Organic chemistry Introduction to organic chemistry Organic chemistry
















- Slides: 53

Organic chemistry

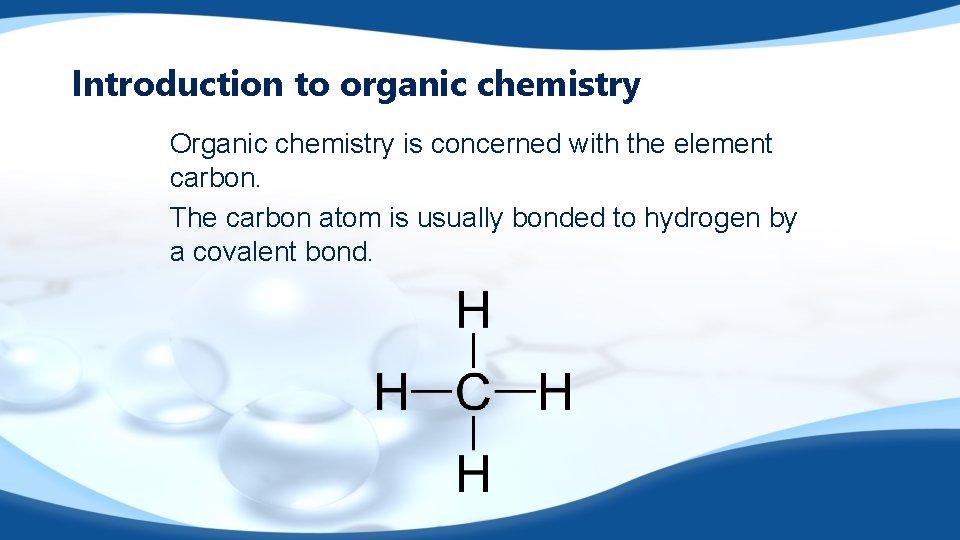
Introduction to organic chemistry Organic chemistry is concerned with the element carbon. The carbon atom is usually bonded to hydrogen by a covalent bond.

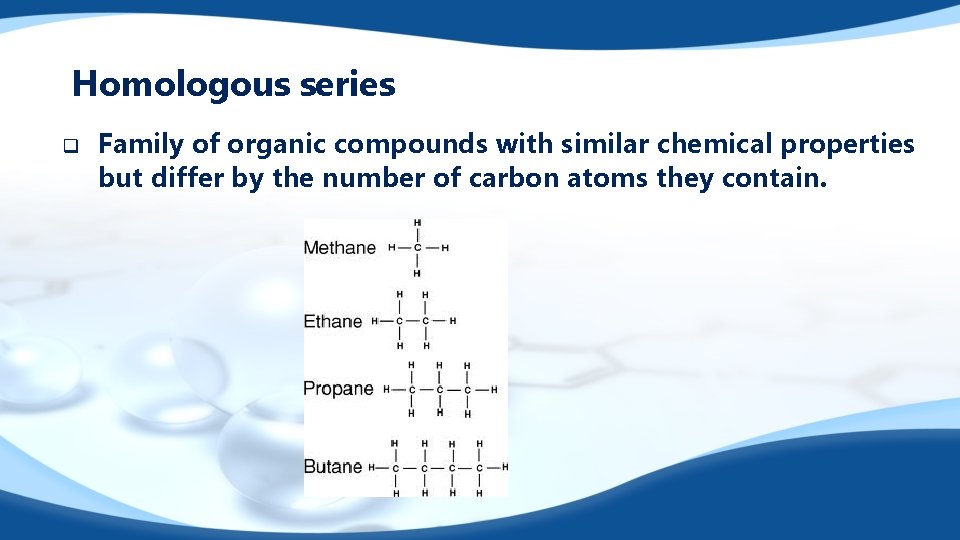
Homologous series q Family of organic compounds with similar chemical properties but differ by the number of carbon atoms they contain.

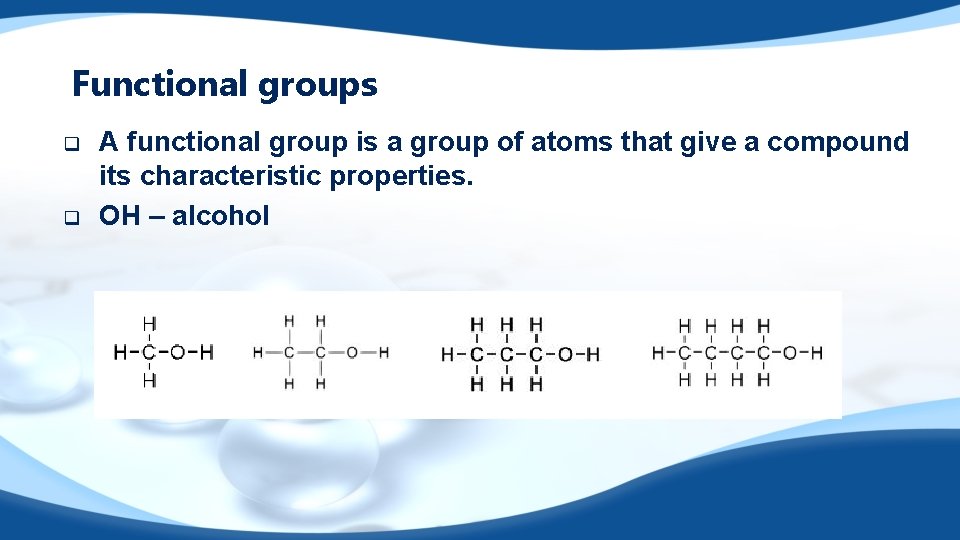
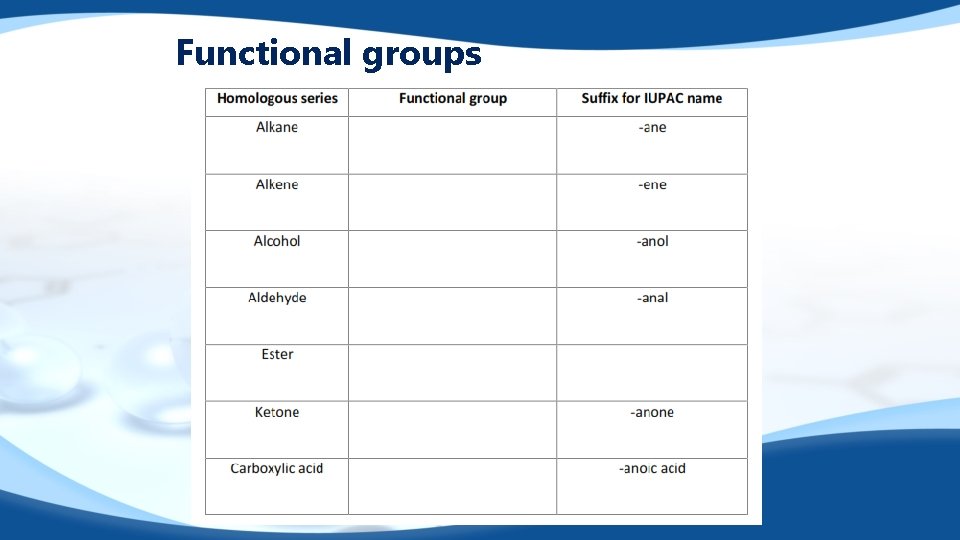
Functional groups q q A functional group is a group of atoms that give a compound its characteristic properties. OH – alcohol

Features of a homologous series q Same functional group q Same general formula q q Differ by CH 2 Show a gradation in physical properties (such as boiling point) q Similar chemical properties

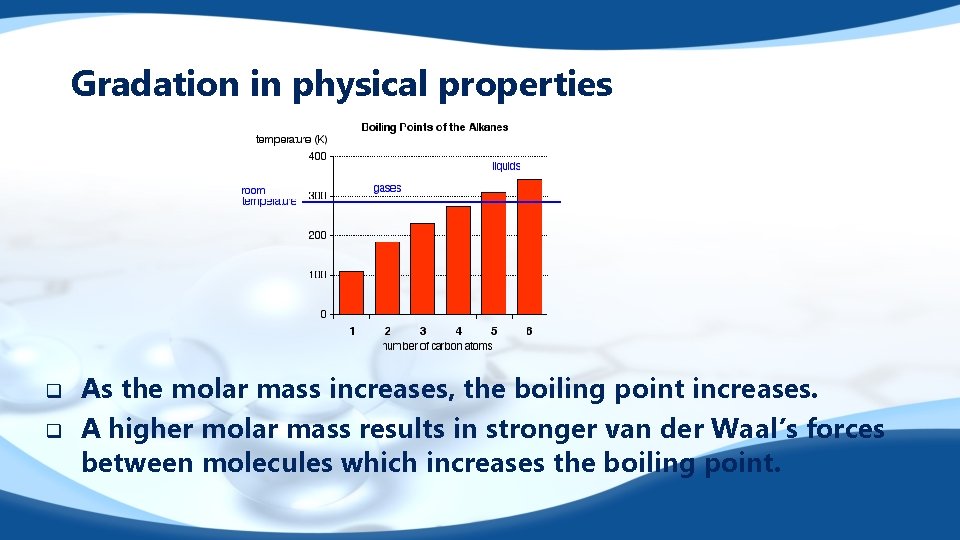
Gradation in physical properties q q As the molar mass increases, the boiling point increases. A higher molar mass results in stronger van der Waal’s forces between molecules which increases the boiling point.

