Organic Chemistry 6 th Edition Chapter 9 Paula


- Slides: 59

Organic Chemistry 6 th Edition Chapter 9 Paula Yurkanis Bruice Elimination Reactions of Alkyl Halides Competition Between Substitution and Elimination 1 © 2011 Pearson Education, Inc.

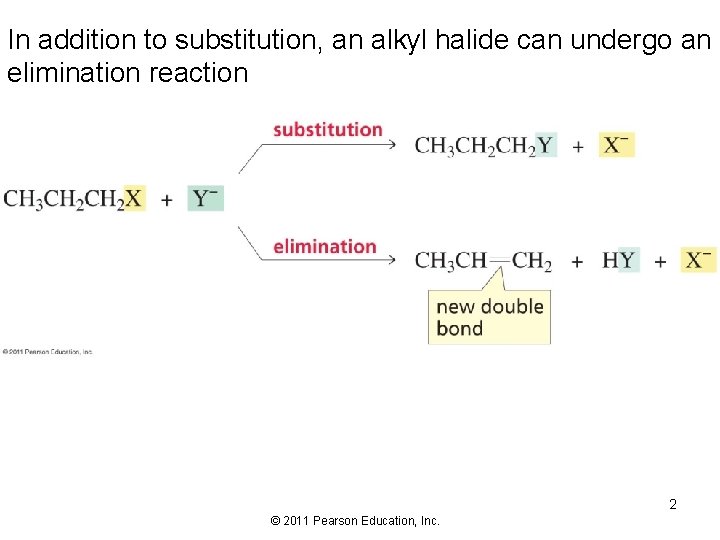
In addition to substitution, an alkyl halide can undergo an elimination reaction 2 © 2011 Pearson Education, Inc.

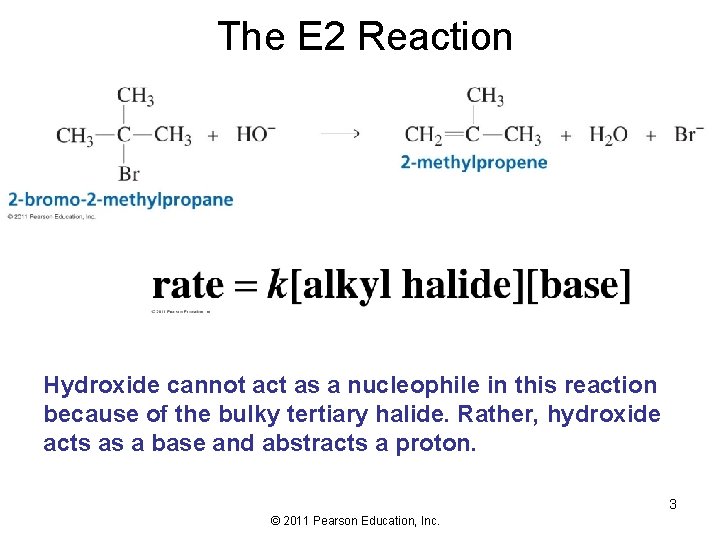
The E 2 Reaction Hydroxide cannot act as a nucleophile in this reaction because of the bulky tertiary halide. Rather, hydroxide acts as a base and abstracts a proton. 3 © 2011 Pearson Education, Inc.

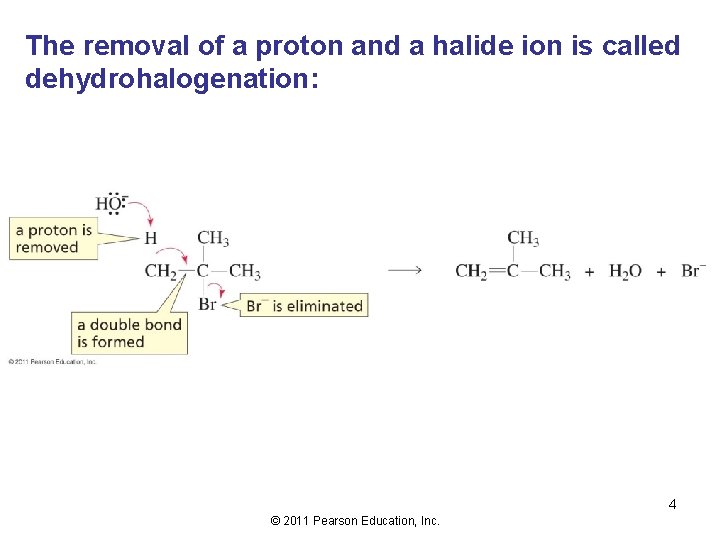
The removal of a proton and a halide ion is called dehydrohalogenation: 4 © 2011 Pearson Education, Inc.

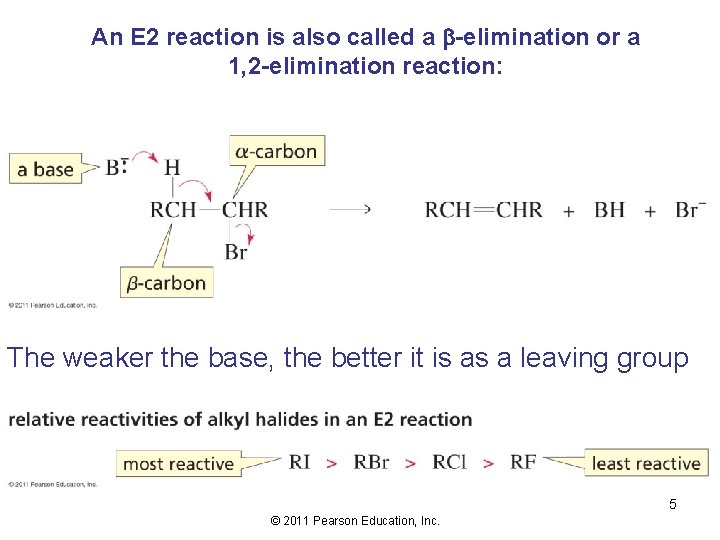
An E 2 reaction is also called a b-elimination or a 1, 2 -elimination reaction: The weaker the base, the better it is as a leaving group 5 © 2011 Pearson Education, Inc.

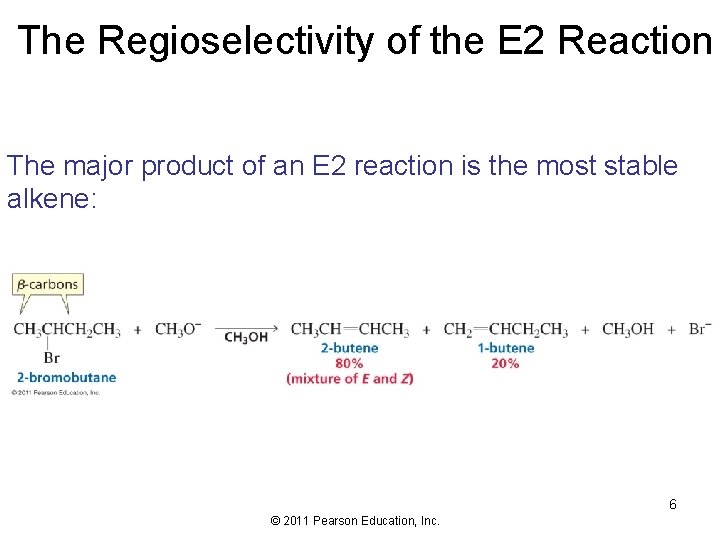
The Regioselectivity of the E 2 Reaction The major product of an E 2 reaction is the most stable alkene: 6 © 2011 Pearson Education, Inc.

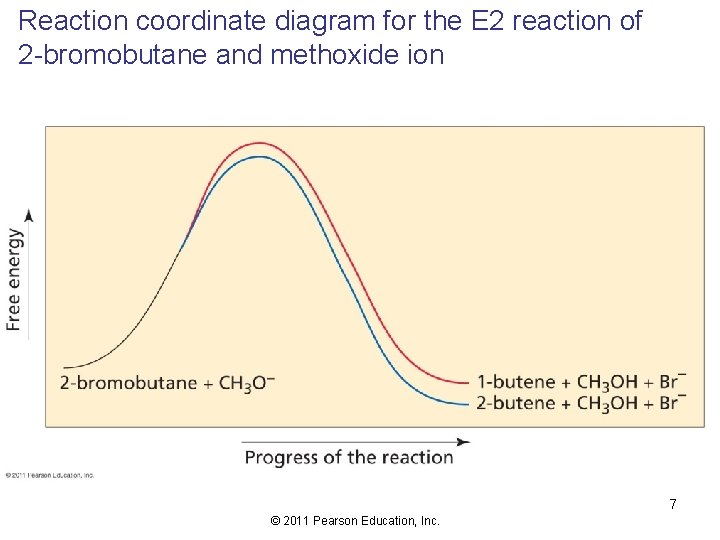
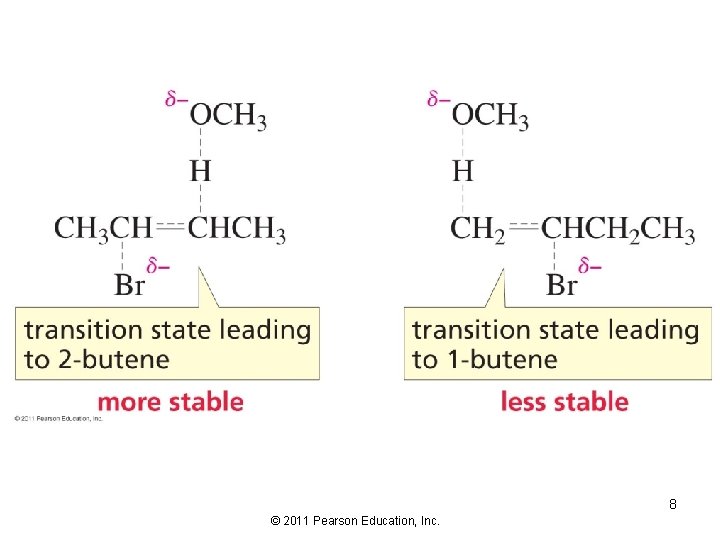
Reaction coordinate diagram for the E 2 reaction of 2 -bromobutane and methoxide ion 7 © 2011 Pearson Education, Inc.

