Organic Chemistry Unit What is Organic Chemistry The

- Slides: 17

Organic Chemistry Unit

What is Organic Chemistry? The study of carbon-containing compounds made up of non-metal elements (covalent bonds)

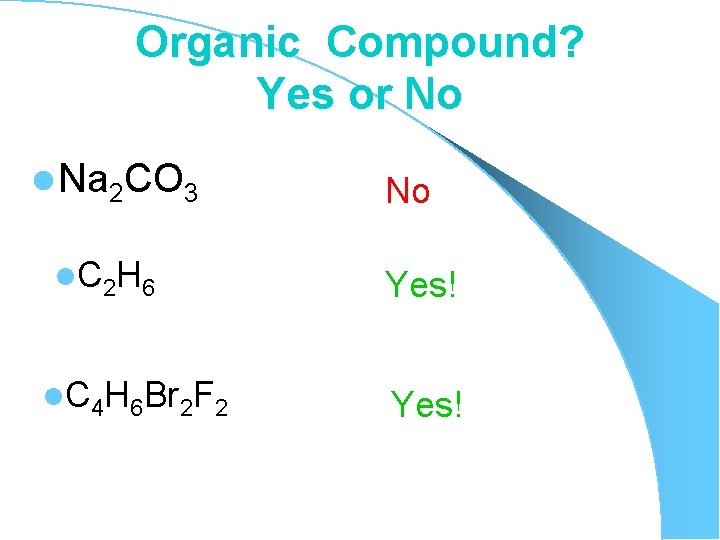
Organic Compound? Yes or No l Na 2 CO 3 l C 2 H 6 l. C 4 H 6 Br 2 F 2 No Yes!

Why Carbon? ? l Found in Nature ( ranked 17 th in crust) l Element l Compound l Found in all living matter l Found in body tissue l Found in food l Found in fuels (coal, wood, petroleum)

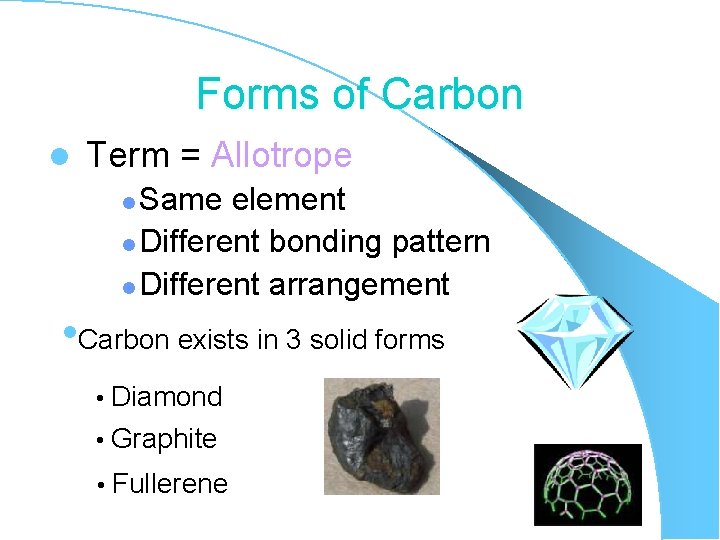
Forms of Carbon l Term = Allotrope Same element l Different bonding pattern l Different arrangement l • Carbon exists in 3 solid forms • Diamond • Graphite • Fullerene

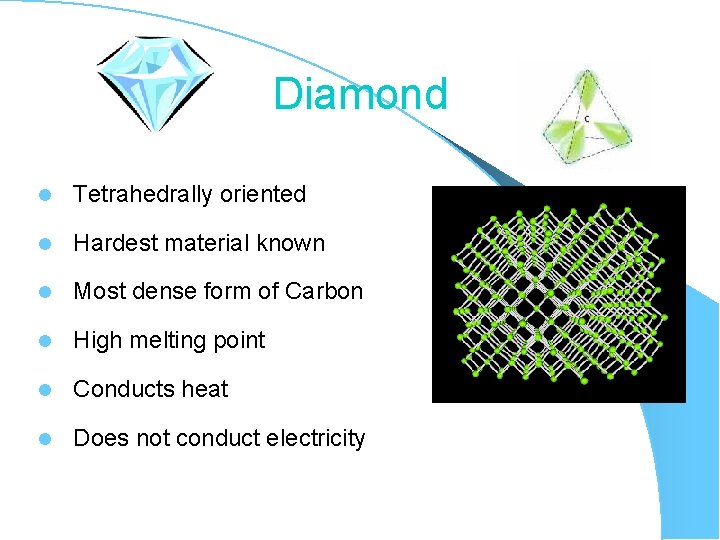
Diamond l Tetrahedrally oriented l Hardest material known l Most dense form of Carbon l High melting point l Conducts heat l Does not conduct electricity

