, C 3 H 6, C 4 H 8

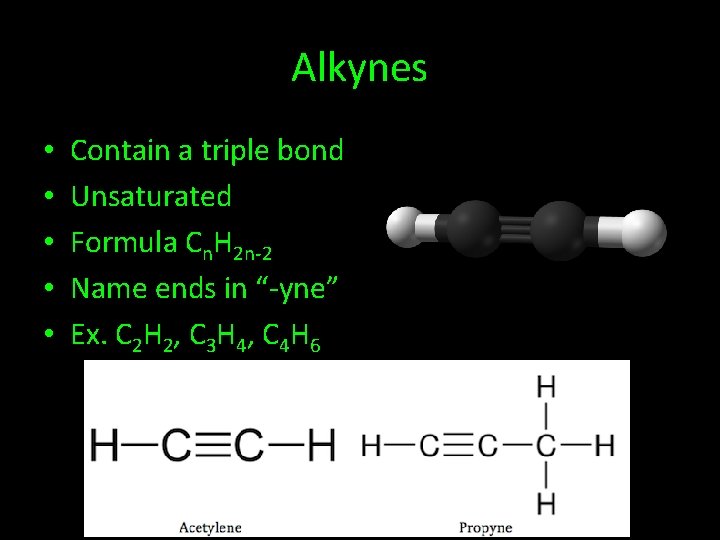
Alkynes • • • Contain a triple bond Unsaturated Formula Cn. H 2 n-2 Name ends in “-yne” Ex. C 2 H 2, C 3 H 4, C 4 H 6

Isomers • SAME MOLECULAR FORMULA, DIFFERENT STRUCTURAL FORMULA

• Practice Problems

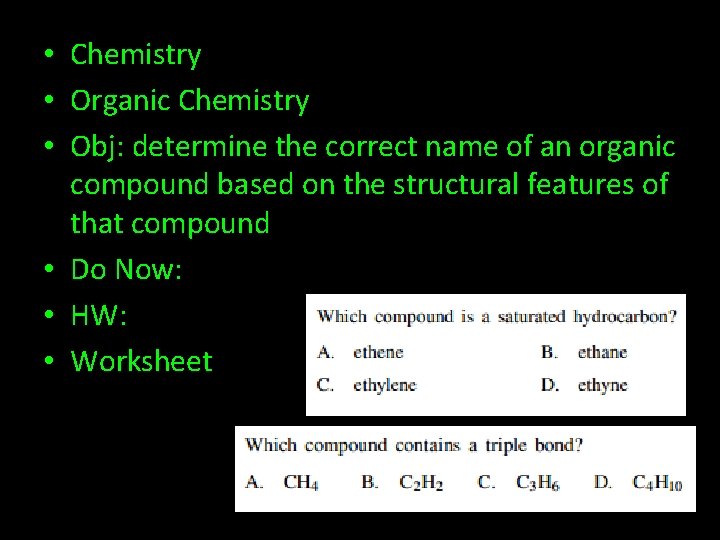
• Chemistry • Organic Chemistry • Obj: determine the correct name of an organic compound based on the structural features of that compound • Do Now: • HW: • Worksheet


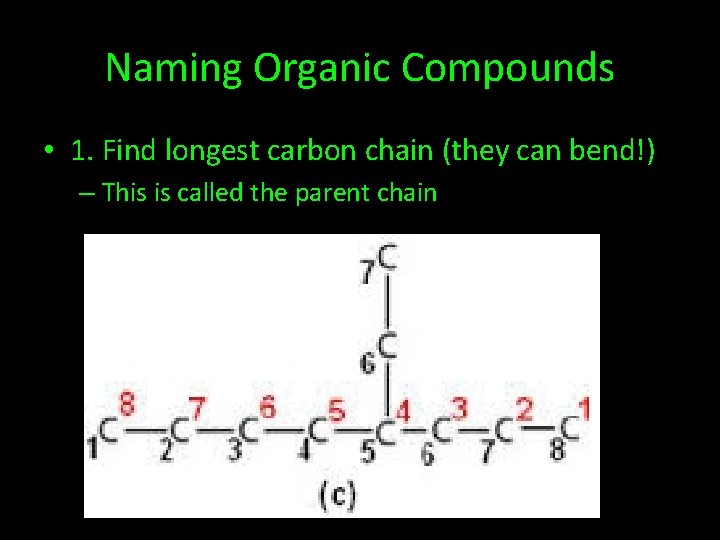
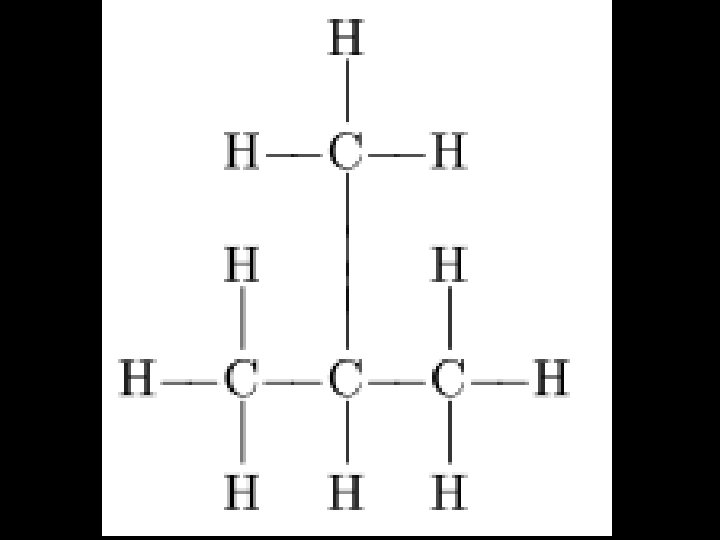
Naming Organic Compounds • 1. Find longest carbon chain (they can bend!) – This is called the parent chain