Empirical and molecular formula q q Empirical formula – the simplest whole number ratio of atoms in a molecule. Molecular formula – the actual number of atoms in a molecule. Full structural formula – shows every atom and bond in a molecule. Condensed structural formula – omits bonds and uses brackets for identical groups.

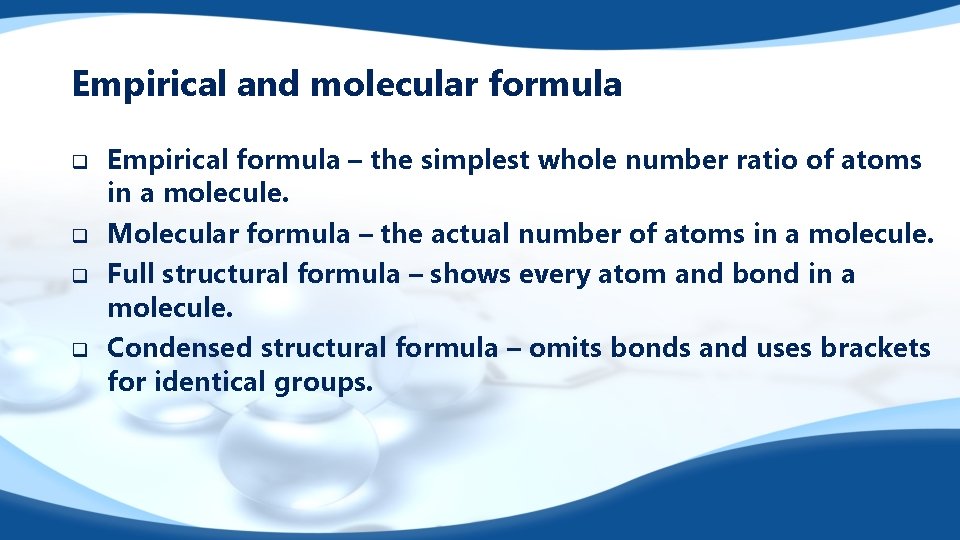
Hexane Full structural formula Molecular formula C 6 H 14 Empirical formula C 3 H 7 Condensed structural formula CH 3(CH 2)4 CH 3

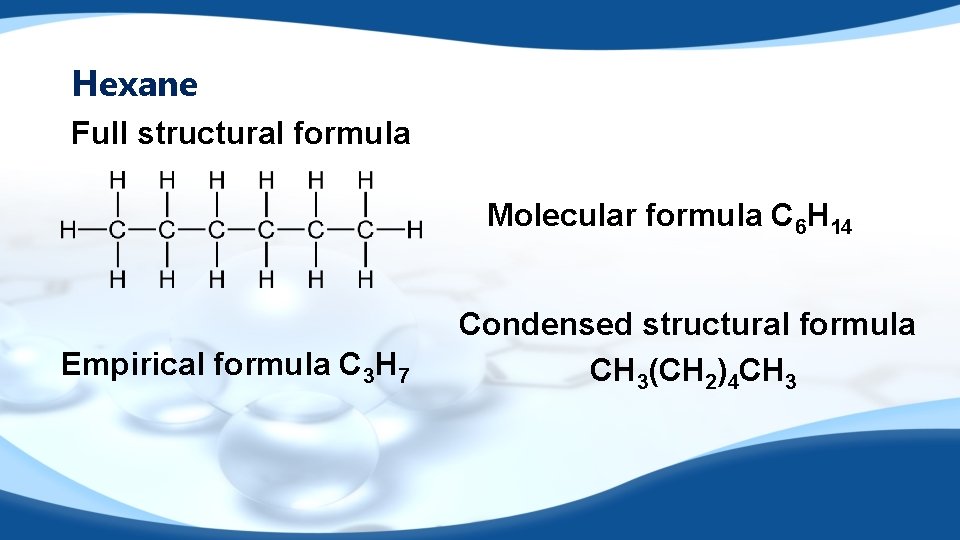
Butan-1 -ol Full structural formula Molecular formula C 4 H 9 OH Empirical formula C 4 H 9 OH Condensed structural formula CH 3 CH 2 CH 2 OH

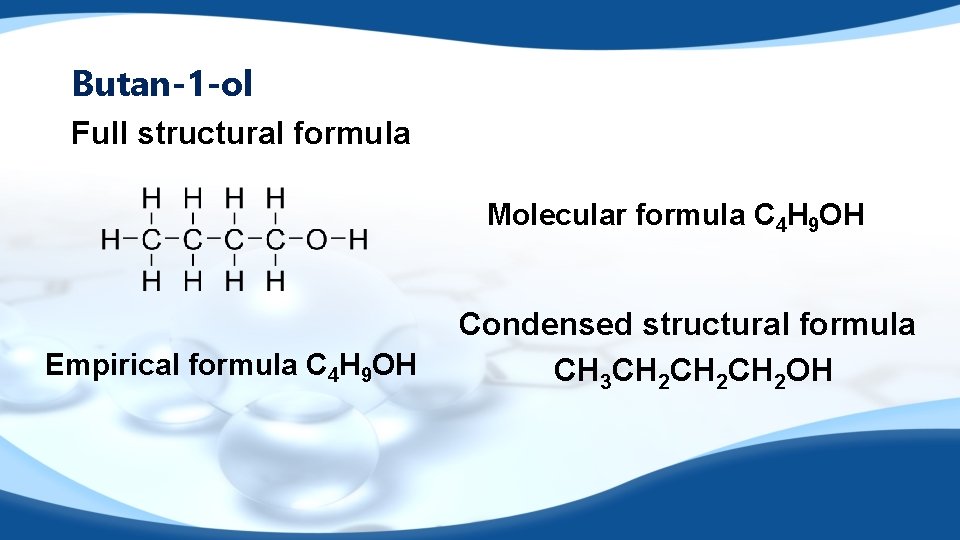
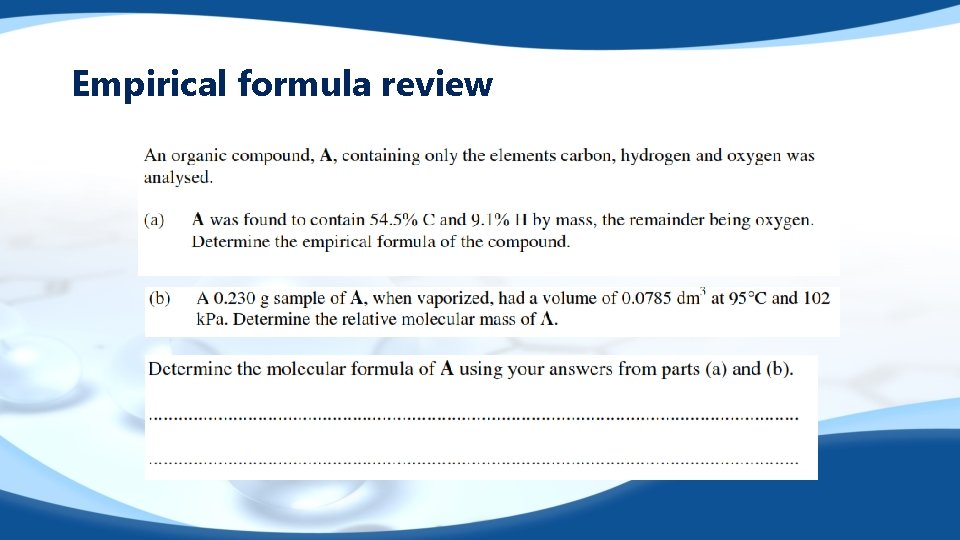
Empirical formula review

