Intro to Organic Chemistry Organic Chemistry Introduction Organic





- Slides: 27

Intro to Organic Chemistry

Organic Chemistry - Introduction • Organic chemistry is the study of carbon compounds. • Animals, plants, and other forms of life consist of organic compounds. – Nucleic acids, proteins, fats, carbohydrates, enzymes, vitamins, and hormones are all organic compounds. • Biochemistry was developed later as the study of the chemical compounds and reactions in living cells. Intro

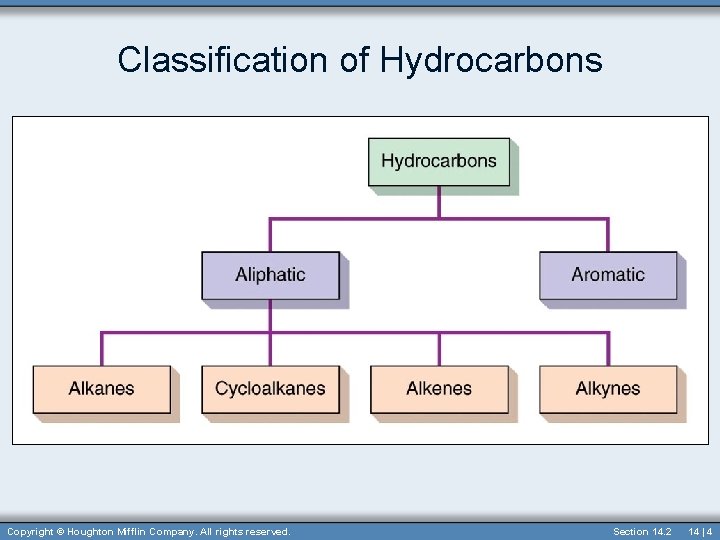
Hydrocarbons • Simplest organic compound • Contain only carbon and hydrogen • Hydrocarbons can be divided into aromatic and aliphatic hydrocarbons. 14 | 3

Classification of Hydrocarbons Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 2 14 | 4

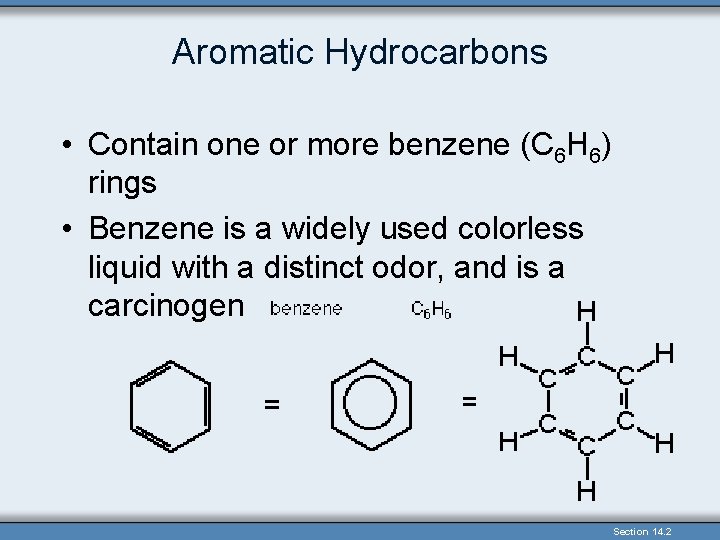
Aromatic Hydrocarbons • Contain one or more benzene (C 6 H 6) rings • Benzene is a widely used colorless liquid with a distinct odor, and is a carcinogen Section 14. 2