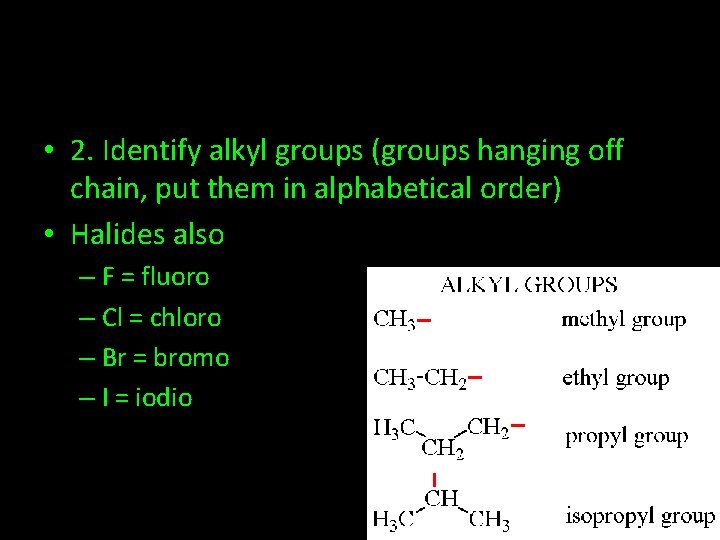
• 2. Identify alkyl groups (groups hanging off chain, put them in alphabetical order) • Halides also – F = fluoro – Cl = chloro – Br = bromo – I = iodio

• 3. If necessary assign numbers to alkyl groups (Final name has lowest possible numbers)

• 4. If more than one of the same alkyl group is present give prefixes – Di for 2 – Tri for 3 – Tetra for 4

Where’s the longest Carbon Chain?
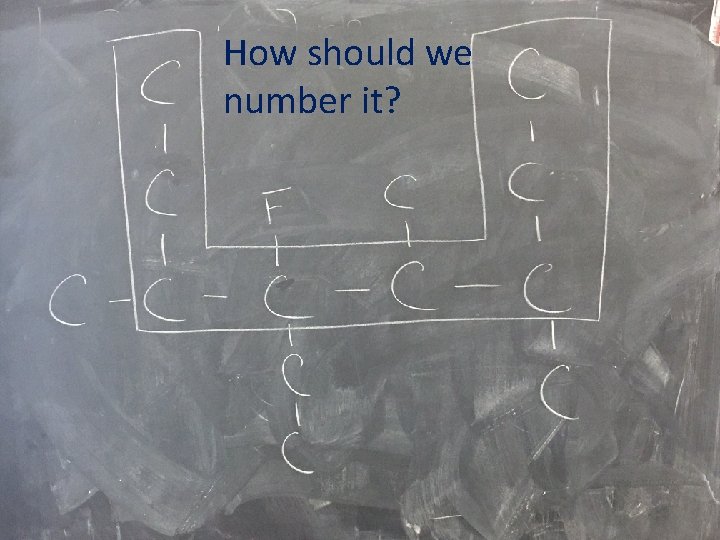
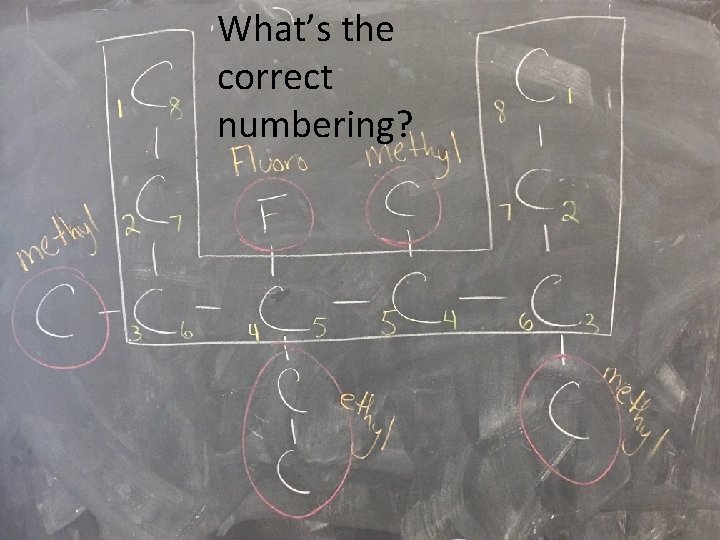
How should we number it?

Are there any groups coming off the parent chain?

What are the names of those groups?

What’s the correct numbering?


What’s the final name?