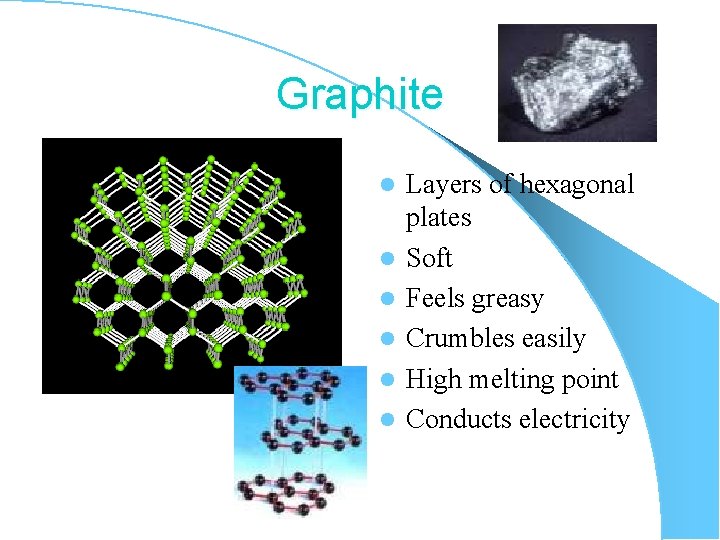
Graphite l l l Layers of hexagonal plates Soft Feels greasy Crumbles easily High melting point Conducts electricity

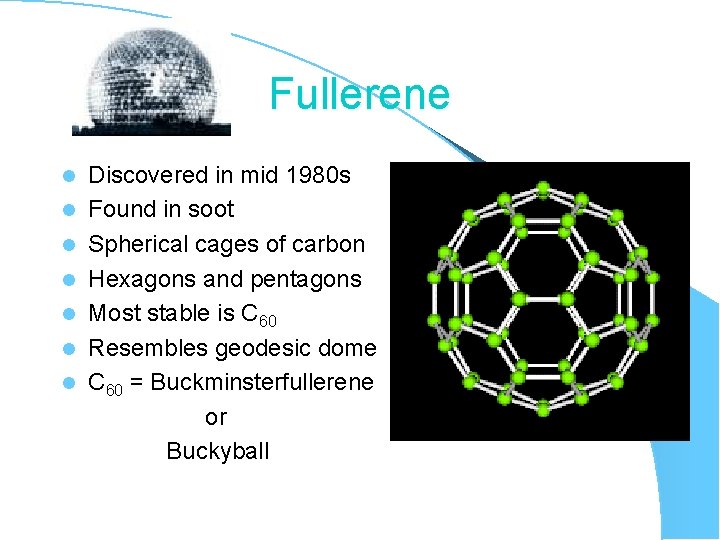
Fullerene l l l l Discovered in mid 1980 s Found in soot Spherical cages of carbon Hexagons and pentagons Most stable is C 60 Resembles geodesic dome C 60 = Buckminsterfullerene or Buckyball

Diversity of Organic Chemistry l Due • to uniqueness of Carbon Can bond to itself covalently • Forms chains and rings • Term = Catenation

Carbon bonds to elements l Carbon readily bonds to : – – – H O N S Halogens l Cl, Br, F, I l Hydrocarbons – Simplest organic compounds – Only contain Carbon and Hydrogen (Cx. Hy)

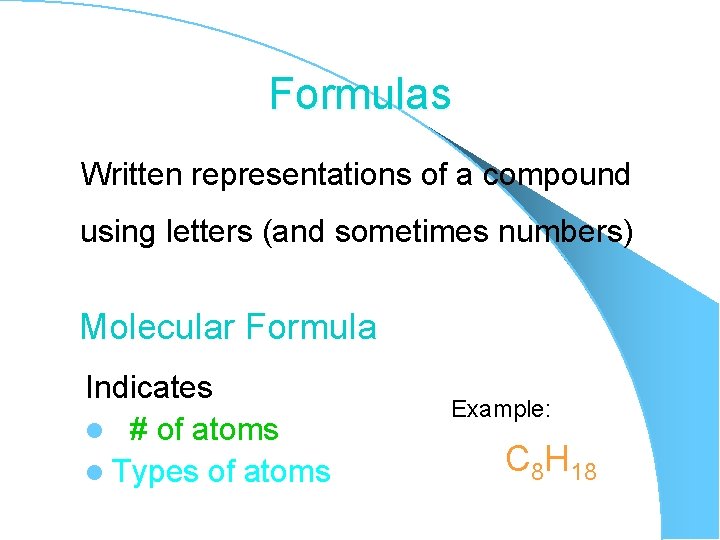
Formulas Written representations of a compound using letters (and sometimes numbers) Molecular Formula Indicates l # of atoms l Types of atoms Example: C 8 H 18

Structural Formula Indicates • • • # of atoms Type of atoms Bonding Arrangement • Shiloh and Dione were here ☻