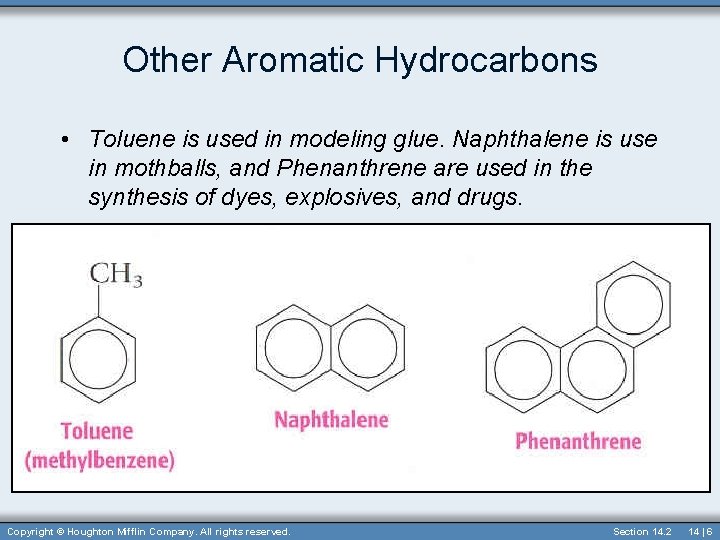
Other Aromatic Hydrocarbons • Toluene is used in modeling glue. Naphthalene is use in mothballs, and Phenanthrene are used in the synthesis of dyes, explosives, and drugs. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 2 14 | 6

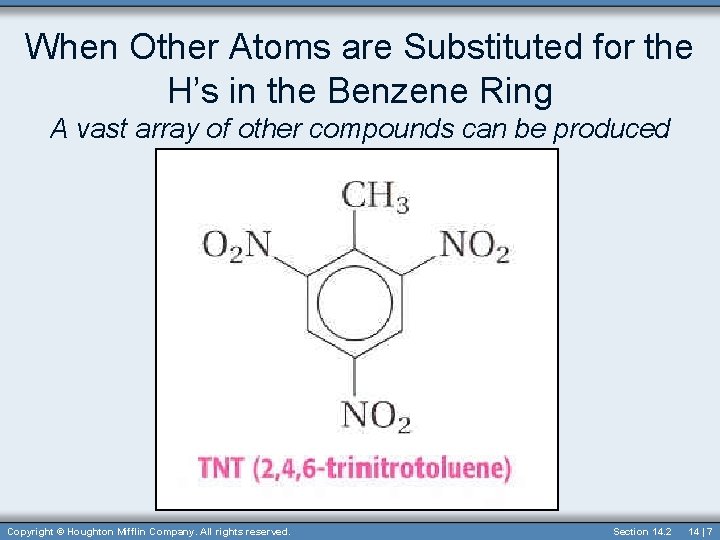
When Other Atoms are Substituted for the H’s in the Benzene Ring A vast array of other compounds can be produced Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 2 14 | 7

Aliphatic Hydrocarbons • Aliphatic hydrocarbons have no benzene rings. • Divided into four major divisions: – Alkanes – Cycloalkanes – ring shaped – Alkenes – contain at least one double bond between carbons – Alkynes – contain at least one tripple bond between carbons Section 14. 3

Alkanes • Alkanes are hydrocarbons that contain only single bonds. • Alkanes are said to be saturated hydrocarbons – Because their hydrogen content is at a maximum. • Alkane general formula Cn. H 2 n + 2 • The names of alkanes all end in “-ane. ” – – Methane – CH 4 Ethane – C 2 H 6 Propane – C 3 H 8 Butane – C 4 H 10 Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 9