8 © 2011 Pearson Education, Inc.

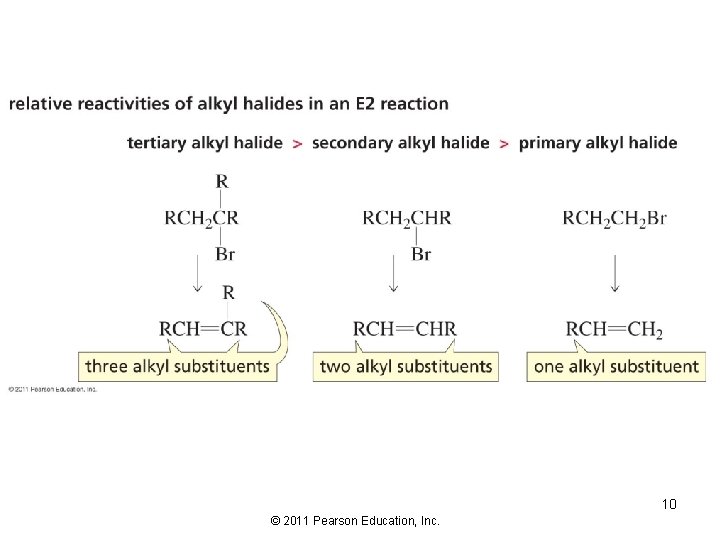
The Zaitsev Rule The more substituted alkene product is obtained when a proton is removed from the b-carbon that is bonded to the fewest hydrogens The most stable alkene is generally (but not always) the most substituted alkene 9 © 2011 Pearson Education, Inc.

10 © 2011 Pearson Education, Inc.

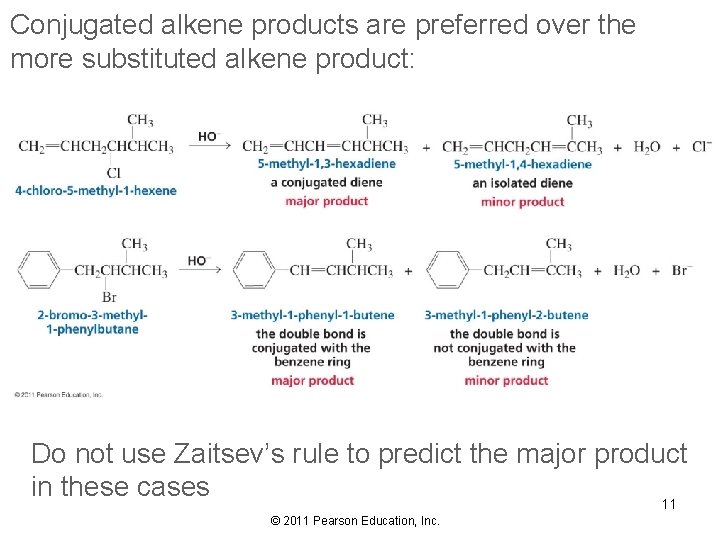
Conjugated alkene products are preferred over the more substituted alkene product: Do not use Zaitsev’s rule to predict the major product in these cases 11 © 2011 Pearson Education, Inc.

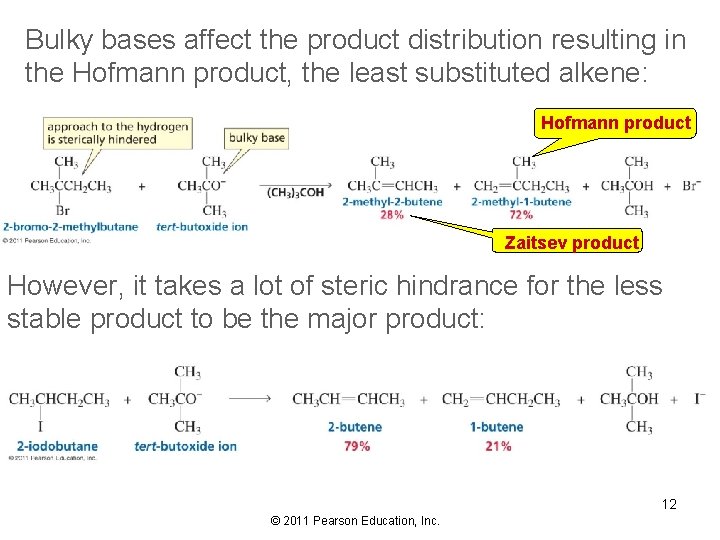
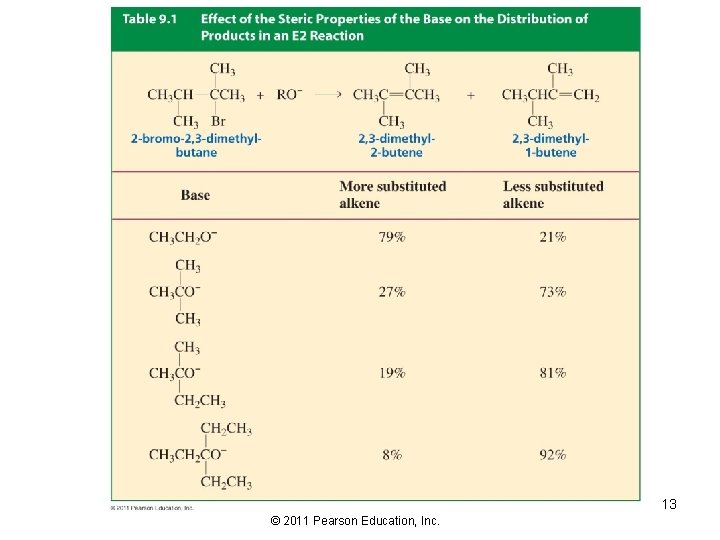
Bulky bases affect the product distribution resulting in the Hofmann product, the least substituted alkene: Hofmann product Zaitsev product However, it takes a lot of steric hindrance for the less stable product to be the major product: 12 © 2011 Pearson Education, Inc.

13 © 2011 Pearson Education, Inc.

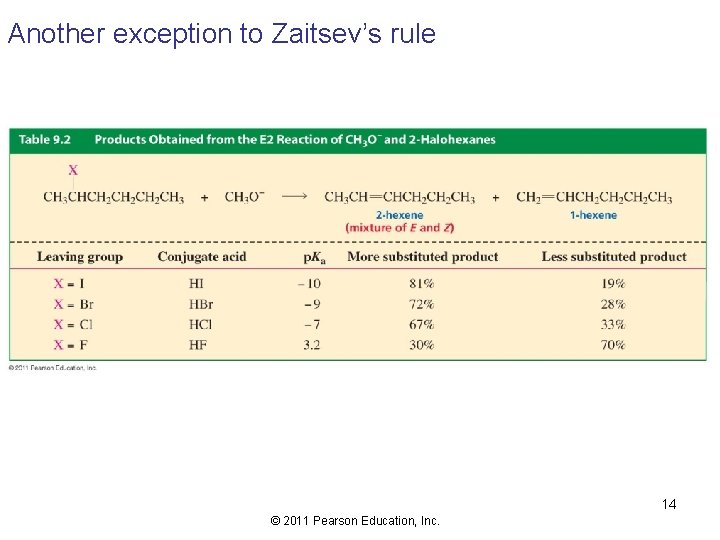
Another exception to Zaitsev’s rule 14 © 2011 Pearson Education, Inc.

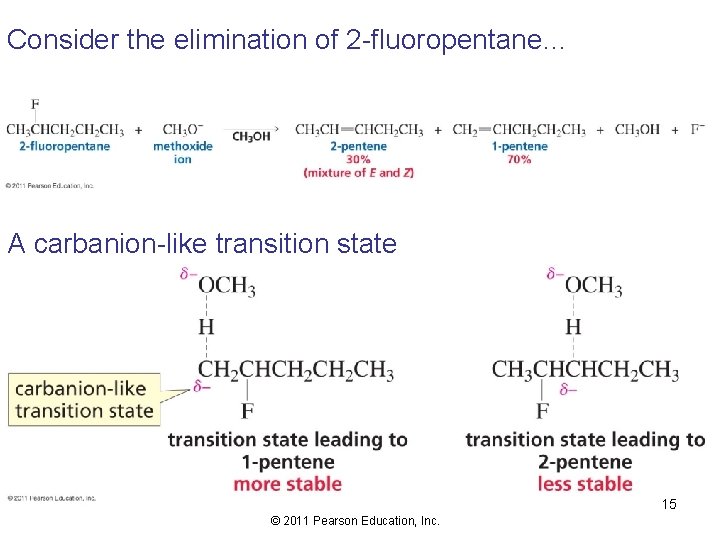
Consider the elimination of 2 -fluoropentane… A carbanion-like transition state 15 © 2011 Pearson Education, Inc.

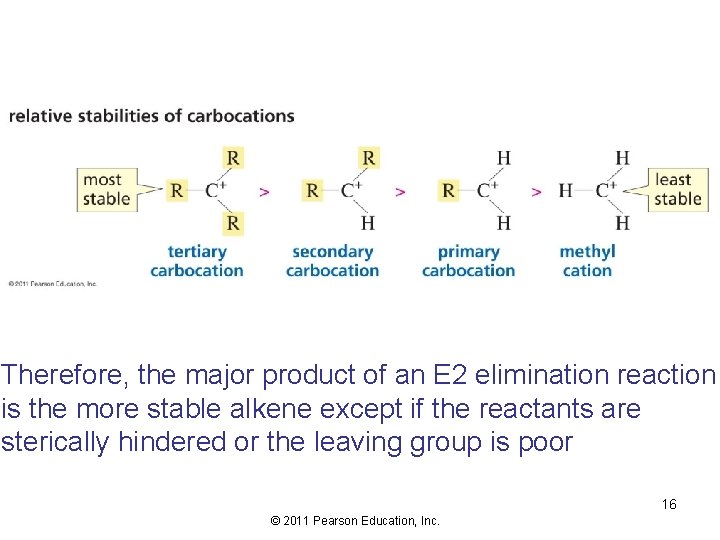
Therefore, the major product of an E 2 elimination reaction is the more stable alkene except if the reactants are sterically hindered or the leaving group is poor 16 © 2011 Pearson Education, Inc.