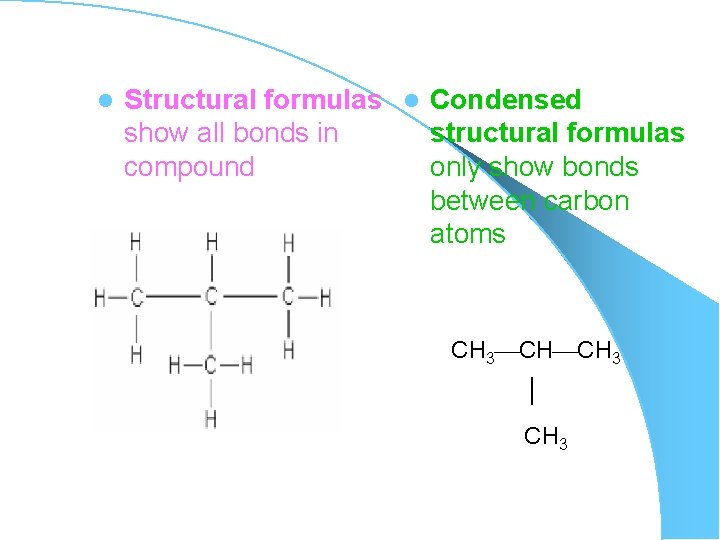
l Structural formulas l Condensed show all bonds in structural formulas compound only show bonds between carbon atoms CH 3 CH CH 3

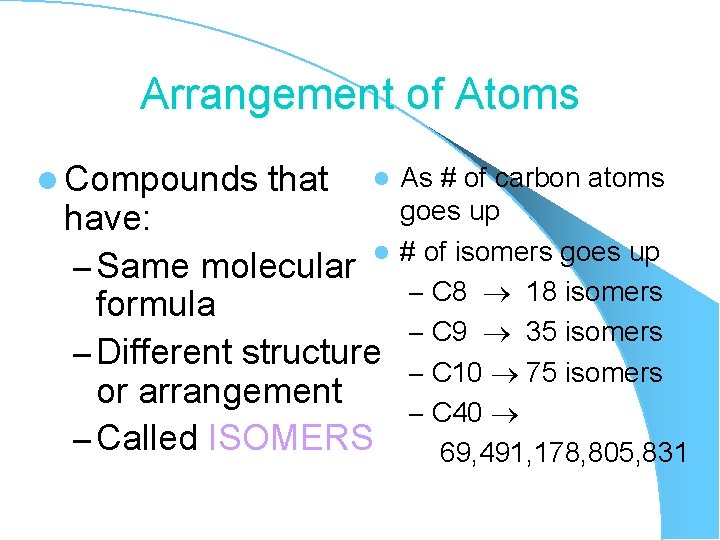
Arrangement of Atoms l Compounds that As # of carbon atoms goes up have: l # of isomers goes up – Same molecular – C 8 18 isomers formula – C 9 35 isomers – Different structure – C 10 75 isomers or arrangement – C 40 – Called ISOMERS 69, 491, 178, 805, 831 l

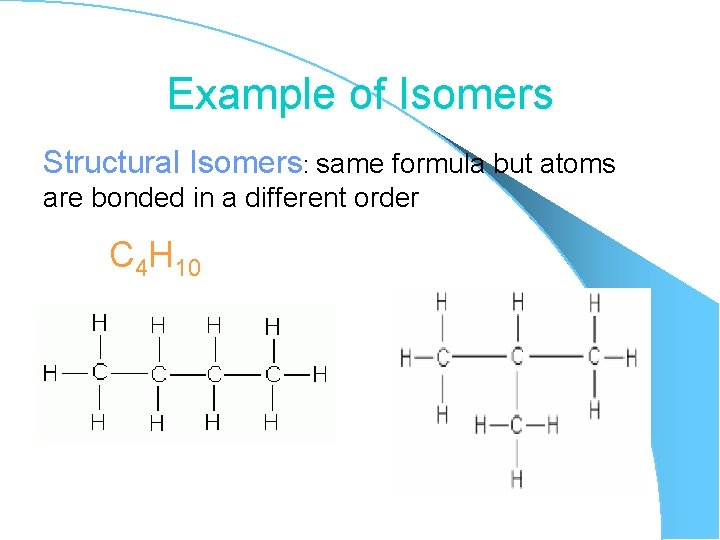
Example of Isomers Structural Isomers: same formula but atoms are bonded in a different order C 4 H 10

Geometric Isomers Order of atoms is the same but the arrangement in space is different Typically need a rigid bond (double or triple bond). Don’t see this with single bonds!

We are going to study: l Alkanes l Ethers l Alkenes l Esters l Alkynes l Aldehydes l Aromatics l Ketones l Alcohols l Amines
 Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Cycloalkanes
Cycloalkanes Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry Structural formula vs displayed formula
Structural formula vs displayed formula Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Nomenclature of ethers
Nomenclature of ethers Is alkane an organic compound
Is alkane an organic compound Leveling effect organic chemistry
Leveling effect organic chemistry What does n mean in nomenclature
What does n mean in nomenclature Objective lab report example
Objective lab report example Britannica.com
Britannica.com Organic chemistry grade 10
Organic chemistry grade 10 Organic chemistry
Organic chemistry