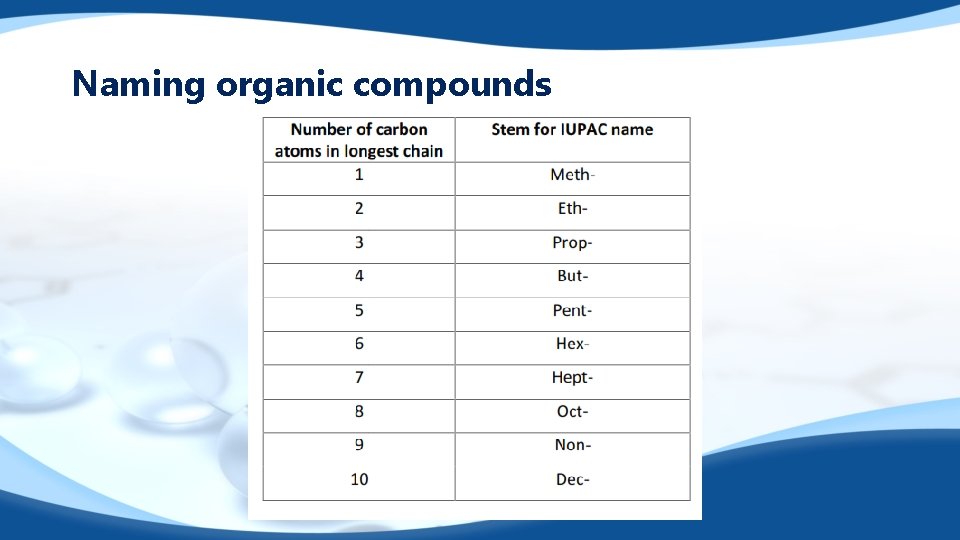
Naming organic compounds

Functional groups

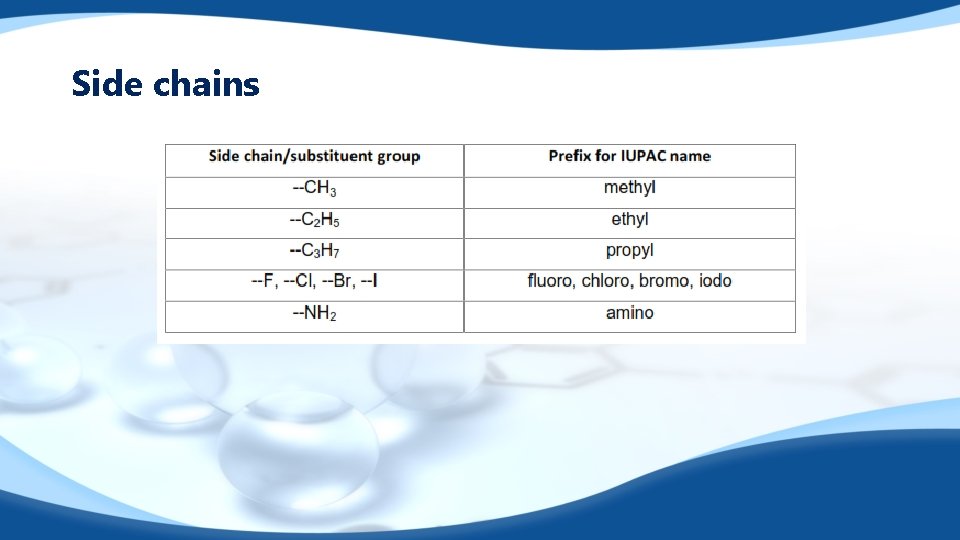
Side chains

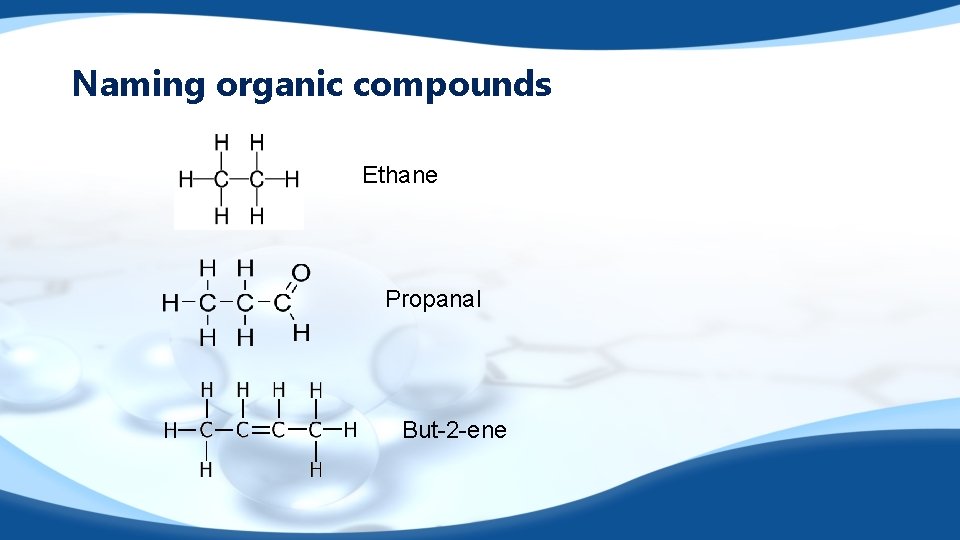
Naming organic compounds Ethane Propanal But-2 -ene

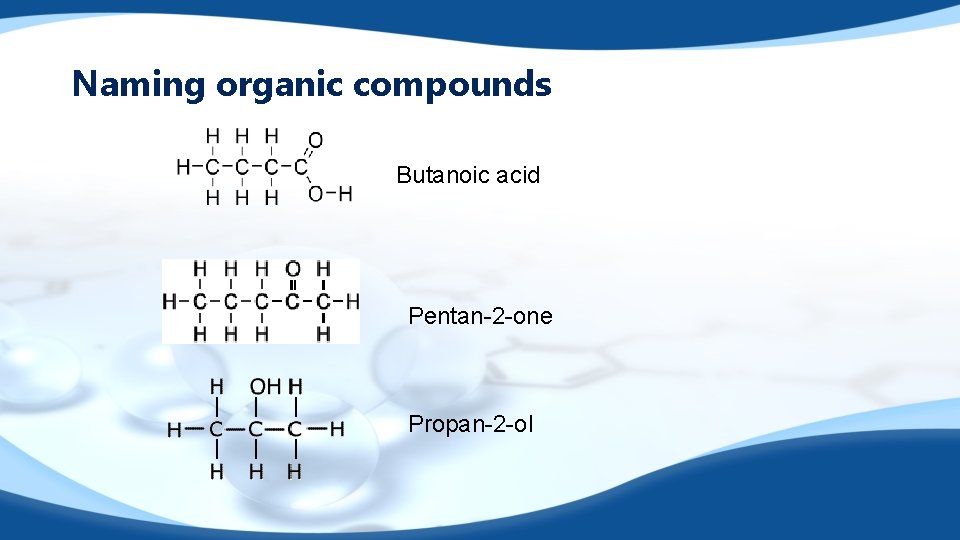
Naming organic compounds Butanoic acid Pentan-2 -one Propan-2 -ol

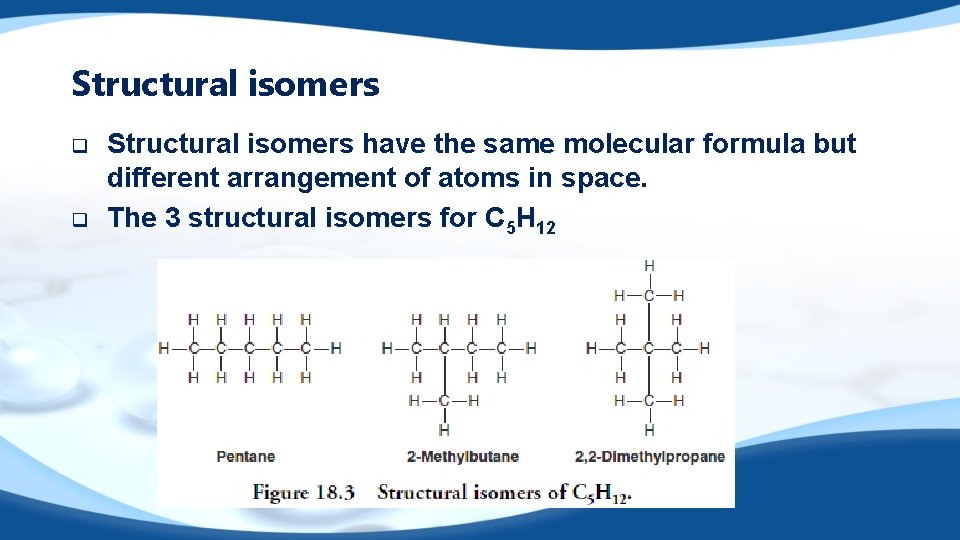
Structural isomers q q Structural isomers have the same molecular formula but different arrangement of atoms in space. The 3 structural isomers for C 5 H 12

Physical properties of organic compounds Physical properties include: q Melting point – the temperature at which a solid becomes a liquid q Boiling point – the temperature at which a liquid becomes a gas q Density – mass per unit volume