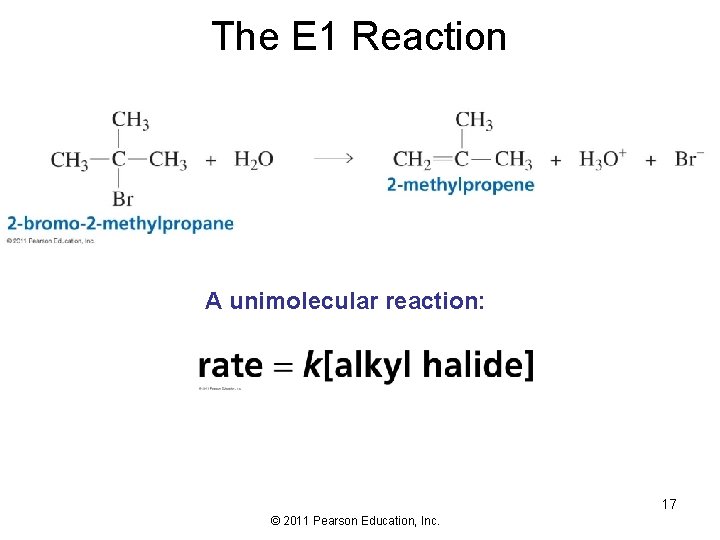
The E 1 Reaction A unimolecular reaction: 17 © 2011 Pearson Education, Inc.

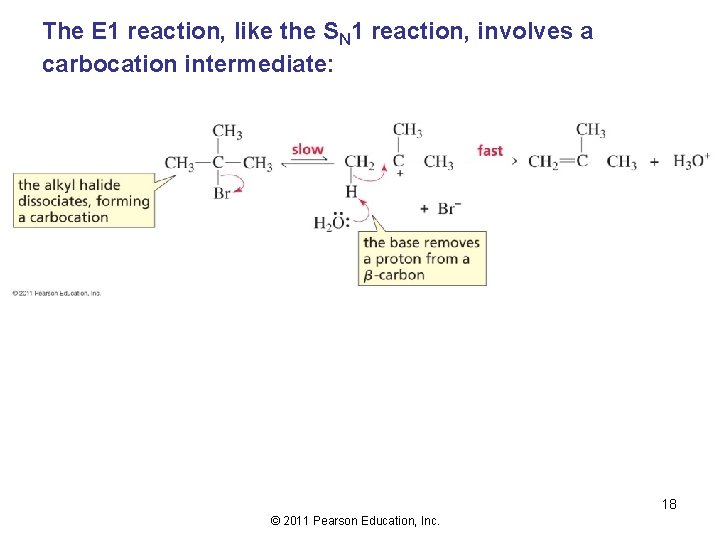
The E 1 reaction, like the SN 1 reaction, involves a carbocation intermediate: 18 © 2011 Pearson Education, Inc.

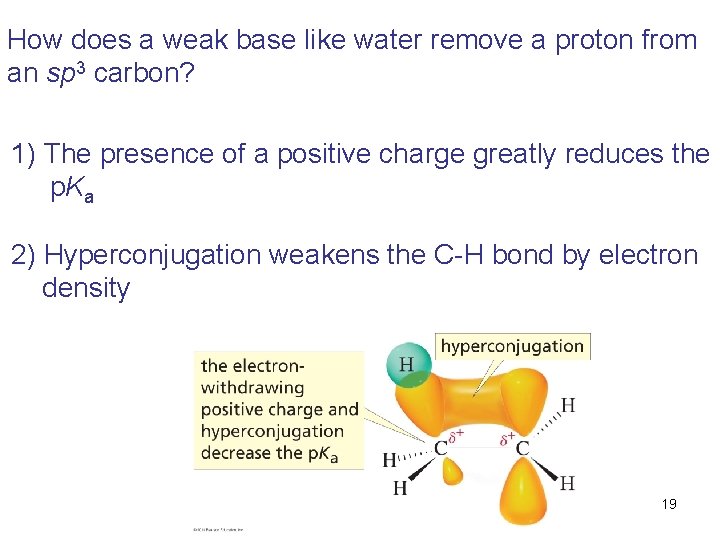
How does a weak base like water remove a proton from an sp 3 carbon? 1) The presence of a positive charge greatly reduces the p. Ka 2) Hyperconjugation weakens the C-H bond by electron density 19 © 2011 Pearson Education, Inc.

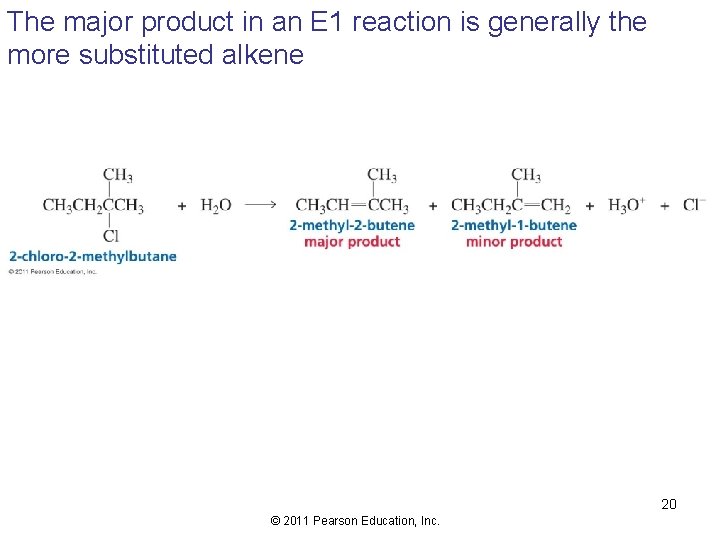
The major product in an E 1 reaction is generally the more substituted alkene 20 © 2011 Pearson Education, Inc.

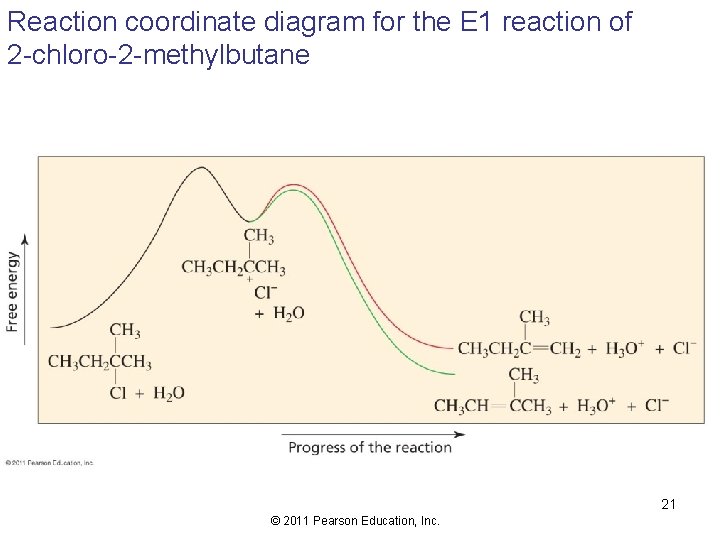
Reaction coordinate diagram for the E 1 reaction of 2 -chloro-2 -methylbutane 21 © 2011 Pearson Education, Inc.

Because the first step is the rate-determining step, the rate of an E 1 reaction depends both on the ease with which the carbocation is formed and how readily the leaving group leaves 22 © 2011 Pearson Education, Inc.

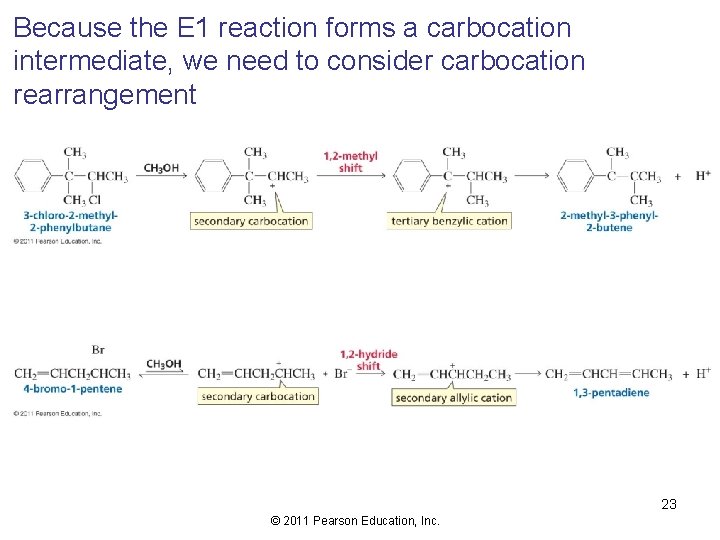
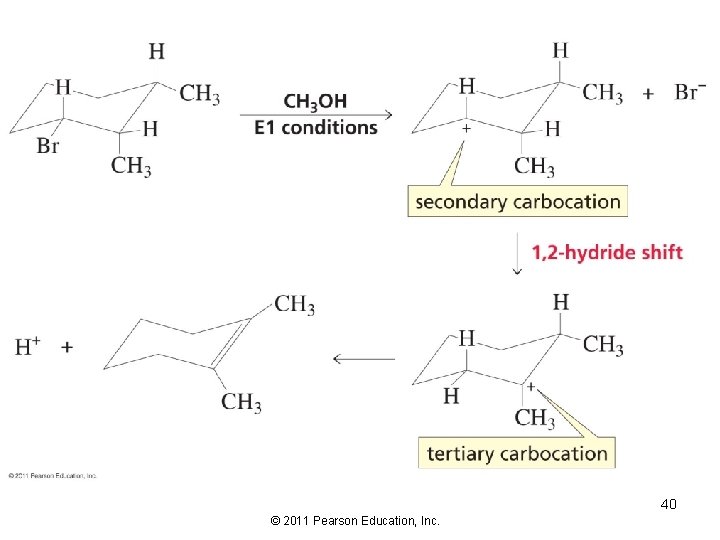
Because the E 1 reaction forms a carbocation intermediate, we need to consider carbocation rearrangement 23 © 2011 Pearson Education, Inc.