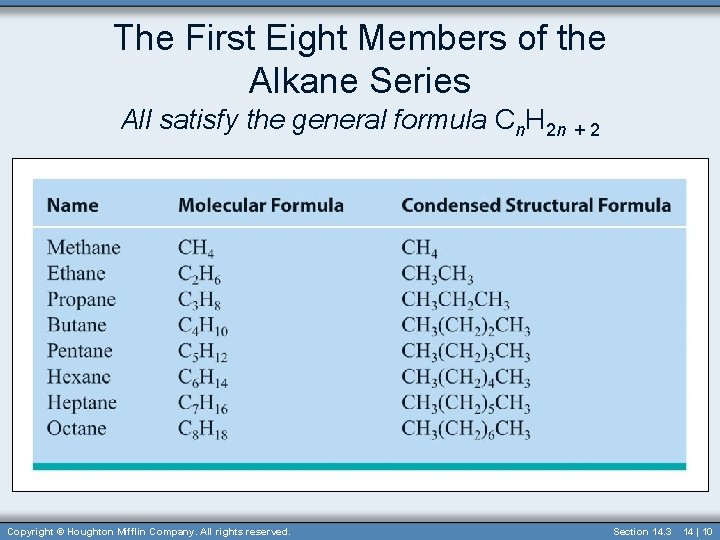
The First Eight Members of the Alkane Series All satisfy the general formula Cn. H 2 n + 2 Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 10

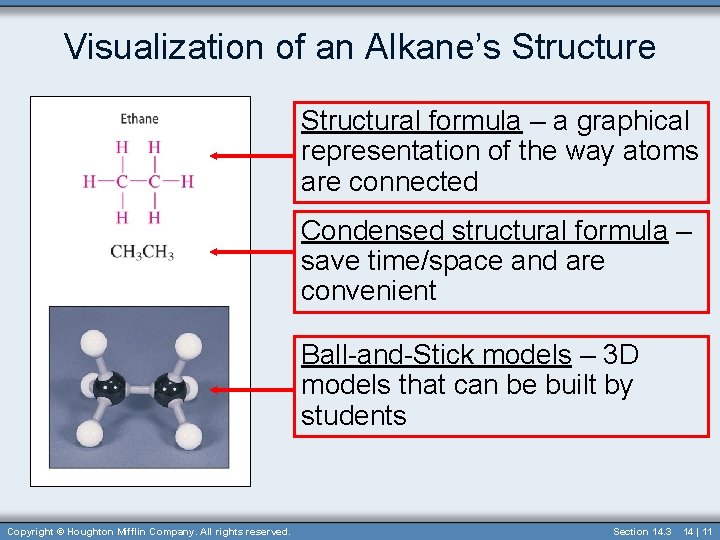
Visualization of an Alkane’s Structure Structural formula – a graphical representation of the way atoms are connected Condensed structural formula – save time/space and are convenient Ball-and-Stick models – 3 D models that can be built by students Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 11

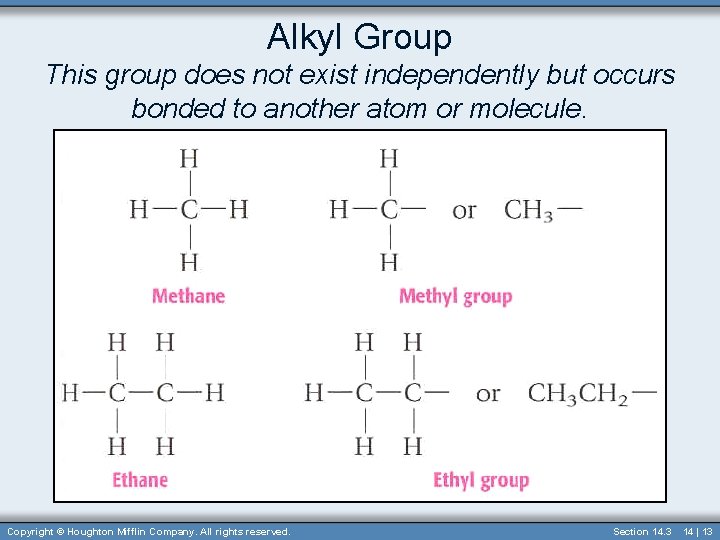
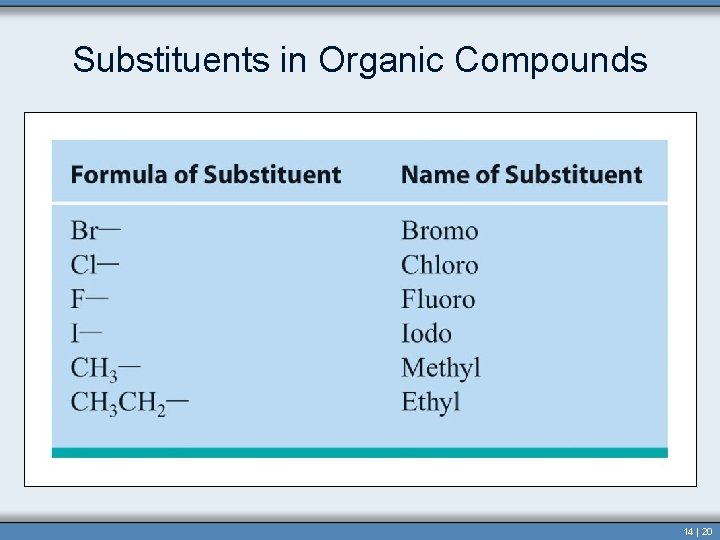
Alkyl Group • Alkyl group contains one less hydrogen than the corresponding alkane. • In naming this group the “-ane” is dropped and “-yl” is added. – Methyl– Ethyl– Propyl– Butyl- • Used as prefixes when naming structures 14 | 12