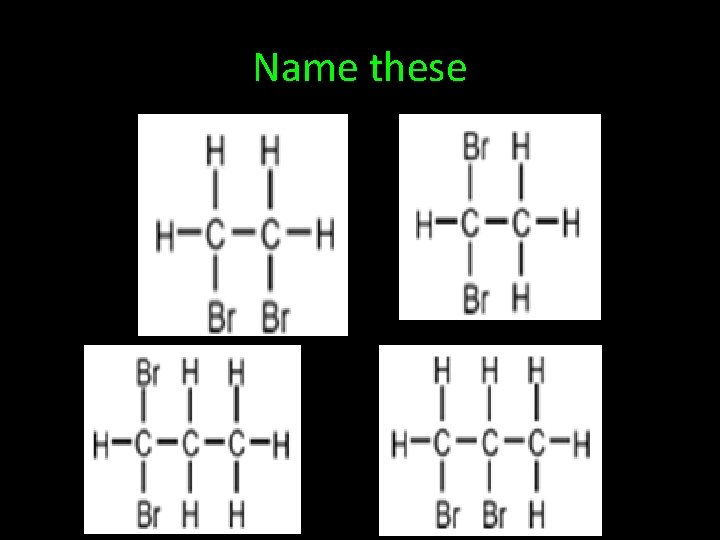
Name these

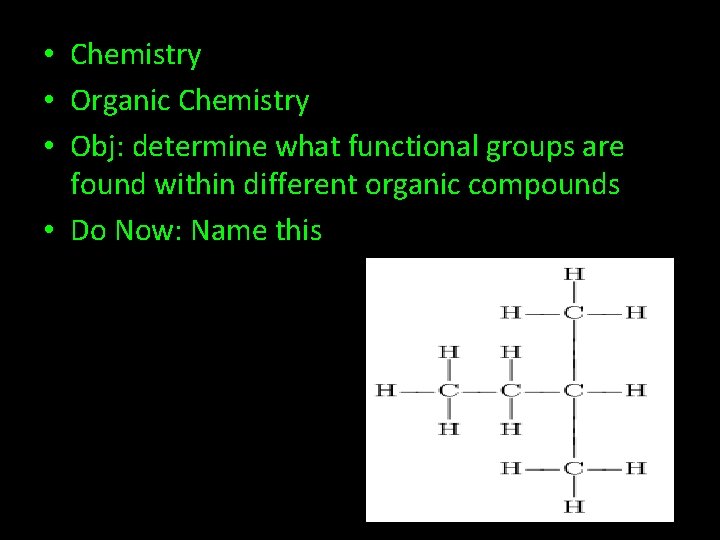
• Chemistry • Organic Chemistry • Obj: determine what functional groups are found within different organic compounds • Do Now: Name this

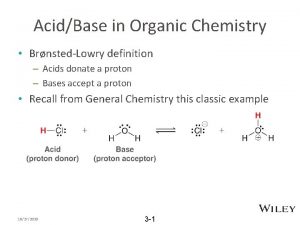
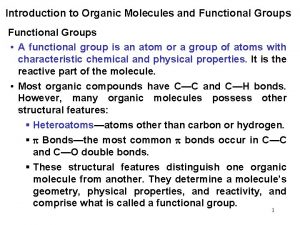
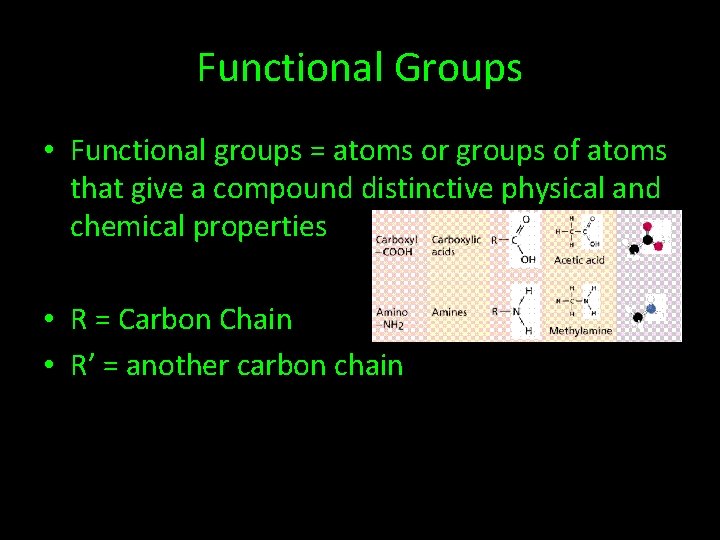
Functional Groups • Functional groups = atoms or groups of atoms that give a compound distinctive physical and chemical properties • R = Carbon Chain • R’ = another carbon chain

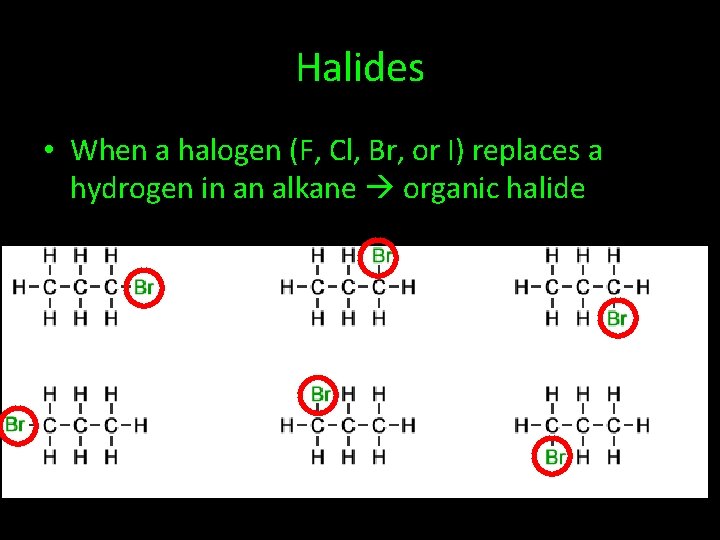
Halides • When a halogen (F, Cl, Br, or I) replaces a hydrogen in an alkane organic halide