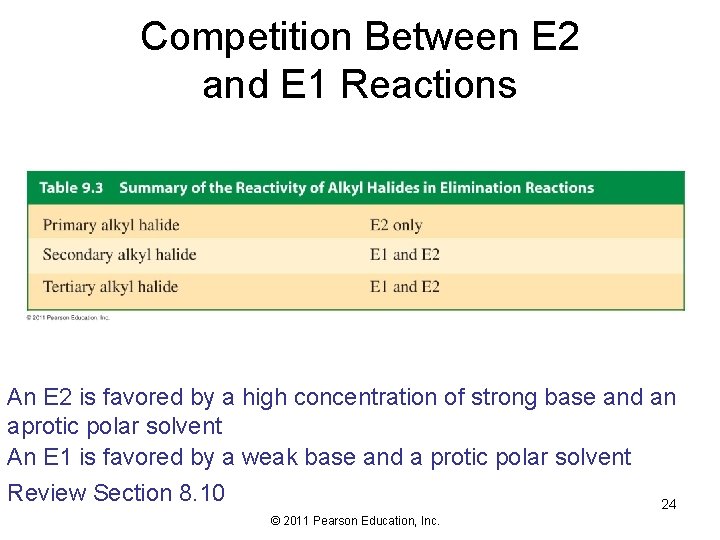
Competition Between E 2 and E 1 Reactions An E 2 is favored by a high concentration of strong base and an aprotic polar solvent An E 1 is favored by a weak base and a protic polar solvent Review Section 8. 10 24 © 2011 Pearson Education, Inc.

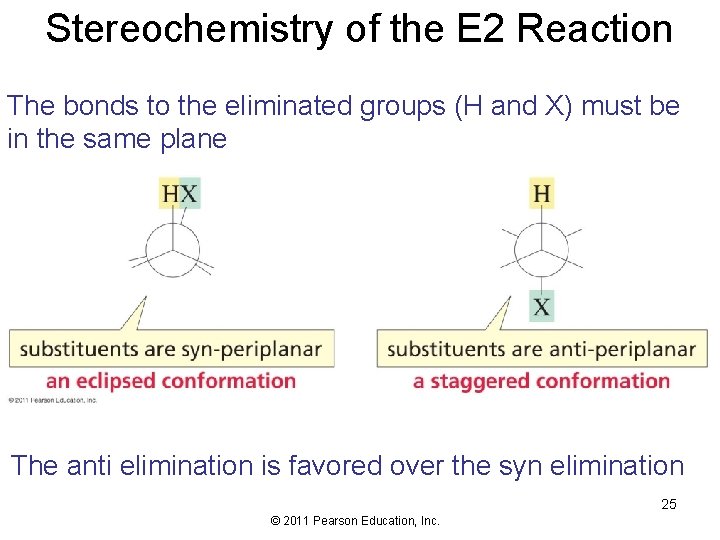
Stereochemistry of the E 2 Reaction The bonds to the eliminated groups (H and X) must be in the same plane The anti elimination is favored over the syn elimination 25 © 2011 Pearson Education, Inc.

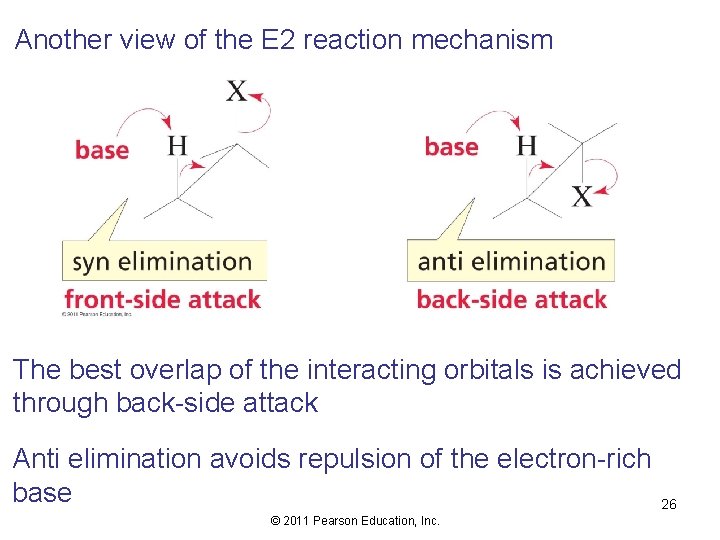
Another view of the E 2 reaction mechanism The best overlap of the interacting orbitals is achieved through back-side attack Anti elimination avoids repulsion of the electron-rich base © 2011 Pearson Education, Inc. 26

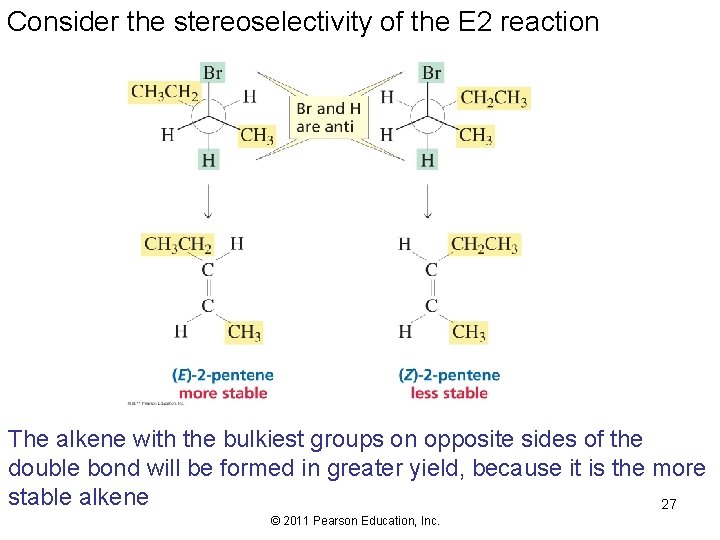
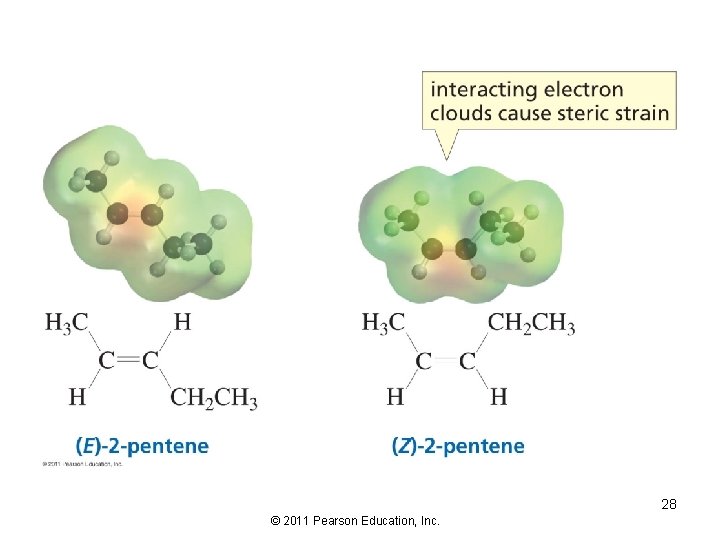
Consider the stereoselectivity of the E 2 reaction The alkene with the bulkiest groups on opposite sides of the double bond will be formed in greater yield, because it is the more stable alkene 27 © 2011 Pearson Education, Inc.

28 © 2011 Pearson Education, Inc.

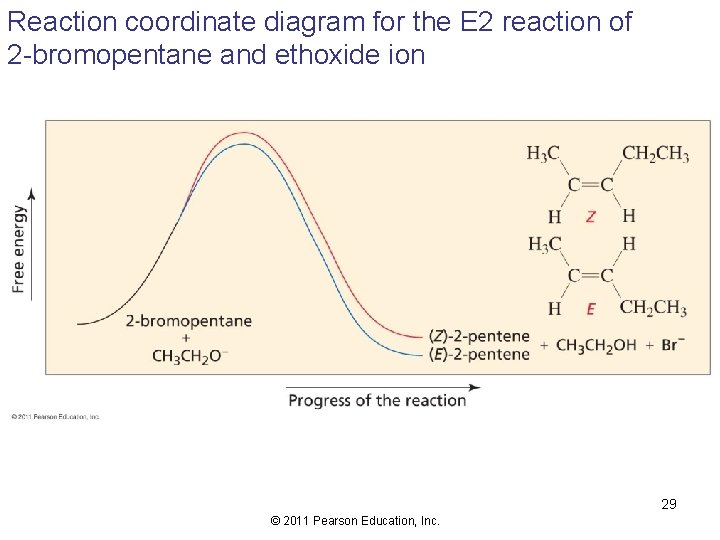
Reaction coordinate diagram for the E 2 reaction of 2 -bromopentane and ethoxide ion 29 © 2011 Pearson Education, Inc.

30 © 2011 Pearson Education, Inc.