Alkyl Group This group does not exist independently but occurs bonded to another atom or molecule. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 13

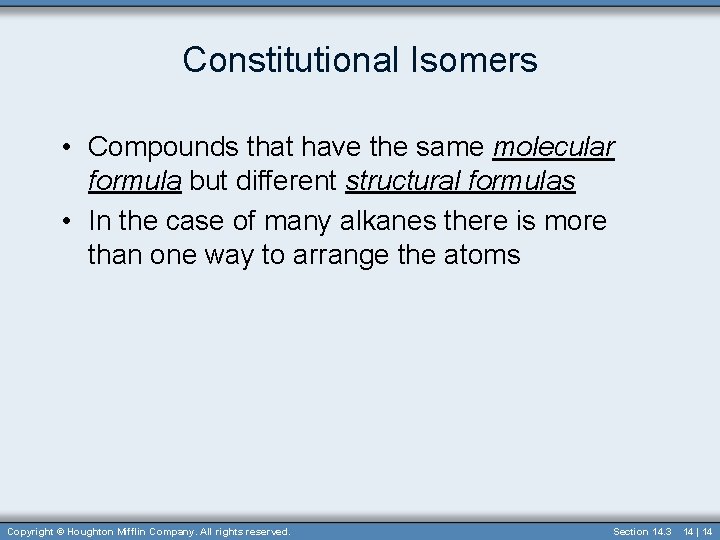
Constitutional Isomers • Compounds that have the same molecular formula but different structural formulas • In the case of many alkanes there is more than one way to arrange the atoms Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 14

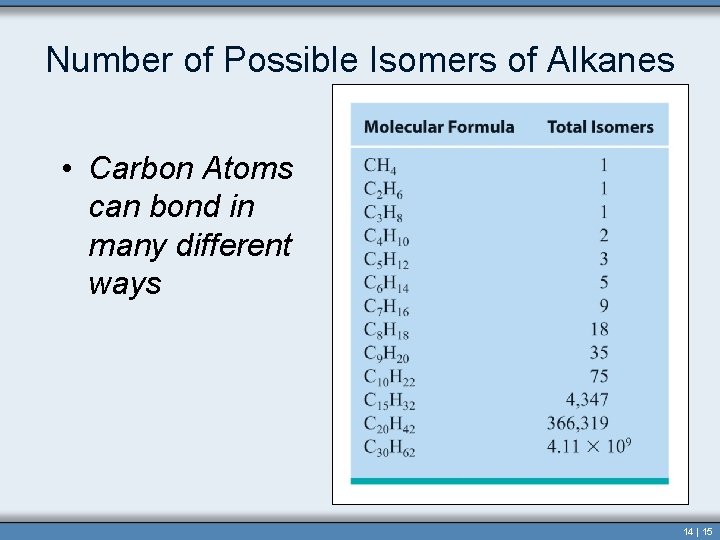
Number of Possible Isomers of Alkanes • Carbon Atoms can bond in many different ways 14 | 15