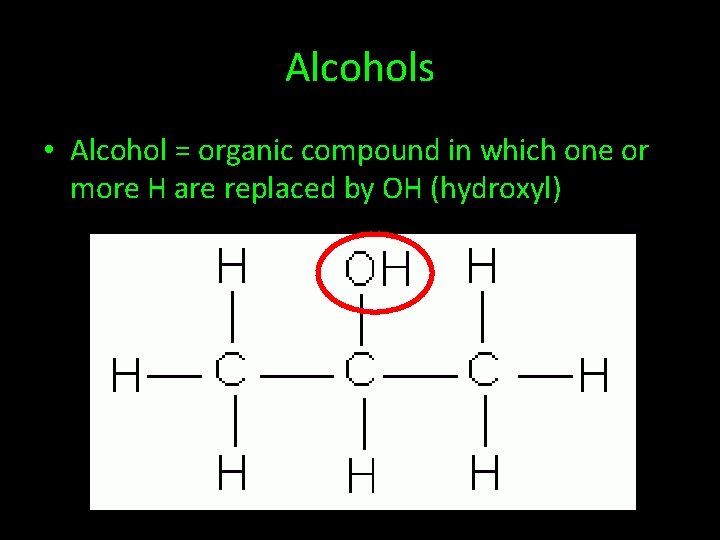
Alcohols • Alcohol = organic compound in which one or more H are replaced by OH (hydroxyl)

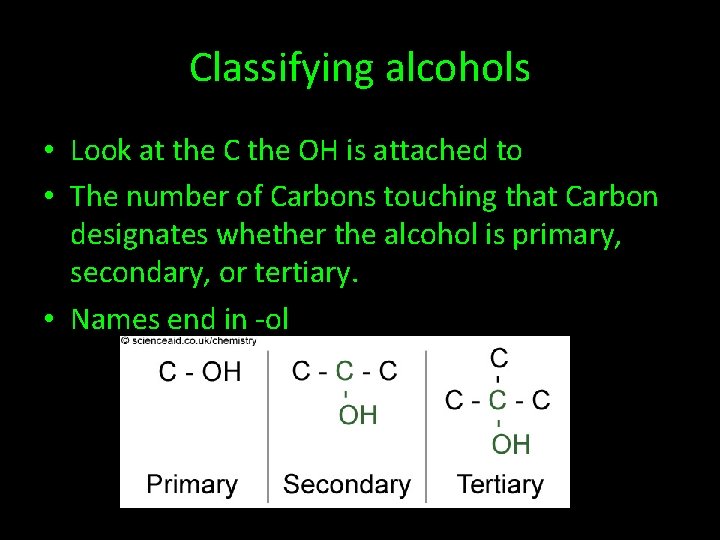
Classifying alcohols • Look at the C the OH is attached to • The number of Carbons touching that Carbon designates whether the alcohol is primary, secondary, or tertiary. • Names end in -ol

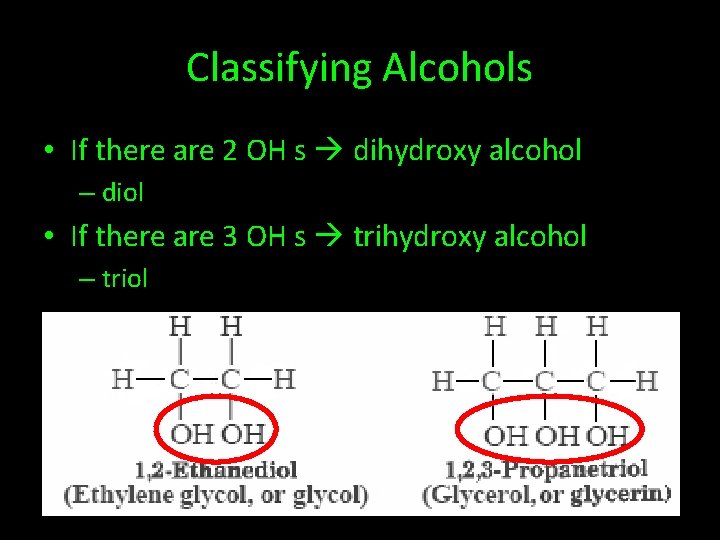
Classifying Alcohols • If there are 2 OH s dihydroxy alcohol – diol • If there are 3 OH s trihydroxy alcohol – triol

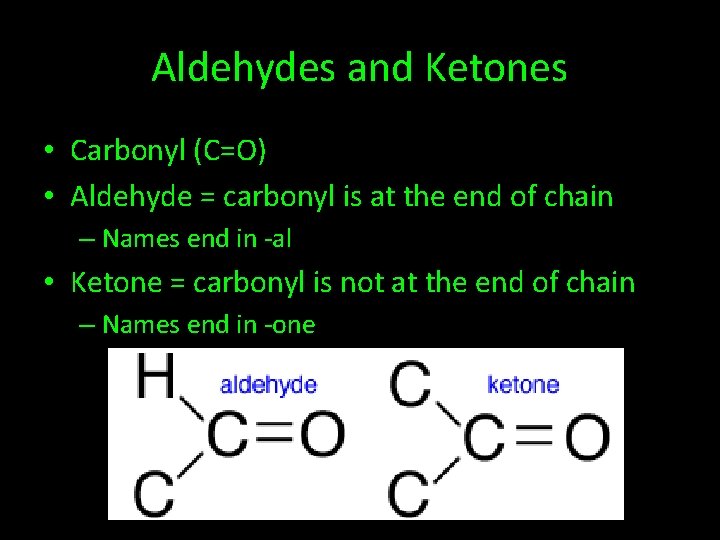
Aldehydes and Ketones • Carbonyl (C=O) • Aldehyde = carbonyl is at the end of chain – Names end in -al • Ketone = carbonyl is not at the end of chain – Names end in -one