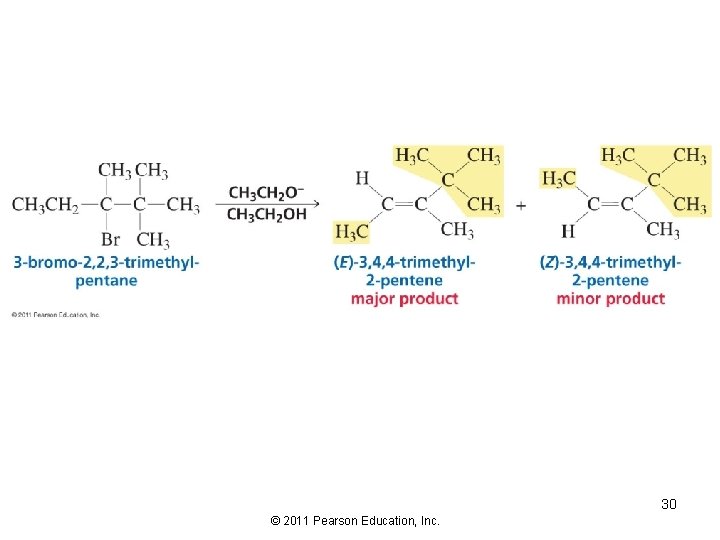
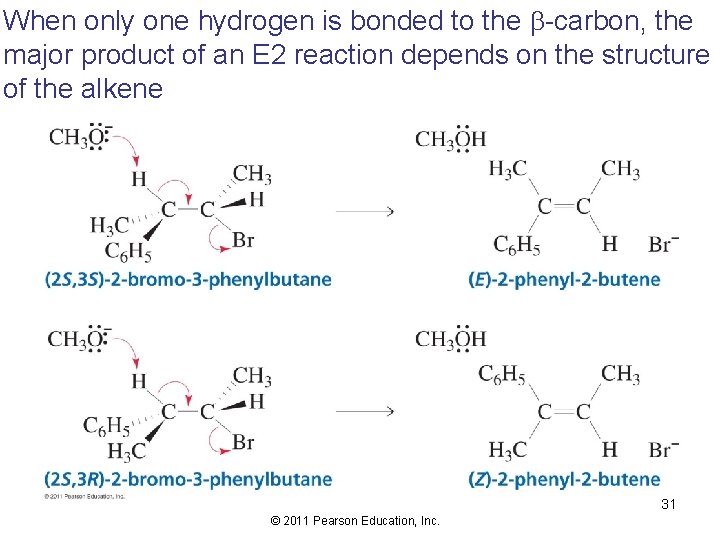
When only one hydrogen is bonded to the b-carbon, the major product of an E 2 reaction depends on the structure of the alkene 31 © 2011 Pearson Education, Inc.

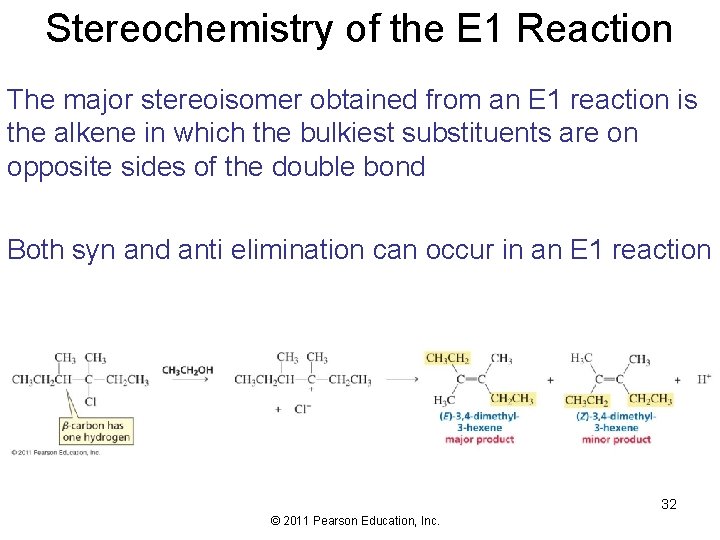
Stereochemistry of the E 1 Reaction The major stereoisomer obtained from an E 1 reaction is the alkene in which the bulkiest substituents are on opposite sides of the double bond Both syn and anti elimination can occur in an E 1 reaction 32 © 2011 Pearson Education, Inc.

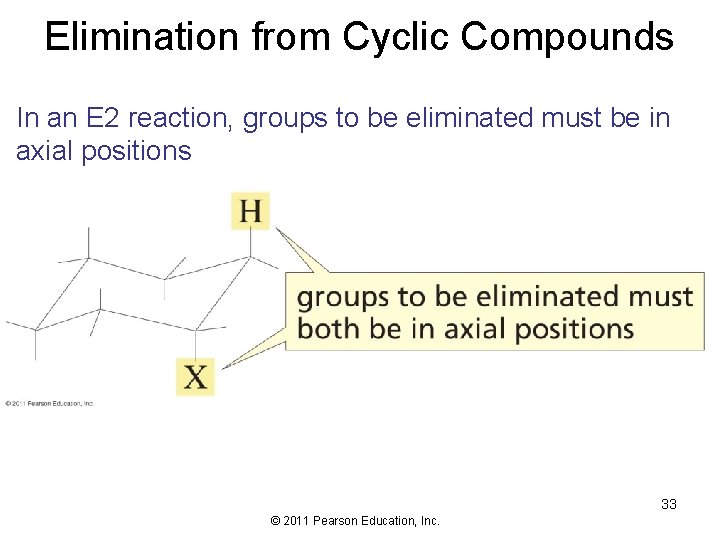
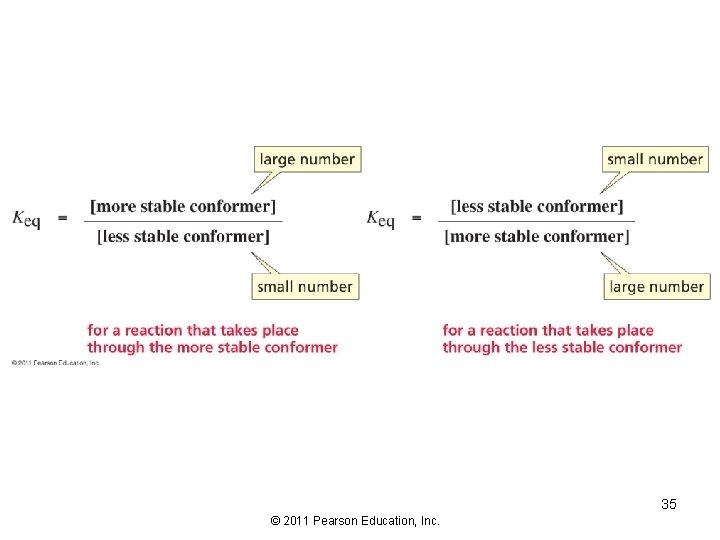
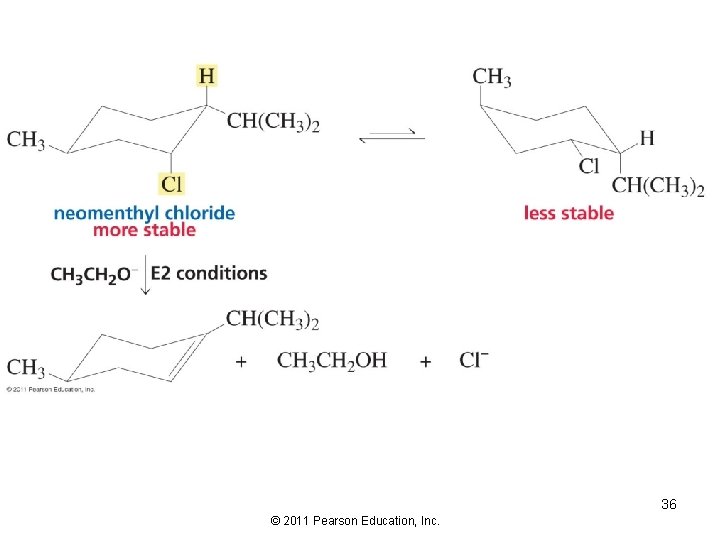
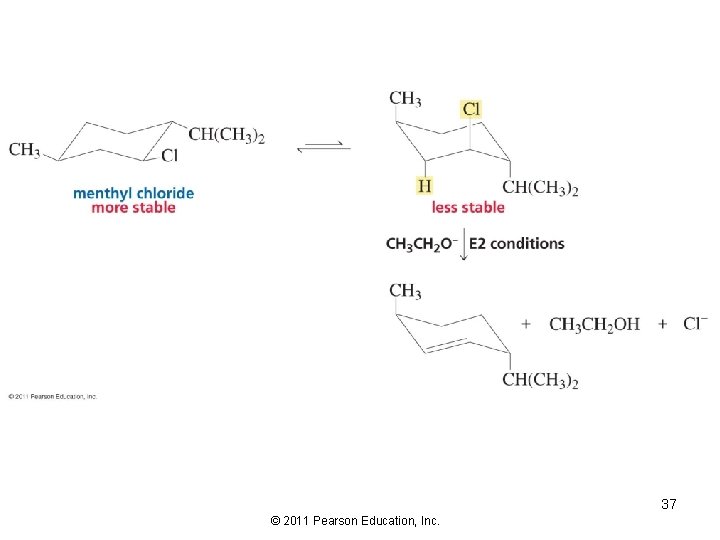
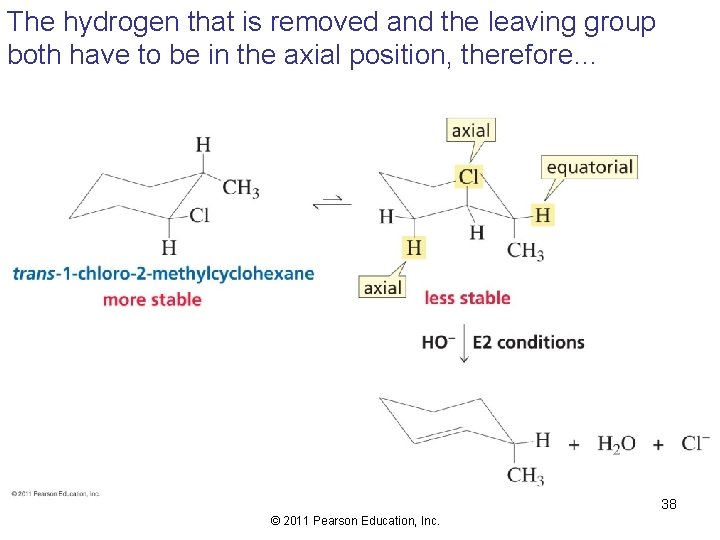
Elimination from Cyclic Compounds In an E 2 reaction, groups to be eliminated must be in axial positions 33 © 2011 Pearson Education, Inc.