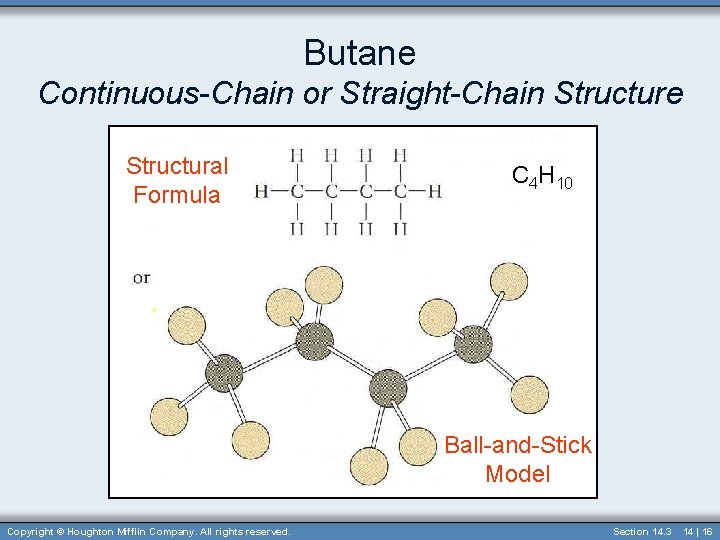
Butane Continuous-Chain or Straight-Chain Structure Structural Formula C 4 H 10 Ball-and-Stick Model Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 16

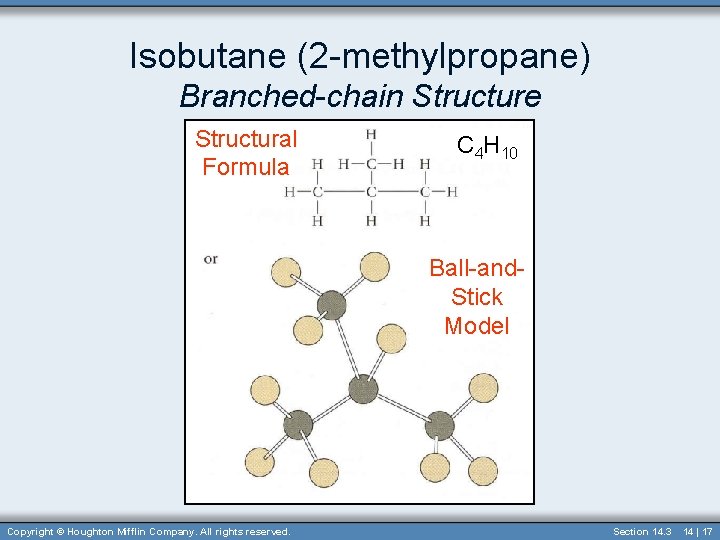
Isobutane (2 -methylpropane) Branched-chain Structure Structural Formula C 4 H 10 Ball-and. Stick Model Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 17