Volatility q q q Volatility is how easily a substance becomes a gas (vaporises) High volatility means that the substance will vaporise at a low temperature (low B. P) Low volatility means that the substance will vaporise at a higher temperature (high B. P)

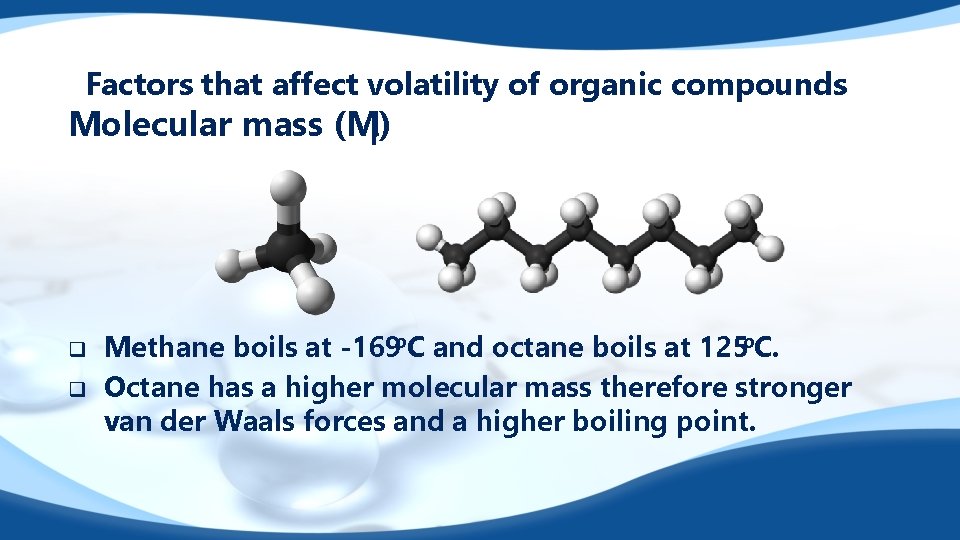
Factors that affect volatility of organic compounds Molecular mass (Mr) q q Methane boils at -169 o. C and octane boils at 125 o. C. Octane has a higher molecular mass therefore stronger van der Waals forces and a higher boiling point.

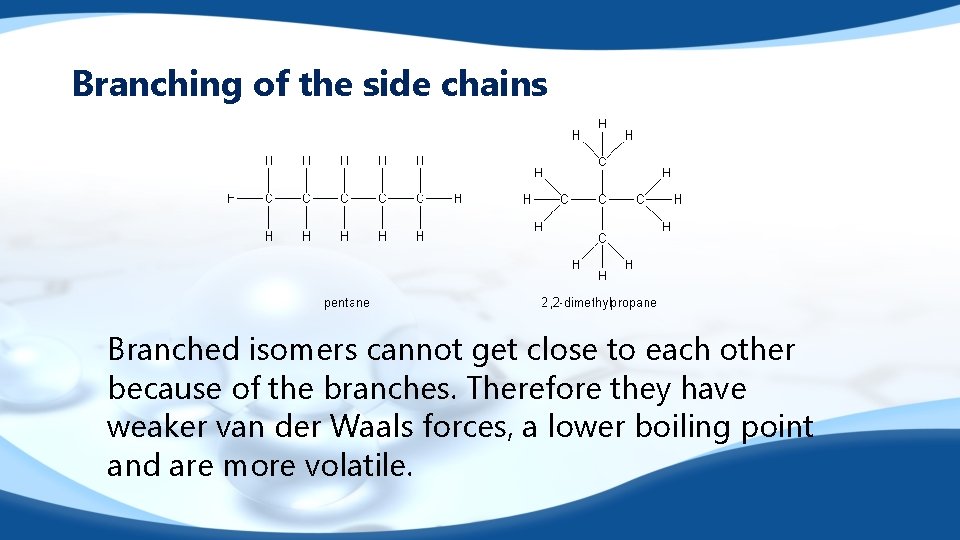
Branching of the side chains Branched isomers cannot get close to each other because of the branches. Therefore they have weaker van der Waals forces, a lower boiling point and are more volatile.

Functional groups q q q Polar functional groups such as aldehydes and ketones have dipole – dipole attractions between molecules. Organic compounds that have hydrogen bonding (alcohols and carboxylic acids) can form hydrogen bonds between molecules. Stronger intermolecular forces between molecules means higher boiling point and lower volatility.

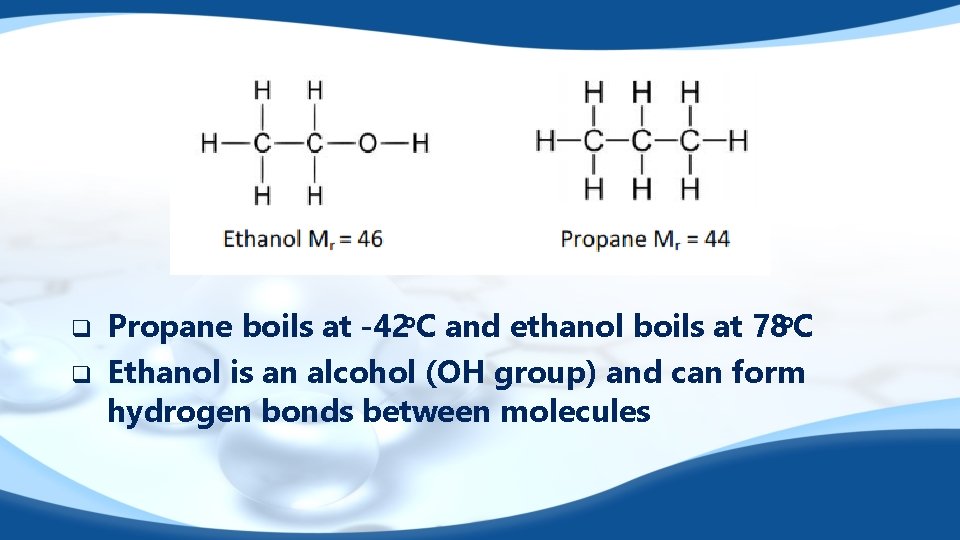
q q Propane boils at -42 o. C and ethanol boils at 78 o. C Ethanol is an alcohol (OH group) and can form hydrogen bonds between molecules

Alkanes q q Alkanes are saturated hydrocarbons. They are made of carbon and hydrogen only. They have single carbon to carbon bonds. They make good fuels because of the energy released during combustion (exothermic reaction).

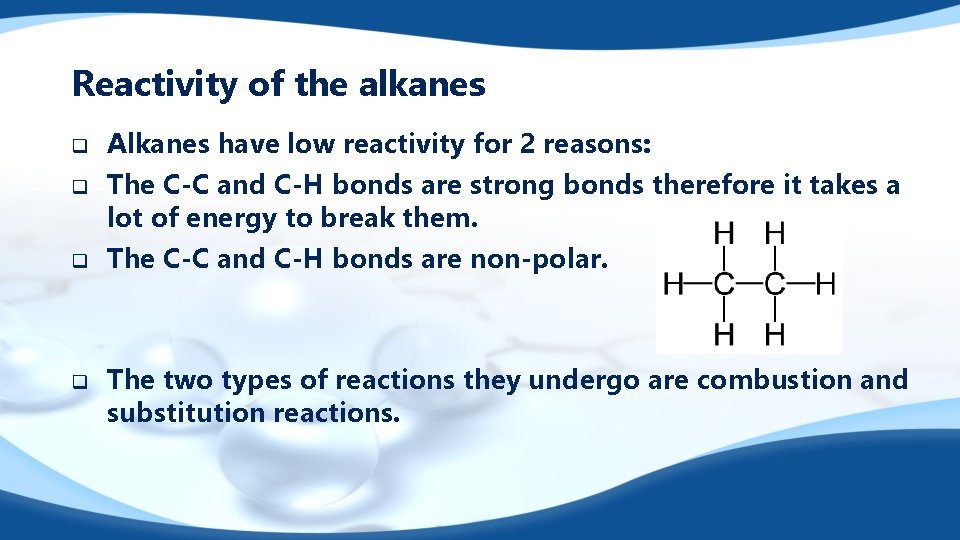
Reactivity of the alkanes q q Alkanes have low reactivity for 2 reasons: The C-C and C-H bonds are strong bonds therefore it takes a lot of energy to break them. The C-C and C-H bonds are non-polar. The two types of reactions they undergo are combustion and substitution reactions.