34 © 2011 Pearson Education, Inc.

35 © 2011 Pearson Education, Inc.

36 © 2011 Pearson Education, Inc.

37 © 2011 Pearson Education, Inc.

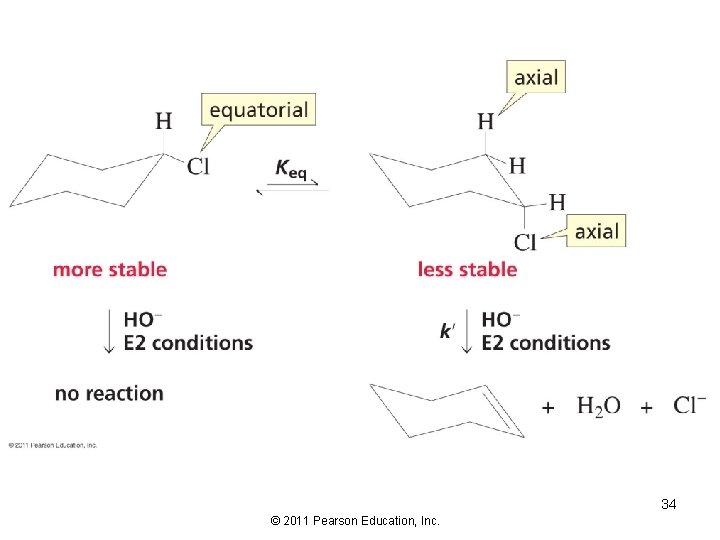
The hydrogen that is removed and the leaving group both have to be in the axial position, therefore… 38 © 2011 Pearson Education, Inc.

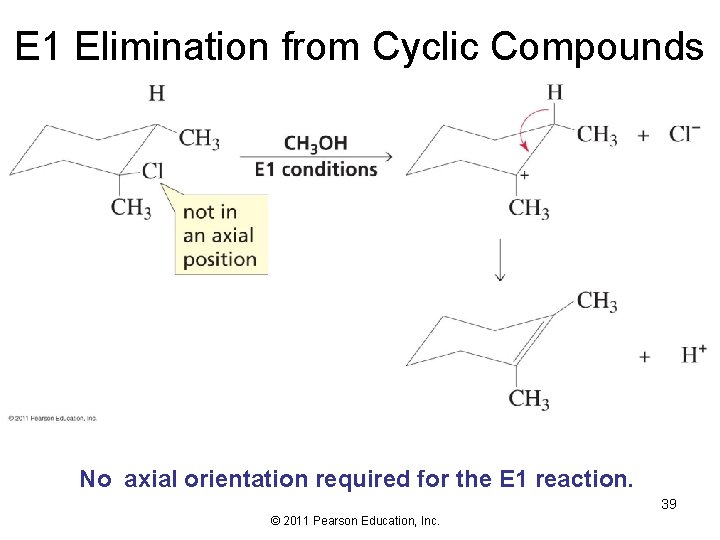
E 1 Elimination from Cyclic Compounds No axial orientation required for the E 1 reaction. 39 © 2011 Pearson Education, Inc.

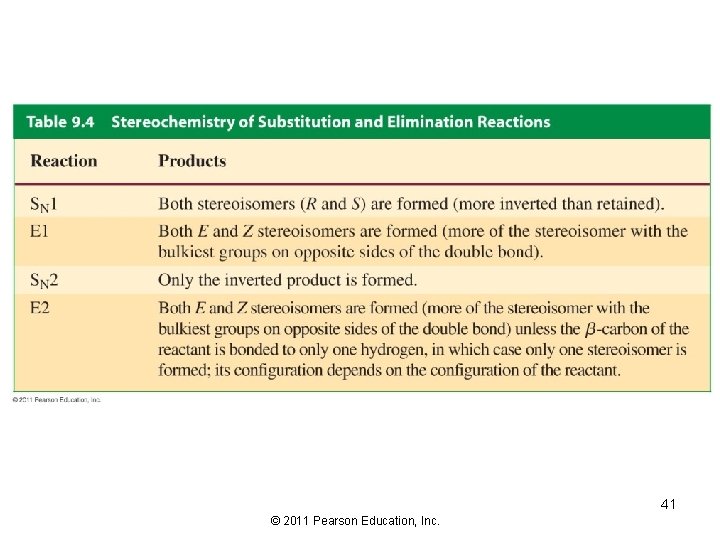
40 © 2011 Pearson Education, Inc.

41 © 2011 Pearson Education, Inc.

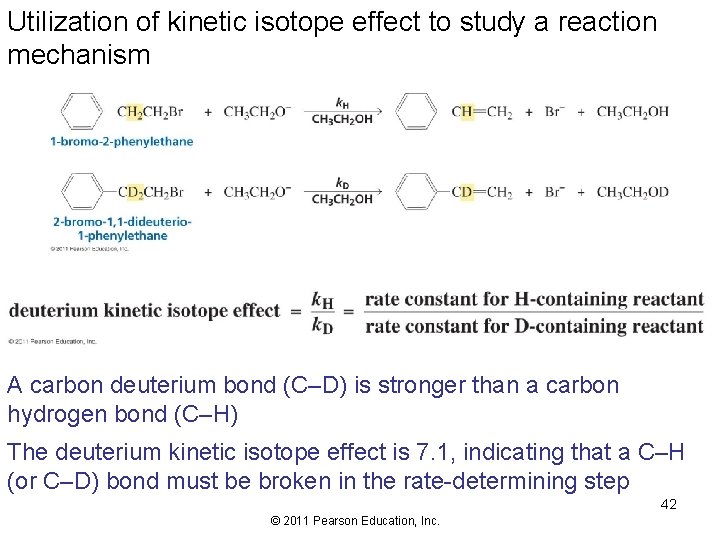
Utilization of kinetic isotope effect to study a reaction mechanism A carbon deuterium bond (C–D) is stronger than a carbon hydrogen bond (C–H) The deuterium kinetic isotope effect is 7. 1, indicating that a C–H (or C–D) bond must be broken in the rate-determining step 42 © 2011 Pearson Education, Inc.

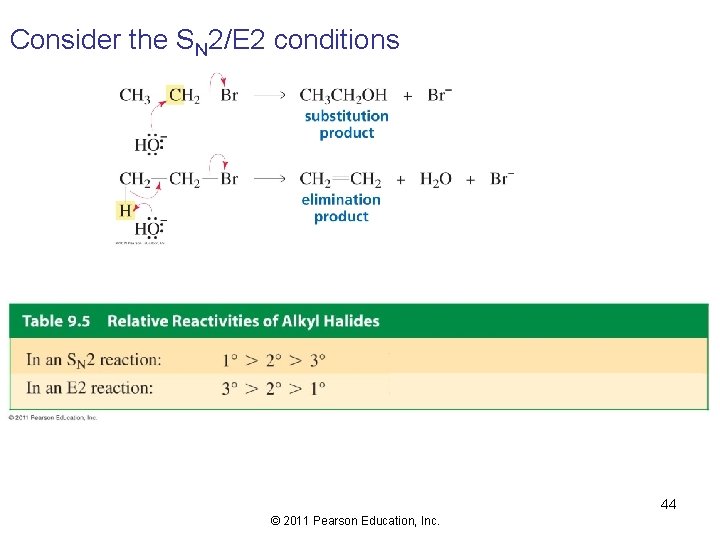
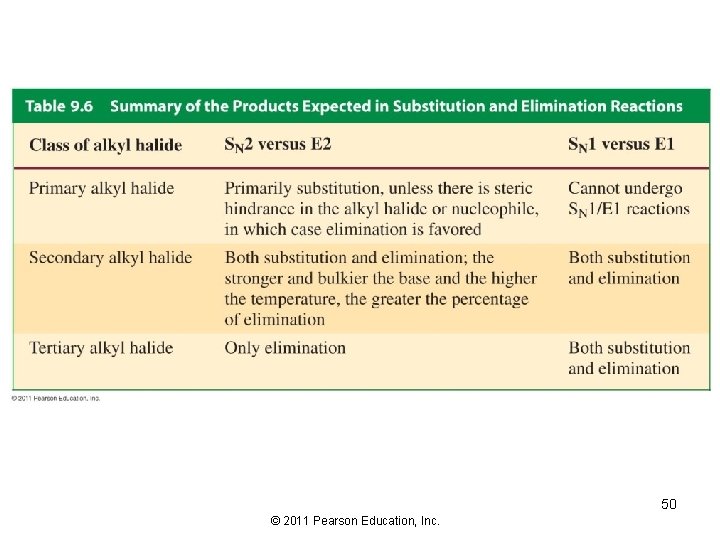
Competition Between Substitution and Elimination Alkyl halides can undergo SN 2, SN 1, E 2, and E 1 1) decide whether the reaction conditions favor SN 2/E 2 or SN 1/E 1 • SN 2/E 2 reactions are favored by a high concentration of a good nucleophile/strong base • SN 1/E 1 reactions are favored by a poor nucleophile/weak base 2) decide how much of the product will be the substitution product and how much of the product will be the elimination product 43 © 2011 Pearson Education, Inc.

Consider the SN 2/E 2 conditions 44 © 2011 Pearson Education, Inc.