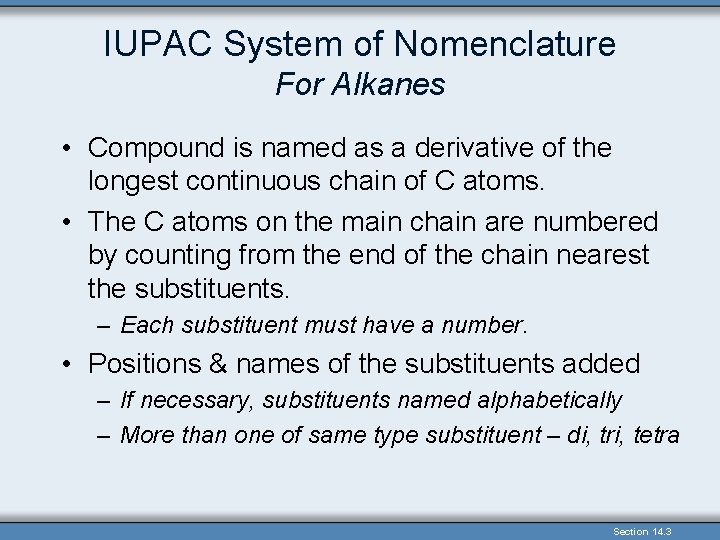
IUPAC System of Nomenclature For Alkanes • Compound is named as a derivative of the longest continuous chain of C atoms. • The C atoms on the main chain are numbered by counting from the end of the chain nearest the substituents. – Each substituent must have a number. • Positions & names of the substituents added – If necessary, substituents named alphabetically – More than one of same type substituent – di, tri, tetra Section 14. 3

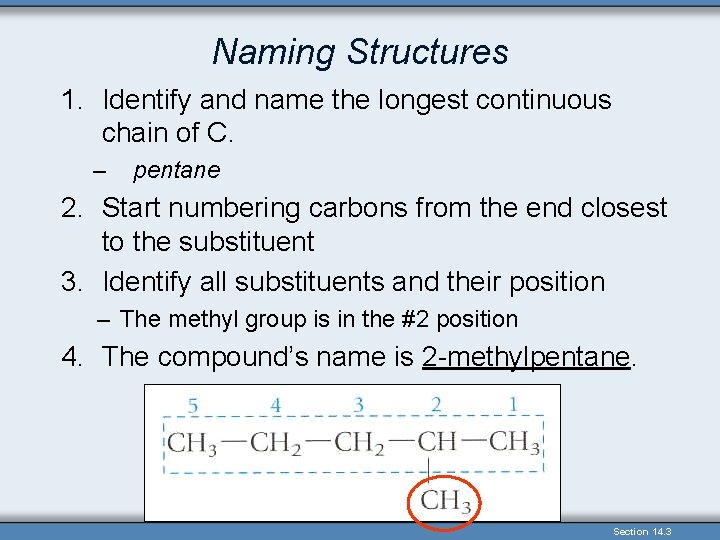
Naming Structures 1. Identify and name the longest continuous chain of C. – pentane 2. Start numbering carbons from the end closest to the substituent 3. Identify all substituents and their position – The methyl group is in the #2 position 4. The compound’s name is 2 -methylpentane. Section 14. 3

Substituents in Organic Compounds 14 | 20

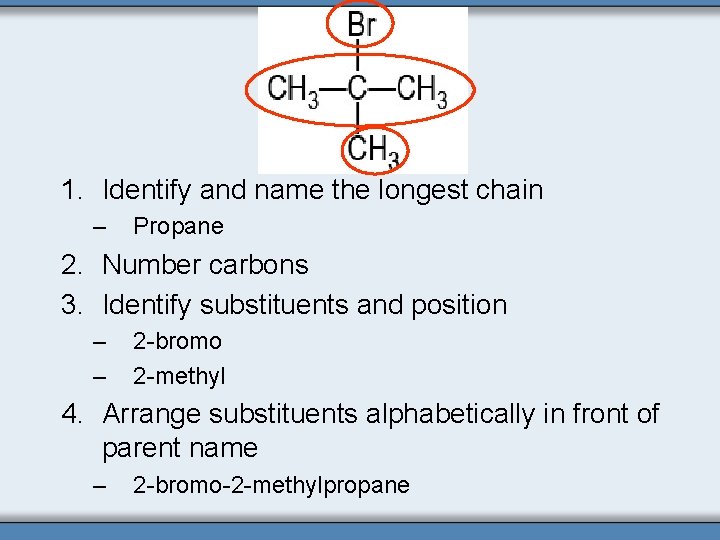
1. Identify and name the longest chain – Propane 2. Number carbons 3. Identify substituents and position – – 2 -bromo 2 -methyl 4. Arrange substituents alphabetically in front of parent name – 2 -bromo-2 -methylpropane