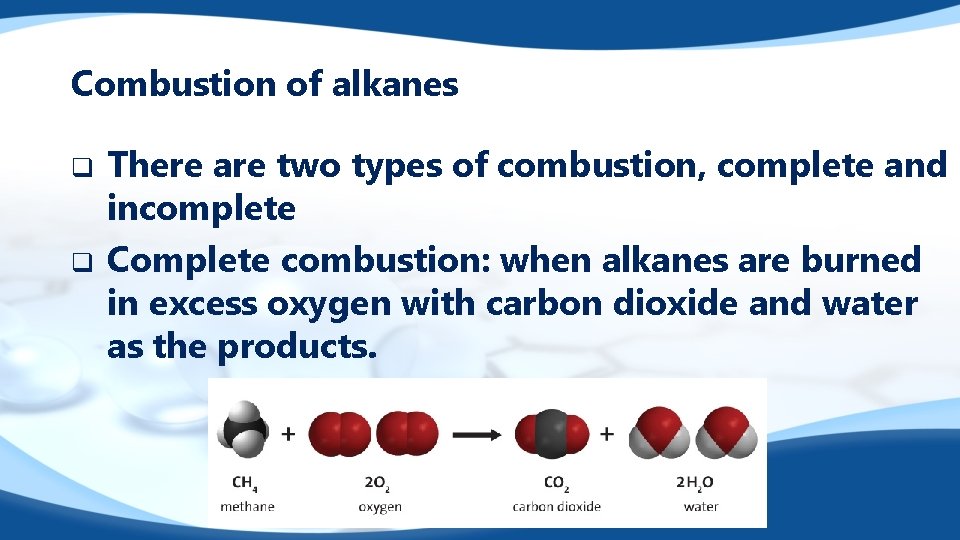
Combustion of alkanes q q There are two types of combustion, complete and incomplete Complete combustion: when alkanes are burned in excess oxygen with carbon dioxide and water as the products.

Incomplete combustion q q q Incomplete combustion happens when alkanes are burned in a lack of oxygen. The products are carbon monoxide (CO) and water. Carbon monoxide is a very poisonous gas.

Substitution reactions of alkanes q q q Alkanes react in the presence of UV light to produce halogenoalkanes. In a substitution reaction, a halogen atom takes the place of a hydrogen atom. There are 3 stages in the reaction, initiation, propagation and termination.

Initiation q q q The bond between the chlorine atoms is broken by UV light. It breaks into 2 chlorine radicals (they have a dot after them). This is called homolytic bond fission (the pair of electrons is shared between each atom).

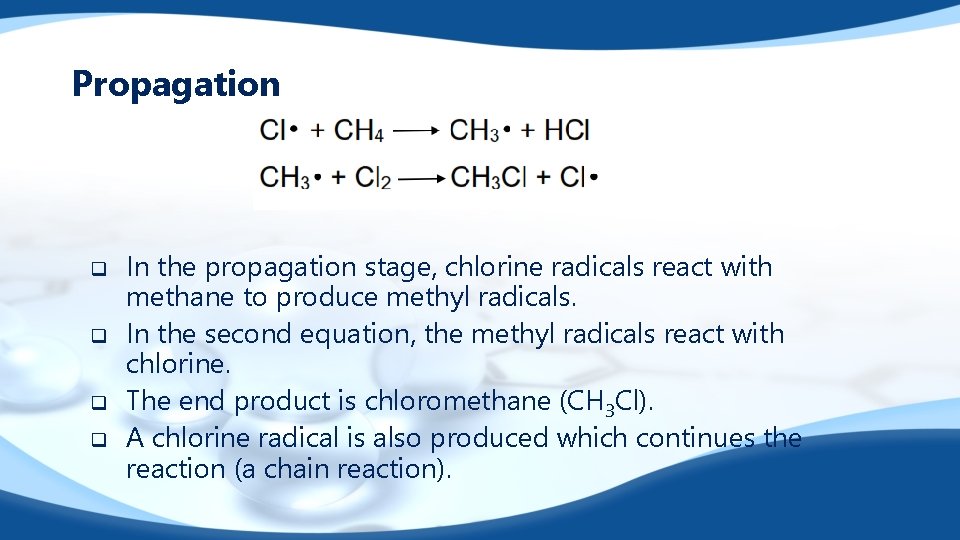
Propagation q q In the propagation stage, chlorine radicals react with methane to produce methyl radicals. In the second equation, the methyl radicals react with chlorine. The end product is chloromethane (CH 3 Cl). A chlorine radical is also produced which continues the reaction (a chain reaction).

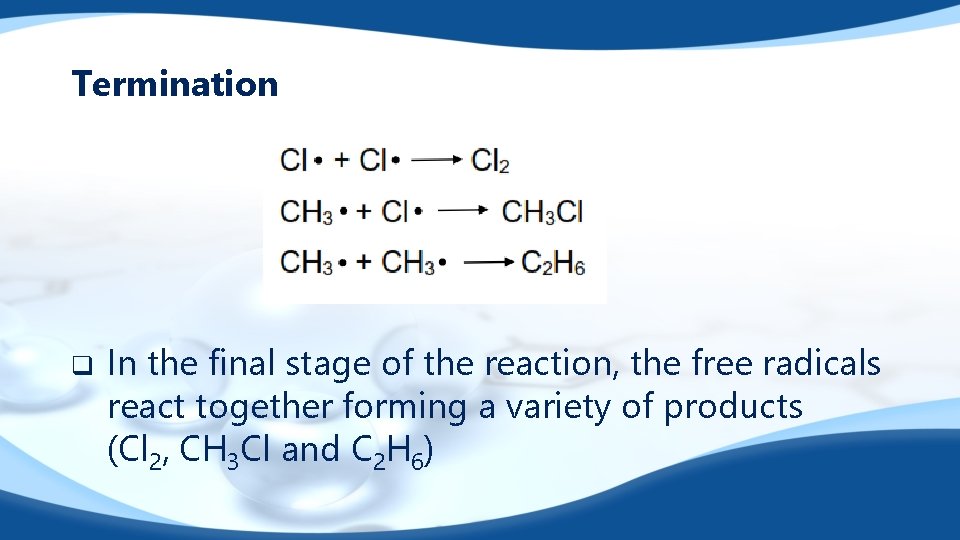
Termination q In the final stage of the reaction, the free radicals react together forming a variety of products (Cl 2, CH 3 Cl and C 2 H 6)

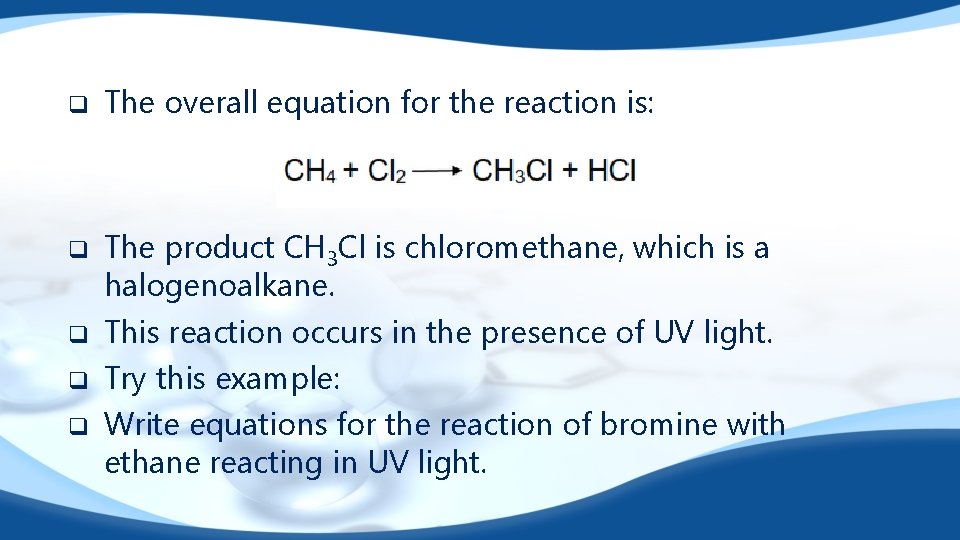
q q q The overall equation for the reaction is: The product CH 3 Cl is chloromethane, which is a halogenoalkane. This reaction occurs in the presence of UV light. Try this example: Write equations for the reaction of bromine with ethane reacting in UV light.