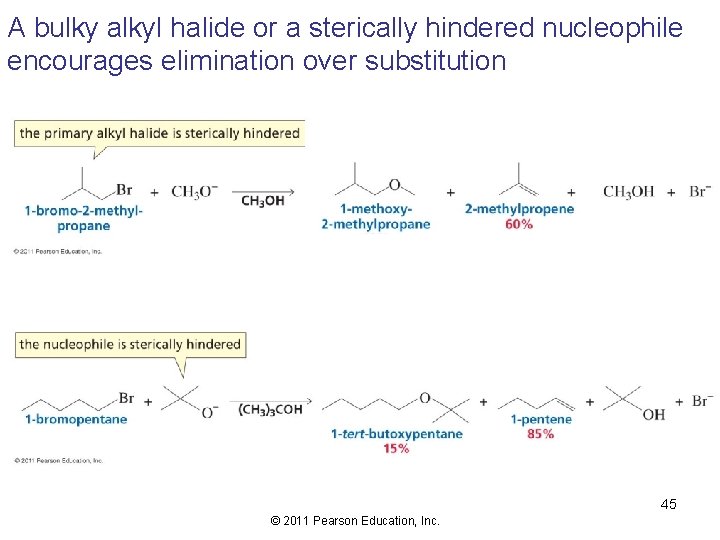
A bulky alkyl halide or a sterically hindered nucleophile encourages elimination over substitution 45 © 2011 Pearson Education, Inc.

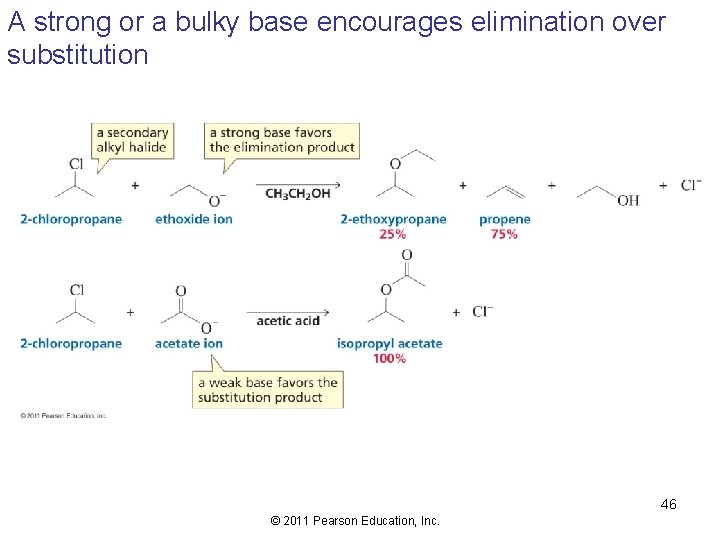
A strong or a bulky base encourages elimination over substitution 46 © 2011 Pearson Education, Inc.

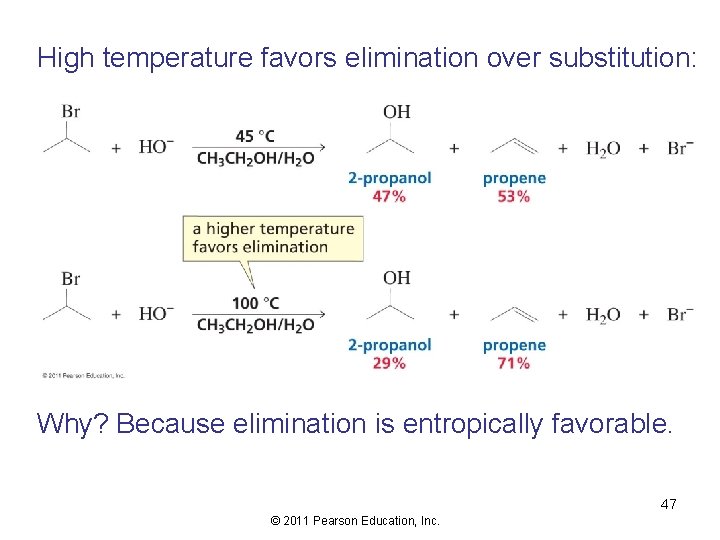
High temperature favors elimination over substitution: Why? Because elimination is entropically favorable. 47 © 2011 Pearson Education, Inc.

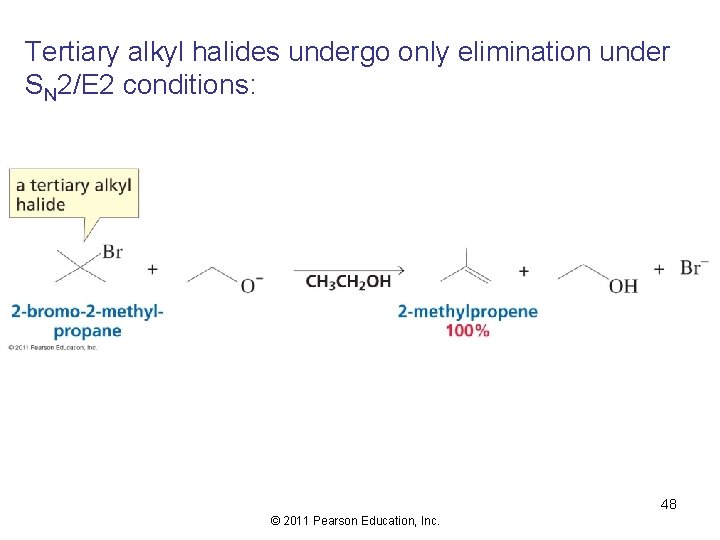
Tertiary alkyl halides undergo only elimination under SN 2/E 2 conditions: 48 © 2011 Pearson Education, Inc.

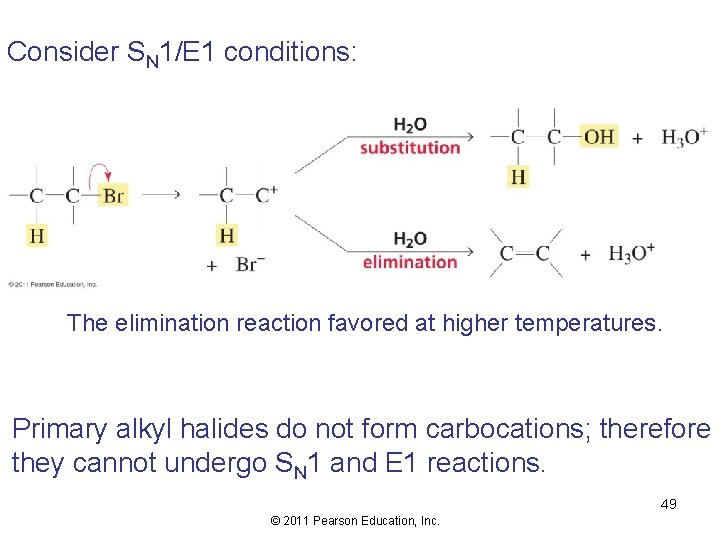
Consider SN 1/E 1 conditions: The elimination reaction favored at higher temperatures. Primary alkyl halides do not form carbocations; therefore they cannot undergo SN 1 and E 1 reactions. 49 © 2011 Pearson Education, Inc.

50 © 2011 Pearson Education, Inc.

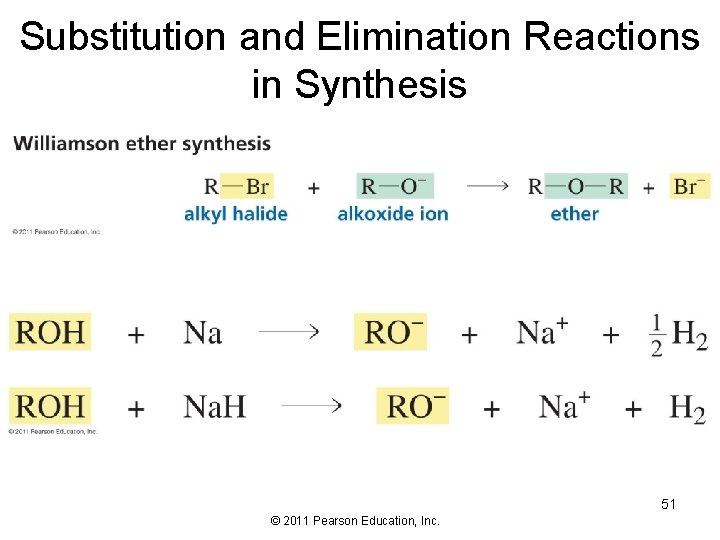
Substitution and Elimination Reactions in Synthesis 51 © 2011 Pearson Education, Inc.

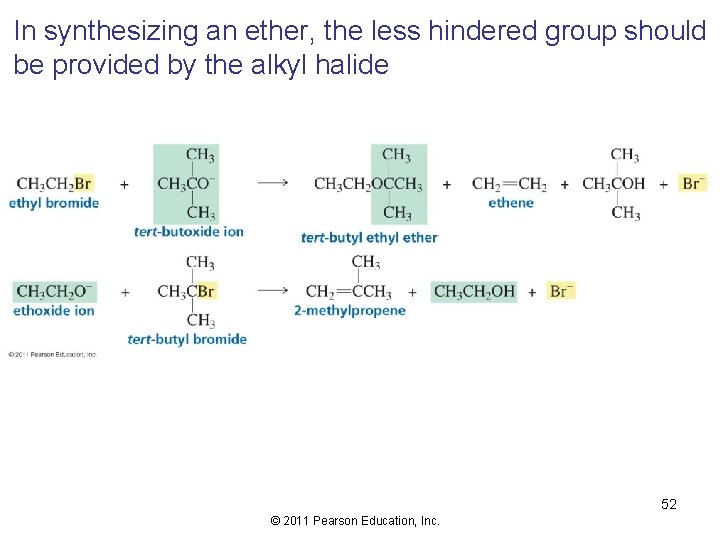
In synthesizing an ether, the less hindered group should be provided by the alkyl halide 52 © 2011 Pearson Education, Inc.