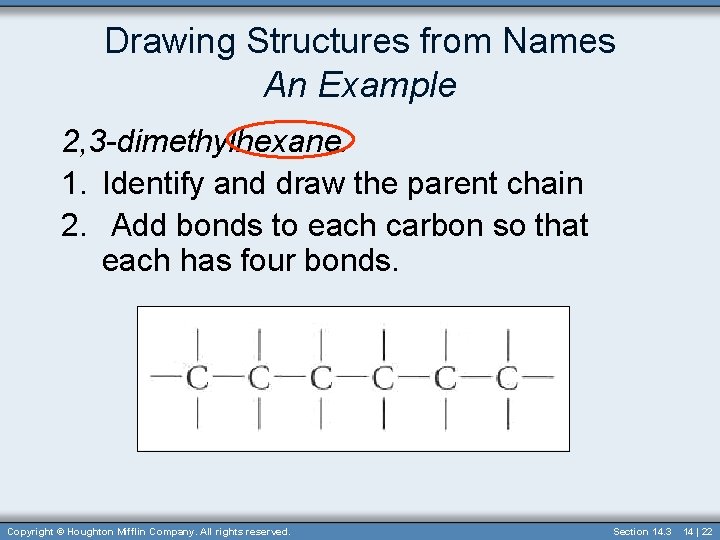
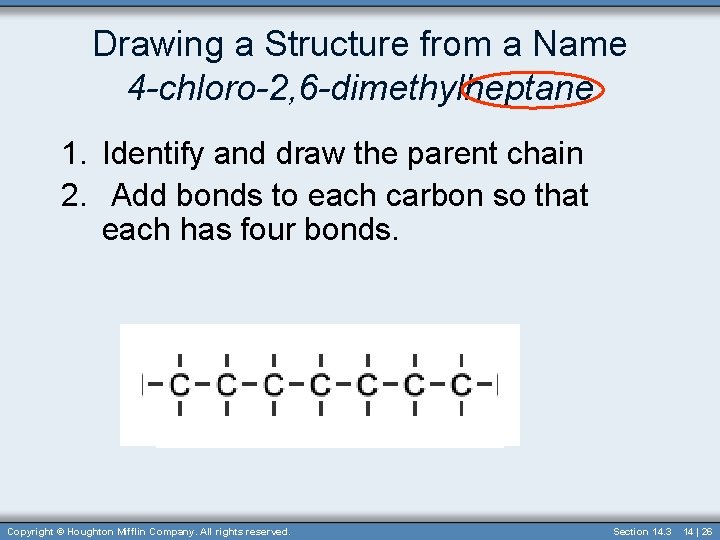
Drawing Structures from Names An Example 2, 3 -dimethylhexane. 1. Identify and draw the parent chain 2. Add bonds to each carbon so that each has four bonds. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 22