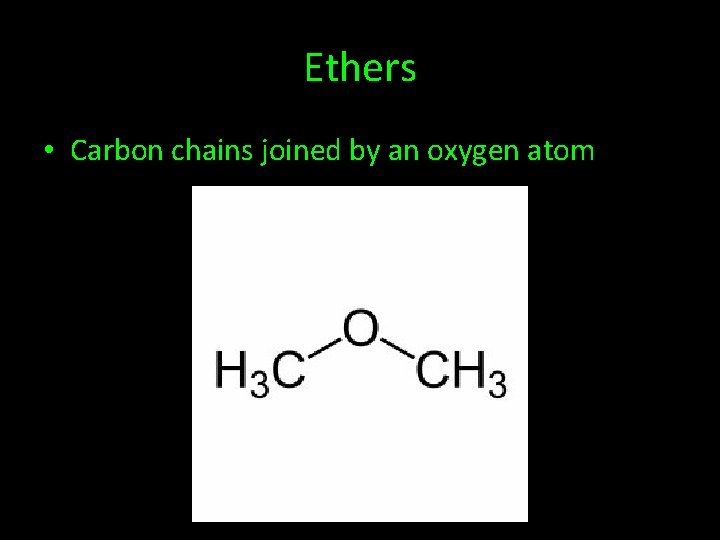
Ethers • Carbon chains joined by an oxygen atom

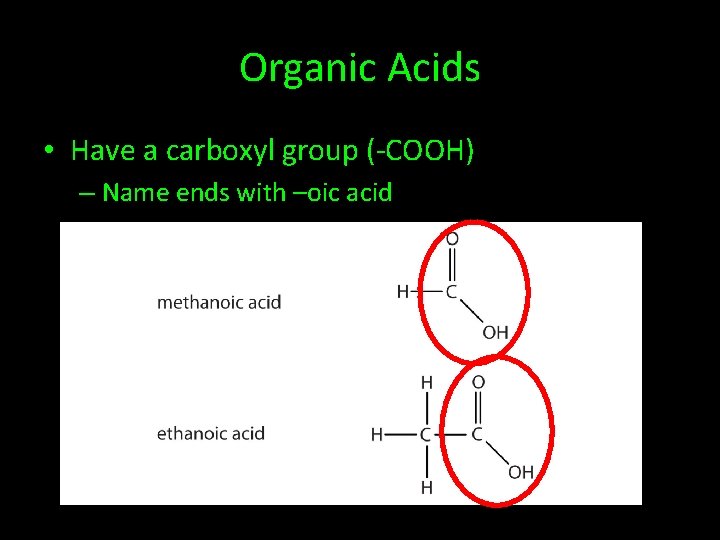
Organic Acids • Have a carboxyl group (-COOH) – Name ends with –oic acid

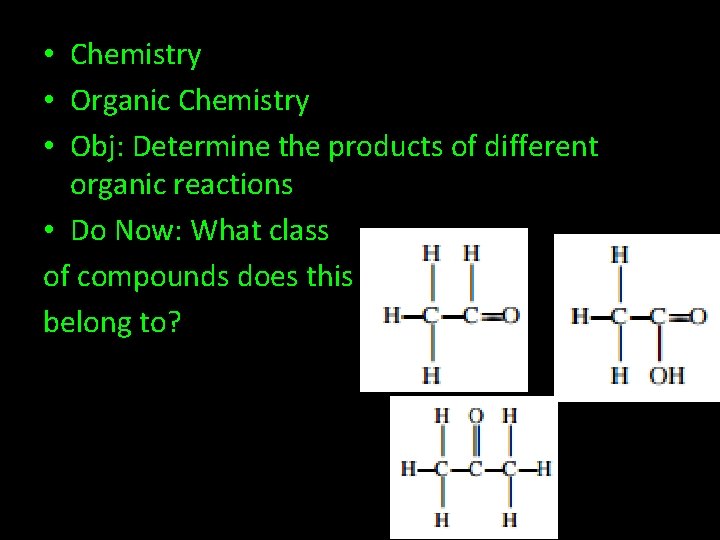
• Chemistry • Organic Chemistry • Obj: Determine the products of different organic reactions • Do Now: What class of compounds does this belong to?