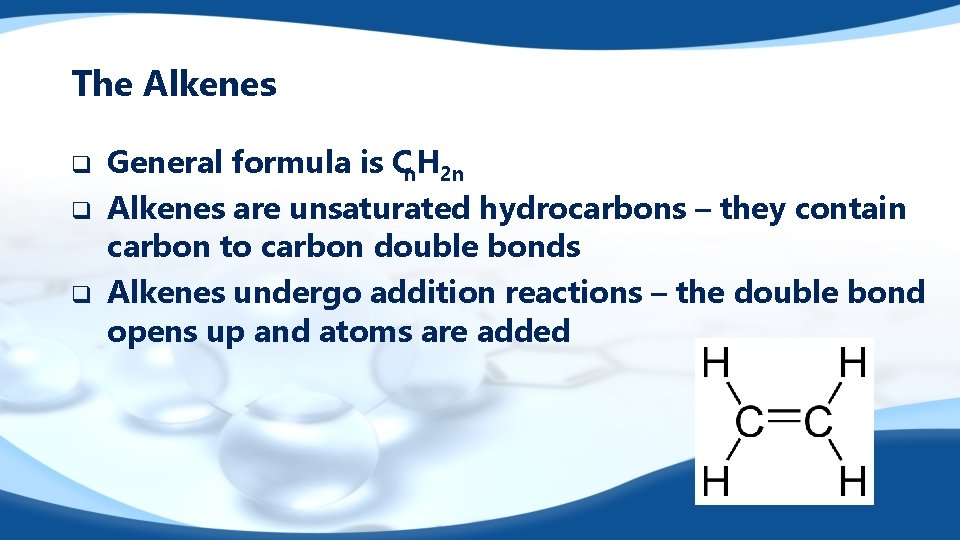
The Alkenes q q q General formula is Cn. H 2 n Alkenes are unsaturated hydrocarbons – they contain carbon to carbon double bonds Alkenes undergo addition reactions – the double bond opens up and atoms are added

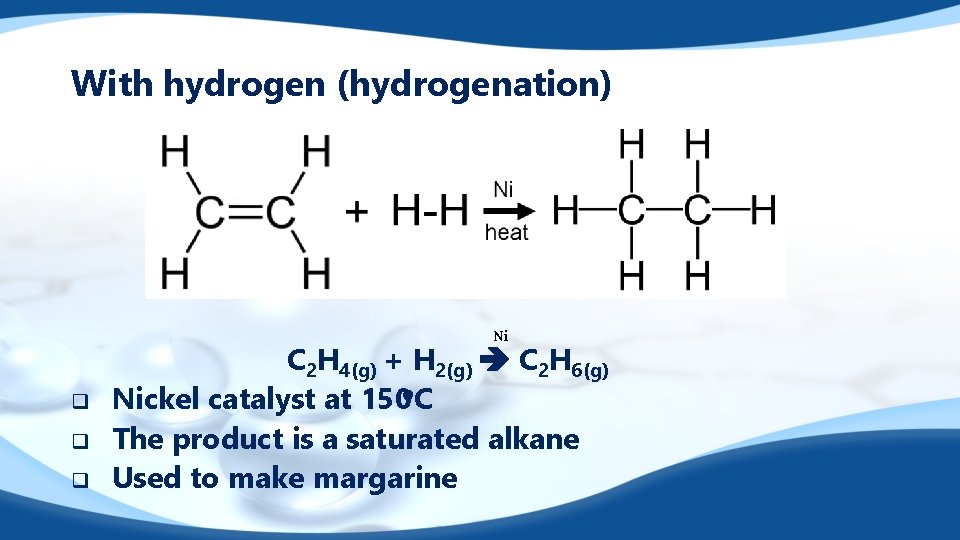
With hydrogen (hydrogenation) Ni q q q C 2 H 4(g) + H 2(g) C 2 H 6(g) Nickel catalyst at 150 o. C The product is a saturated alkane Used to make margarine

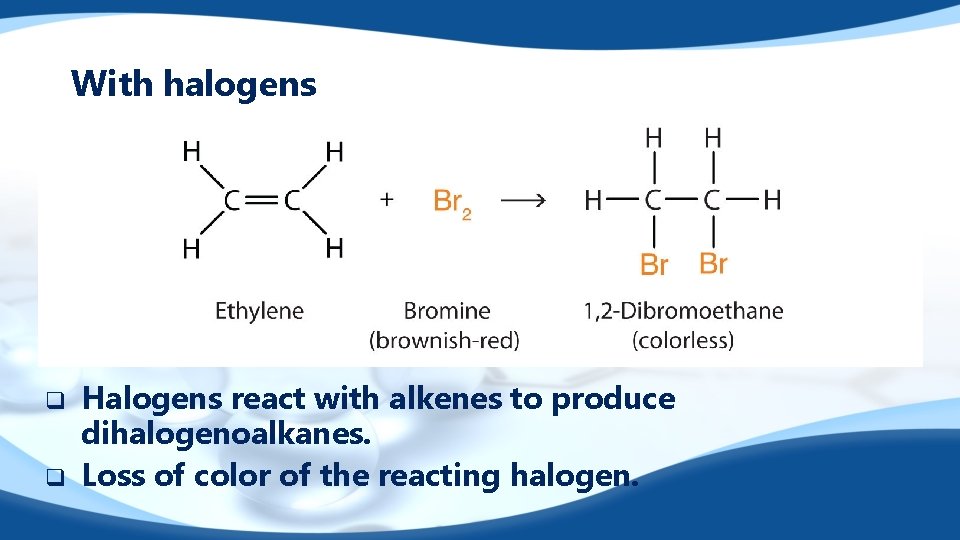
With halogens q q Halogens react with alkenes to produce dihalogenoalkanes. Loss of color of the reacting halogen.

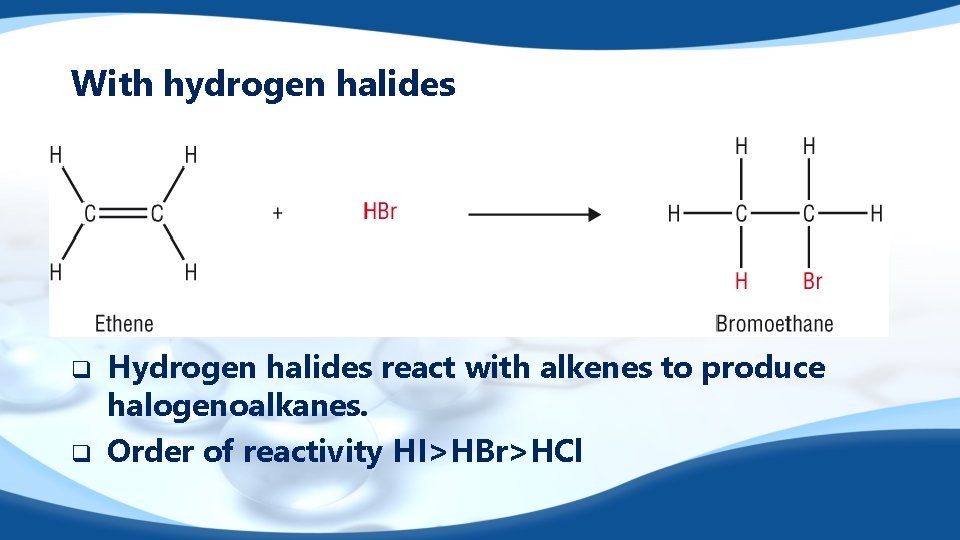
With hydrogen halides q q Hydrogen halides react with alkenes to produce halogenoalkanes. Order of reactivity HI>HBr>HCl

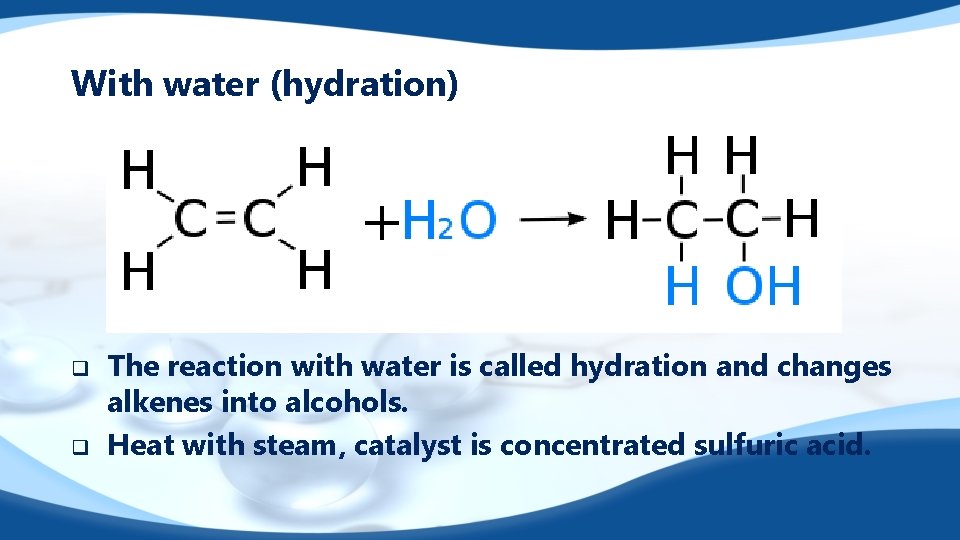
With water (hydration) q q The reaction with water is called hydration and changes alkenes into alcohols. Heat with steam, catalyst is concentrated sulfuric acid.