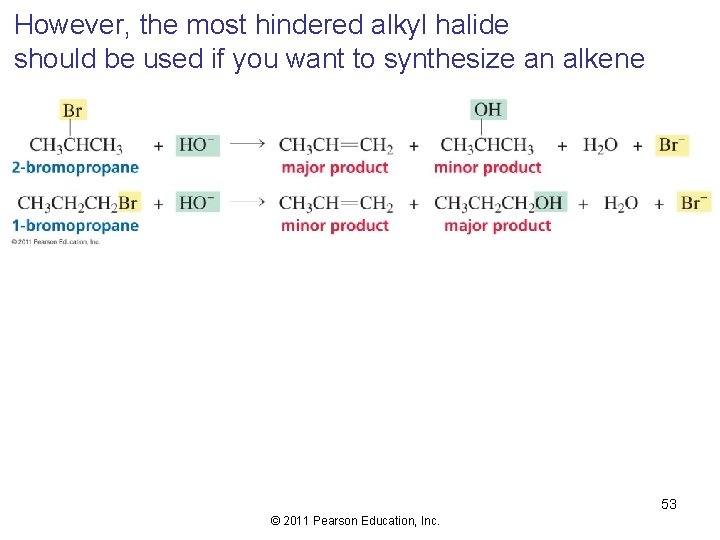
However, the most hindered alkyl halide should be used if you want to synthesize an alkene 53 © 2011 Pearson Education, Inc.

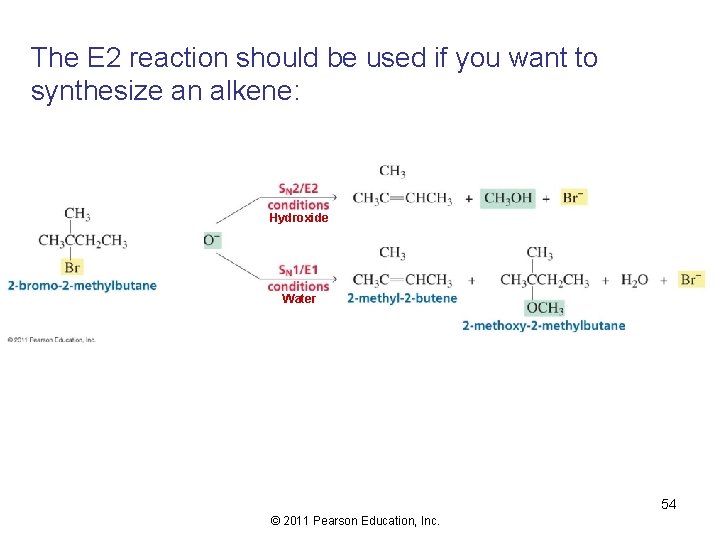
The E 2 reaction should be used if you want to synthesize an alkene: Hydroxide Water 54 © 2011 Pearson Education, Inc.

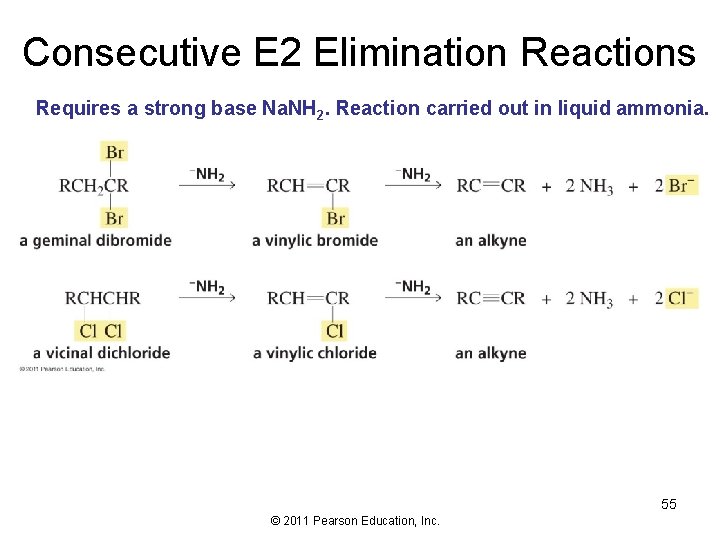
Consecutive E 2 Elimination Reactions Requires a strong base Na. NH 2. Reaction carried out in liquid ammonia. 55 © 2011 Pearson Education, Inc.

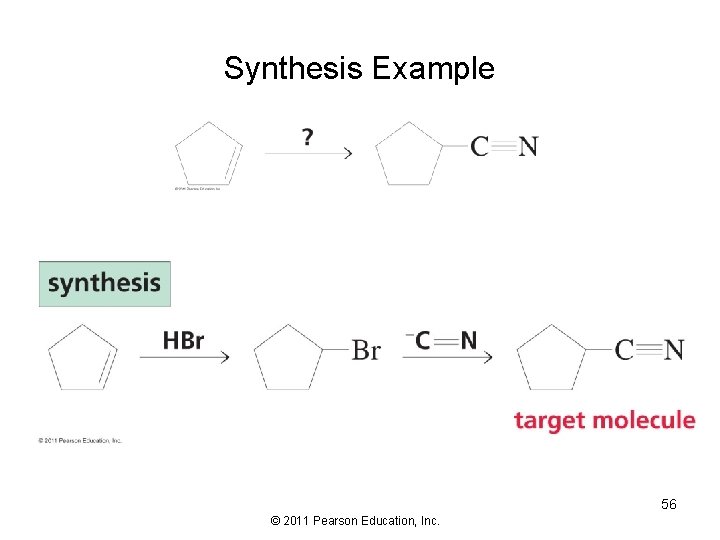
Synthesis Example 56 © 2011 Pearson Education, Inc.

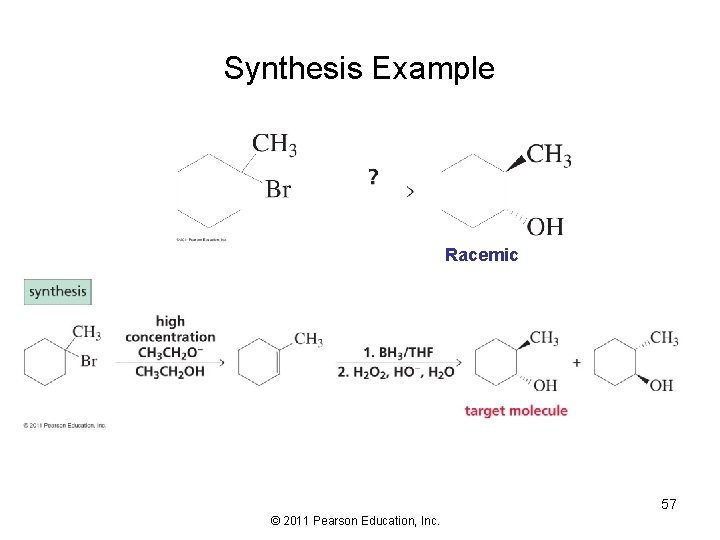
Synthesis Example Racemic 57 © 2011 Pearson Education, Inc.

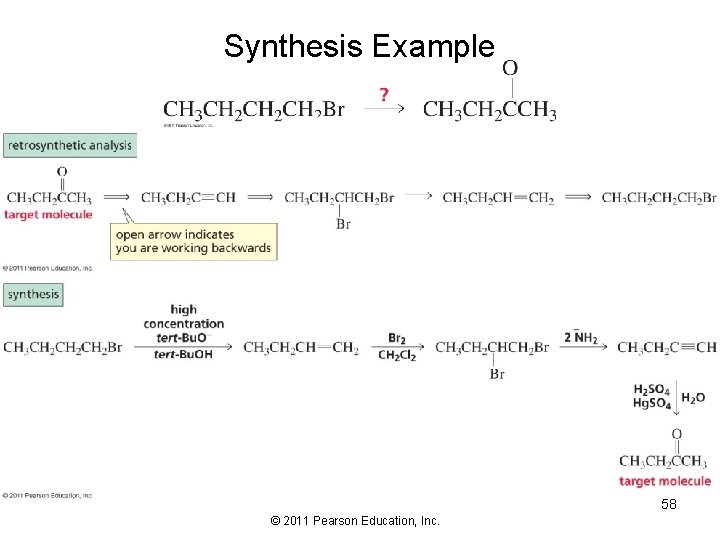
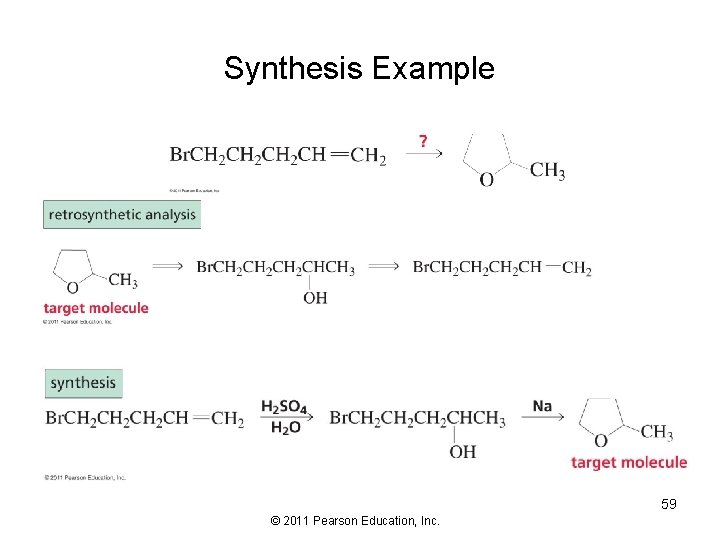
Synthesis Example 58 © 2011 Pearson Education, Inc.

Synthesis Example 59 © 2011 Pearson Education, Inc.