Esters • Contain R-COO-R’ • Formed from an alcohol and organic acid – Have strong fragrances (found in fruits and perfumes) – Names end in -oate

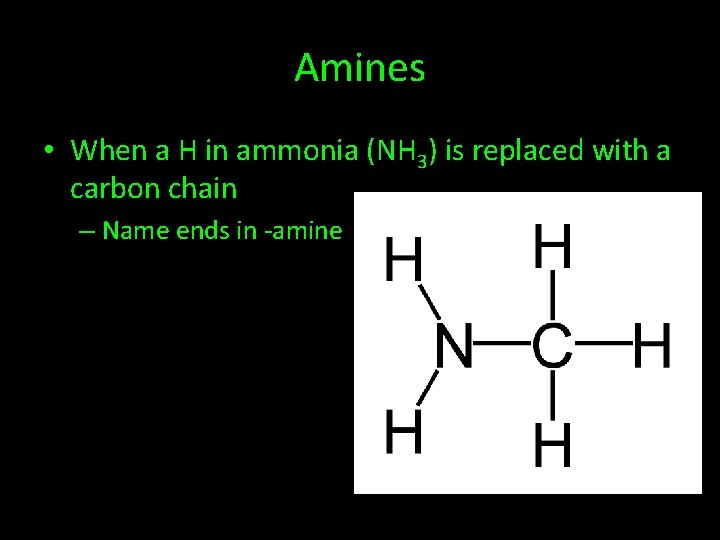
Amines • When a H in ammonia (NH 3) is replaced with a carbon chain – Name ends in -amine

Amino Acid • Have carboxylic (COOH) group and amine group – Building blocks of proteins

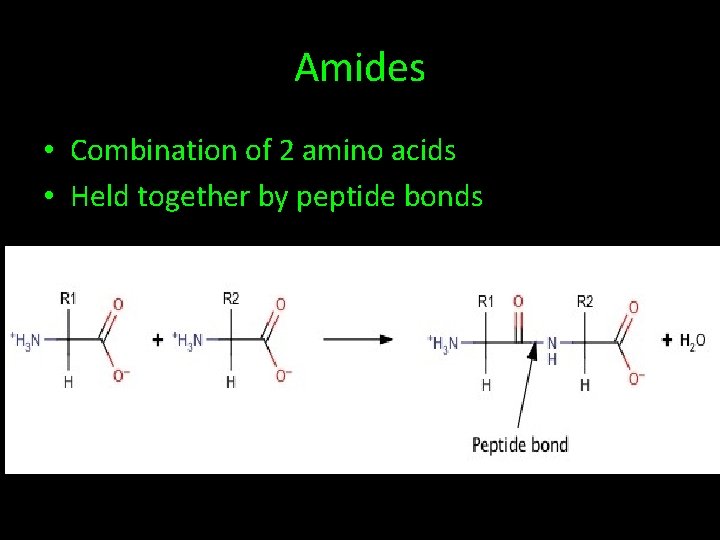
Amides • Combination of 2 amino acids • Held together by peptide bonds

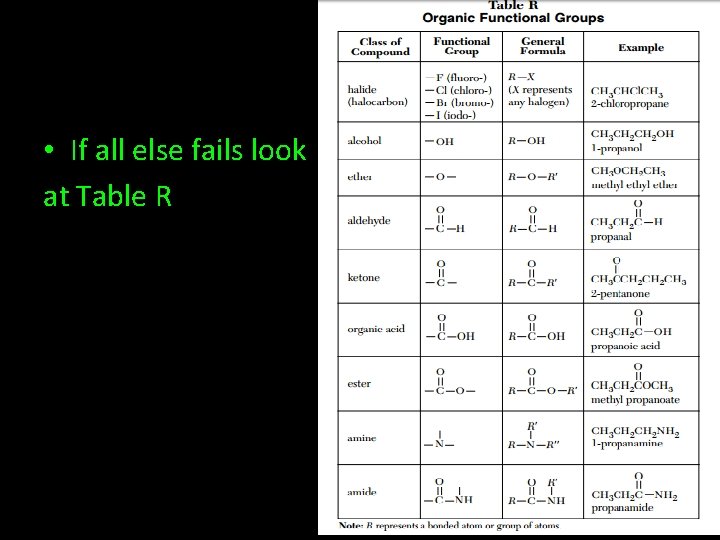
• If all else fails look at Table R

Combustion • Hydrocarbon + oxygen CO 2 + H 2 O

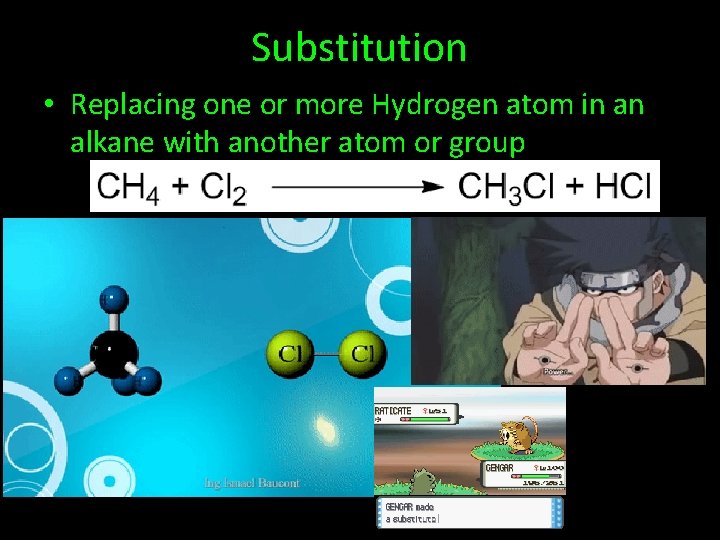
Substitution • Replacing one or more Hydrogen atom in an alkane with another atom or group