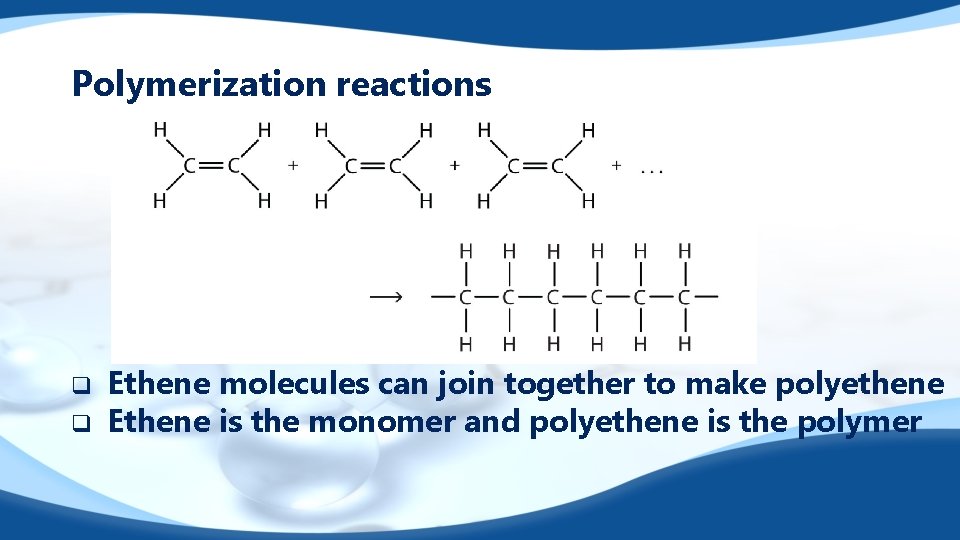
Polymerization reactions q q Ethene molecules can join together to make polyethene Ethene is the monomer and polyethene is the polymer

Alcohols q q q General formula Cn. H 2 n+1 OH They are polar molecules and are soluble in water because of the OH group (form hydrogen bonds with water molecules) They are good fuels – burn in oxygen to produce carbon dioxide and water

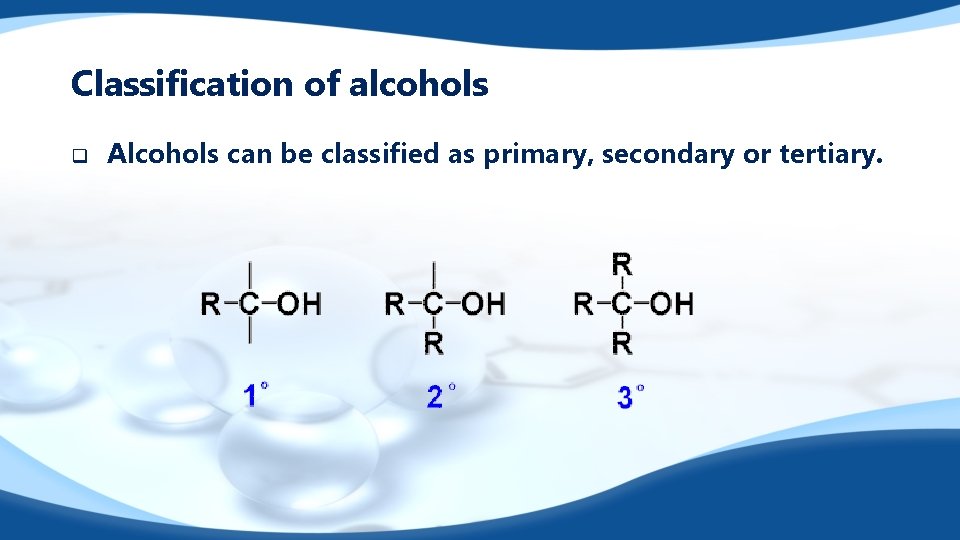
Classification of alcohols q Alcohols can be classified as primary, secondary or tertiary.

Oxidation of alcohols q q Alcohols can be oxidized into other organic compounds. The common oxidizing agent is acidified potassium dichromate (VI) H+/ K 2 Cr 2 O 7

Primary alcohols q q Primary alcohols can be oxidized into aldehydes or carboxylic acids, depending on the conditions used. Conditions needed for oxidation to aldehyde: Heat with acidified potassium dichromate with distillation. This method can be used because the aldehyde has a lower boiling point than the alcohol.

Distillation

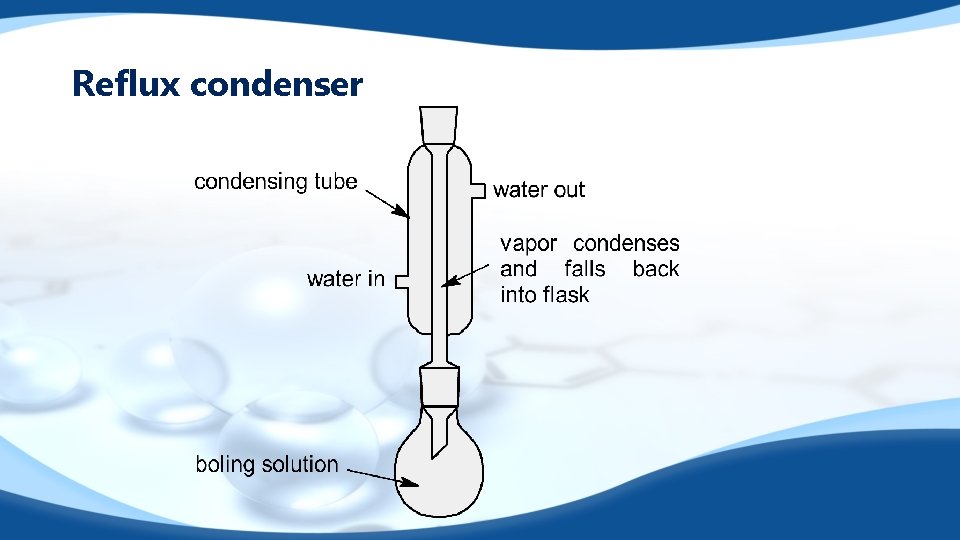
Primary alcohols q q q Primary alcohols can be oxidized to carboxylic acids under different conditions Heat under reflux with acidified potassium dichromate Alcohol and oxidising agent are kept together for as along as possible

Reflux condenser

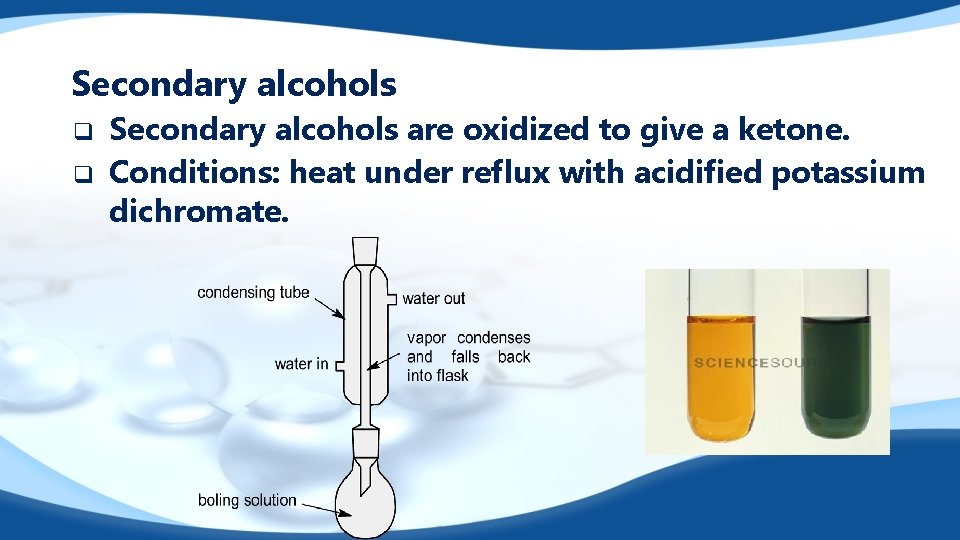
Secondary alcohols q q Secondary alcohols are oxidized to give a ketone. Conditions: heat under reflux with acidified potassium dichromate.