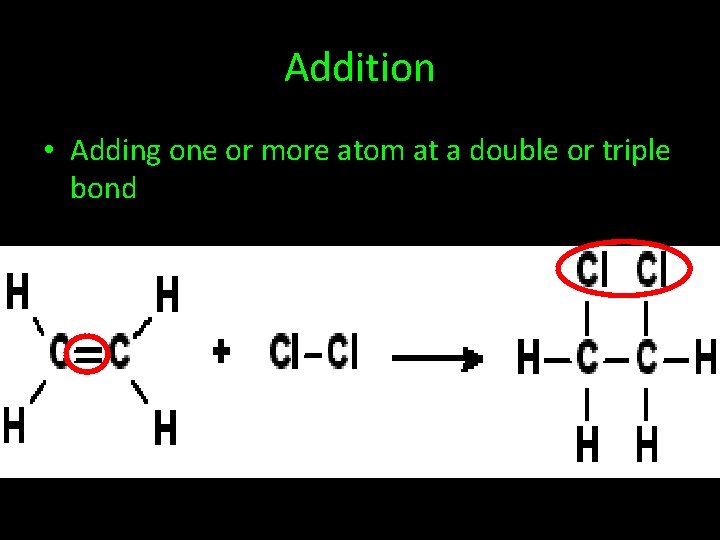
Addition • Adding one or more atom at a double or triple bond

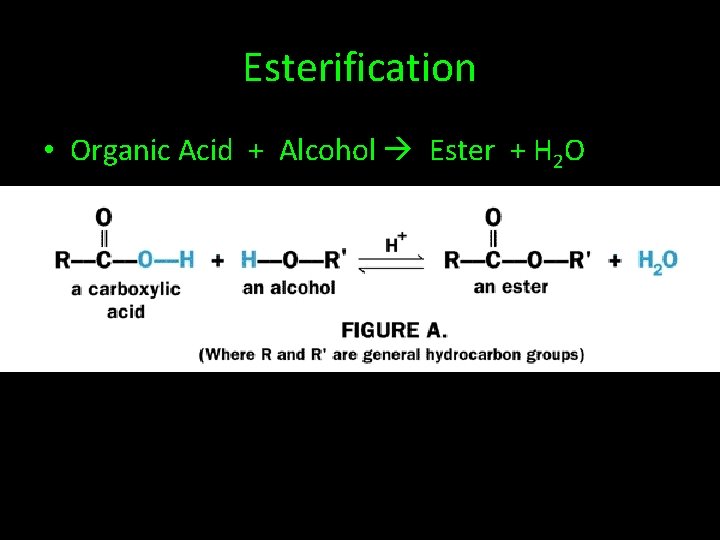
Esterification • Organic Acid + Alcohol Ester + H 2 O

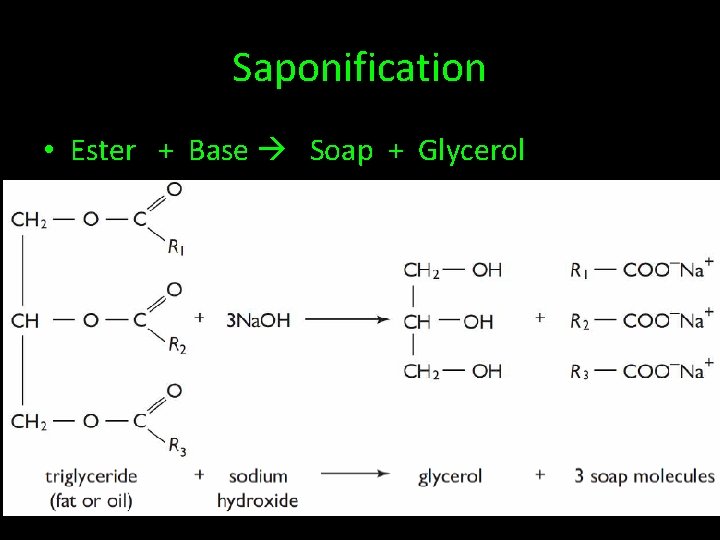
Saponification • Ester + Base Soap + Glycerol

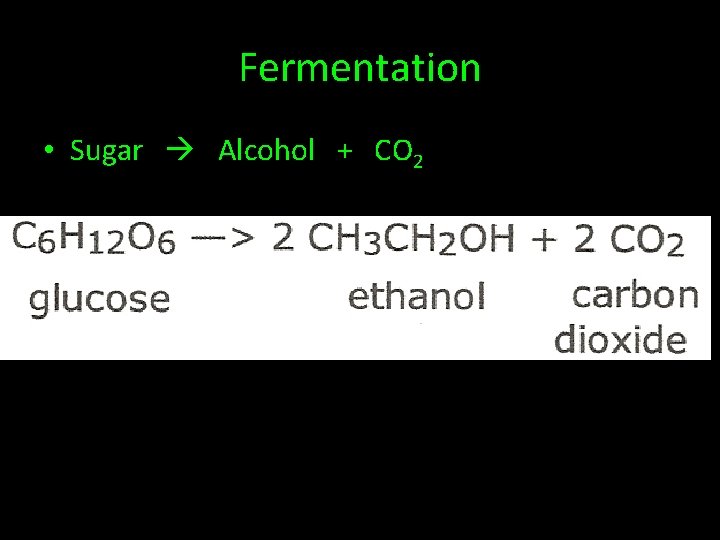
Fermentation • Sugar Alcohol + CO 2

Polymerization • Polymers = organic compounds made up of chains or smaller units – Ex. Nylon, rayon, polyethylene, proteins, starches