Tertiary alcohols: q Tertiary alcohols do not oxidize

Summary q Primary alcohols can be oxidised to aldehydes or carboxylic acids Aldehyde: distil with acidified potassium dichromate Carboxylic acid: heat under reflux with acidified potassium dichromate Secondary alcohols can be oxidised to a ketone Heat under reflux with acidified potassium dichromate q Tertiary alcohols do not oxidise q q

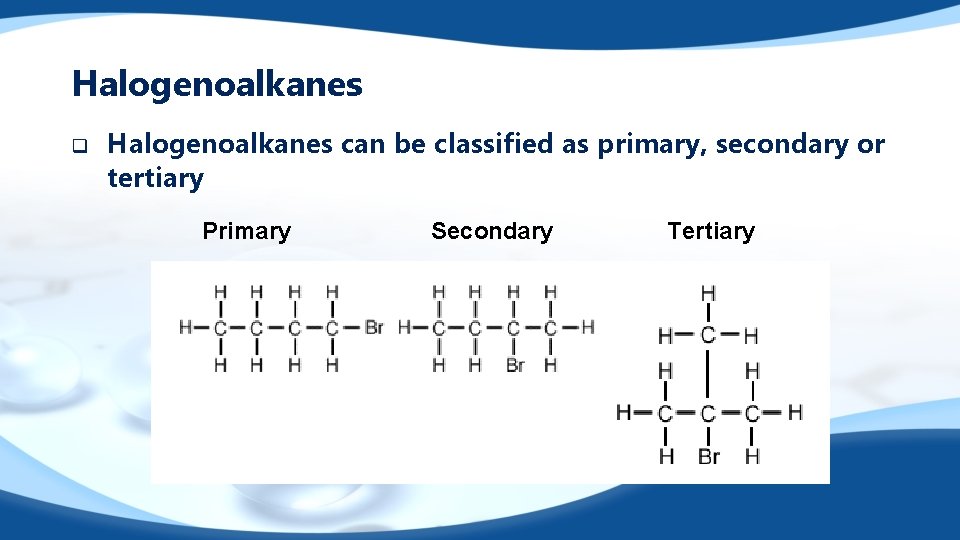
Halogenoalkanes q Halogenoalkanes can be classified as primary, secondary or tertiary Primary Secondary Tertiary

Substitution Nucleophilic reactions of the halogenoalkanes q q Halogenoalkanes react with sodium hydroxide (Na. OH) in substitution reactions. The hydroxide ion (OH-) is a nucleophile (it is attracted to a region of positive charge). The hydroxide ion will take the place of (substitute) the halogen. The halogenoalkane is converted to an alcohol.

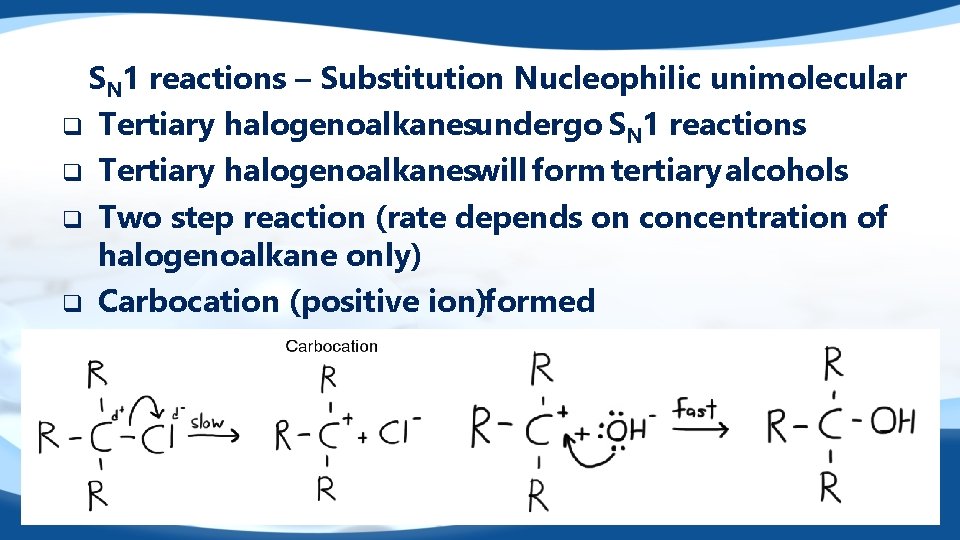
SN 1 reactions – Substitution Nucleophilic unimolecular q Tertiary halogenoalkanesundergo SN 1 reactions q Tertiary halogenoalkaneswill form tertiary alcohols q Two step reaction (rate depends on concentration of halogenoalkane only) q Carbocation (positive ion)formed

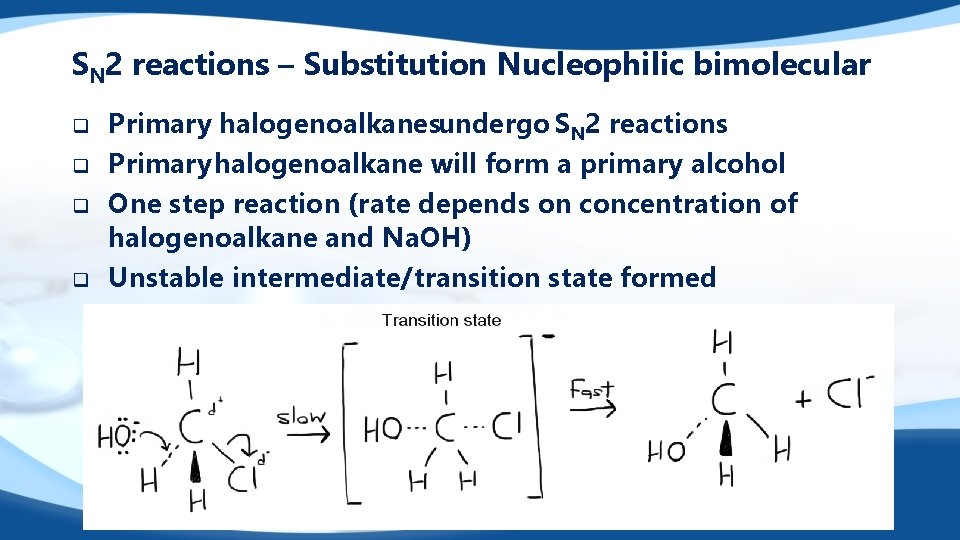
SN 2 reactions – Substitution Nucleophilic bimolecular q q Primary halogenoalkanesundergo SN 2 reactions Primary halogenoalkane will form a primary alcohol One step reaction (rate depends on concentration of halogenoalkane and Na. OH) Unstable intermediate/transition state formed

Summary q q A substitution nucleophilic reaction involves the replacement of an atom by a nucleophile (species with a lone pair of electrons that is attracted to a region of positive charge) Primary halogenoalkanes undergo SN 2 reactions to form a primary alcohol Tertiary halogenoalkanes undergo SN 1 reactions to form a tertiary alcohol Secondary halogenoalkanes undergo both SN 1 and SN 2 reactions

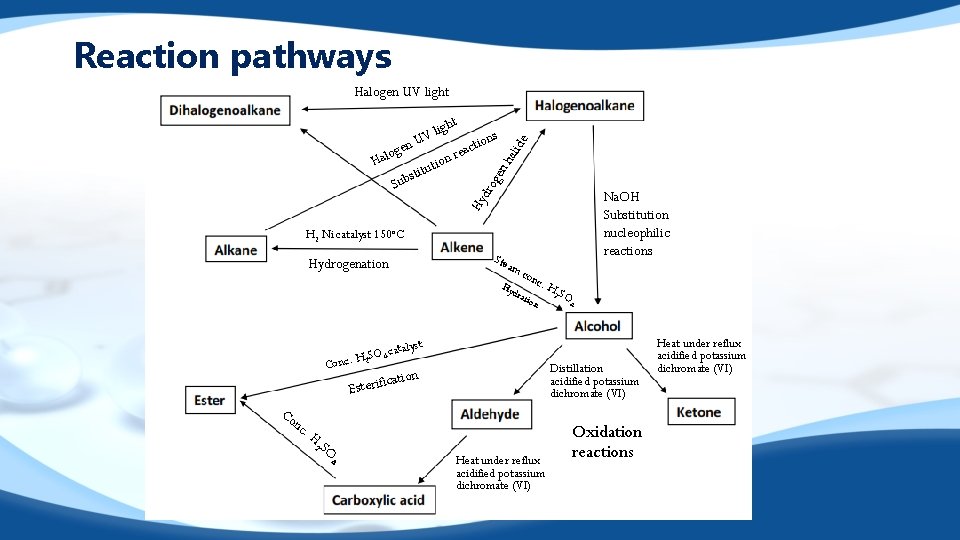
Reaction pathways Halogen UV light t ide Na. OH Substitution nucleophilic reactions Hy Sub ion t u t sti hal o Hal ns tio reac gen U gen dro gh V li H 2 Ni catalyst 150 o. C Ste Hydrogenation am c Hy dra onc tion . H talyst O ca c. H 2 S 4 Con Co Distillation acidified potassium dichromate (VI) ation c i f i r e t Es nc . H 2 SO 4 Heat under reflux acidified potassium dichromate (VI) Oxidation reactions Heat under reflux acidified potassium dichromate (VI)