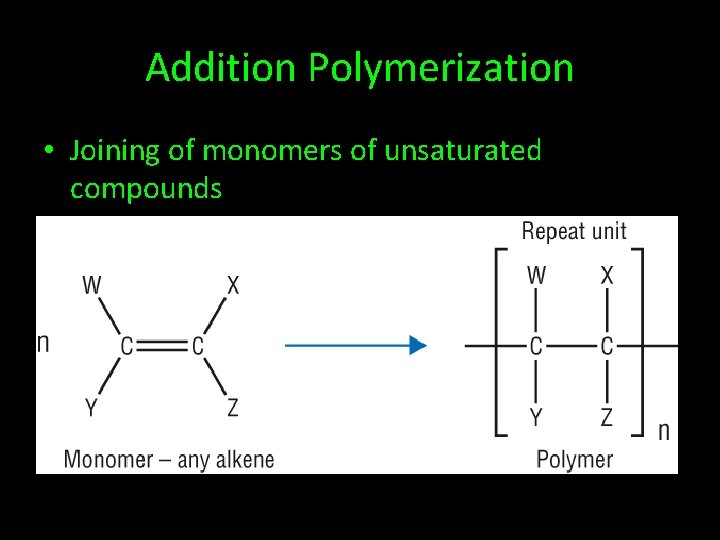
Addition Polymerization • Joining of monomers of unsaturated compounds

Condensation Polymerization • Bonding of monomers by removing water

• Chemistry • Organic Chemistry • Obj: SWBAT review concepts of Organic Chemistry through practice problems and offering explanations backed by their knowledge. • Do Now: Take out homework from last night to be checked.

• What careers did you learn about today? • Which did you find most interesting? Why? • Did any change your mind about your future?

• Go to codeacademy and work on HTML and CSS • By the end of the class, you should be at least 30% done with the course. If not, please ask for help if needed.

• Chemistry • Organic Chemistry • Obj: SWBAT review concepts of Organic Chemistry through practice problems and offering explanations backed by their knowledge. • Do Now: What are the reactants and products of saponification? • Get worksheet

• Chemistry • Organic Chemistry • Obj: SWBAT review concepts of Organic Chemistry through practice problems and offering explanations backed by their knowledge. • Do Now: What are the reactants and products of esterification? • Homework: STUDY!!!

Test Instructions • Get Test (doesn’t matter which version), Zipgrade, and Reference Tables from Desk • START ON NEW SIDE OF ZIPGRADE!!!! – PUT YOUR NAME ON EVERYTHING AND YOUR ID # ON ZIPGRADE (DON’T FORGET YOUR VERSION) • START AT #1 • Answer extended response on test itself • If extended response is in the middle of the test, skip it on zipgrade. Keep the numbering the same.
 Topic 11 organic chemistry
Topic 11 organic chemistry Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Narrow topic examples
Narrow topic examples Paragraph writing strategy
Paragraph writing strategy But pent hex hept
But pent hex hept Ir spectroscopy
Ir spectroscopy How to name compounds in organic chemistry
How to name compounds in organic chemistry Lewis dot structure ch4
Lewis dot structure ch4 Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry
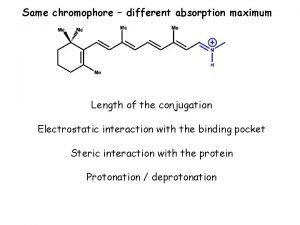
Organic chemistry Conjugation organic chemistry
Conjugation organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry
Organic chemistry Structure of pentanoic acid
Structure of pentanoic acid Propyl bromide
Propyl bromide Organic chemistry formulas
Organic chemistry formulas Propagation organic chemistry
Propagation organic chemistry Organic chemistry
Organic chemistry Macromolecules cheat sheet
Macromolecules cheat sheet Cyclo organic chemistry
Cyclo organic chemistry Nonene
Nonene Eth meth prop but pent hex
Eth meth prop but pent hex Organic synthesis via enolates
Organic synthesis via enolates Hybridisation
Hybridisation Ario+
Ario+ Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Pericyclic
Pericyclic What is organic chemistry like
What is organic chemistry like Organic chemistry
Organic chemistry Cracking organic chemistry
Cracking organic chemistry Father of organic chemistry
Father of organic chemistry Organic chemistry lab report format
Organic chemistry lab report format Oxidation of carbohydrates
Oxidation of carbohydrates Organic chemistry chapter 9
Organic chemistry chapter 9 C-c-c-c-c chemistry
C-c-c-c-c chemistry Organic chemistry
Organic chemistry Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry
Organic chemistry Alpha cleavage
Alpha cleavage Organic chemistry
Organic chemistry Mind map organic chemistry
Mind map organic chemistry Organic chemistry introduction
Organic chemistry introduction Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry What is a branched hydrocarbon
What is a branched hydrocarbon Calculating percentage yield
Calculating percentage yield Wiley
Wiley Organic chemistry
Organic chemistry David klein organic chemistry
David klein organic chemistry Neon organic or inorganic
Neon organic or inorganic Rhodopsin cgmp
Rhodopsin cgmp What is chemistry
What is chemistry Canola oil
Canola oil Britannica.com
Britannica.com Organic chemistry
Organic chemistry -oate functional group
-oate functional group Sodalime test
Sodalime test