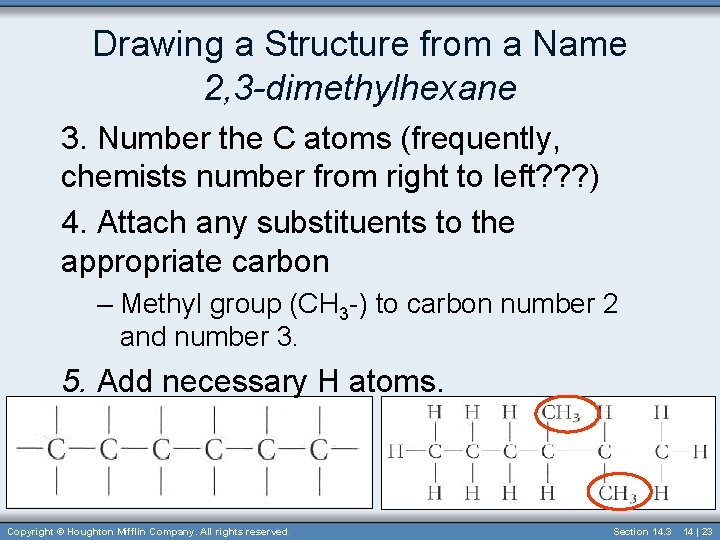
Drawing a Structure from a Name 2, 3 -dimethylhexane 3. Number the C atoms (frequently, chemists number from right to left? ? ? ) 4. Attach any substituents to the appropriate carbon – Methyl group (CH 3 -) to carbon number 2 and number 3. 5. Add necessary H atoms. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 23

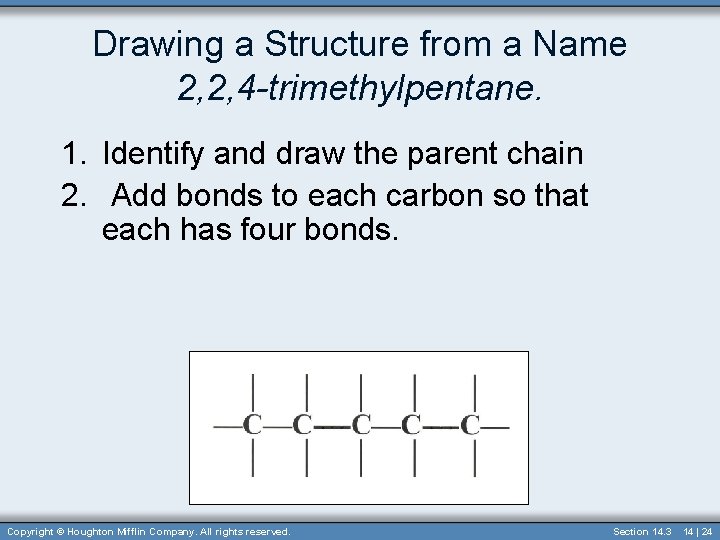
Drawing a Structure from a Name 2, 2, 4 -trimethylpentane. 1. Identify and draw the parent chain 2. Add bonds to each carbon so that each has four bonds. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 24

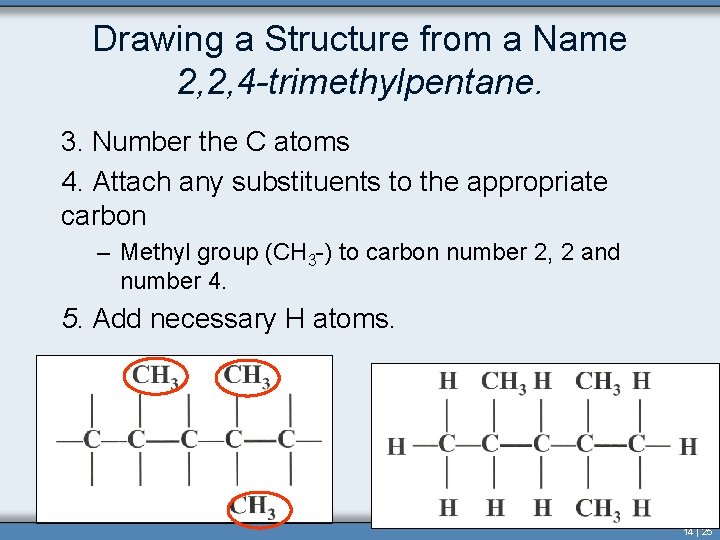
Drawing a Structure from a Name 2, 2, 4 -trimethylpentane. 3. Number the C atoms 4. Attach any substituents to the appropriate carbon – Methyl group (CH 3 -) to carbon number 2, 2 and number 4. 5. Add necessary H atoms. 14 | 25

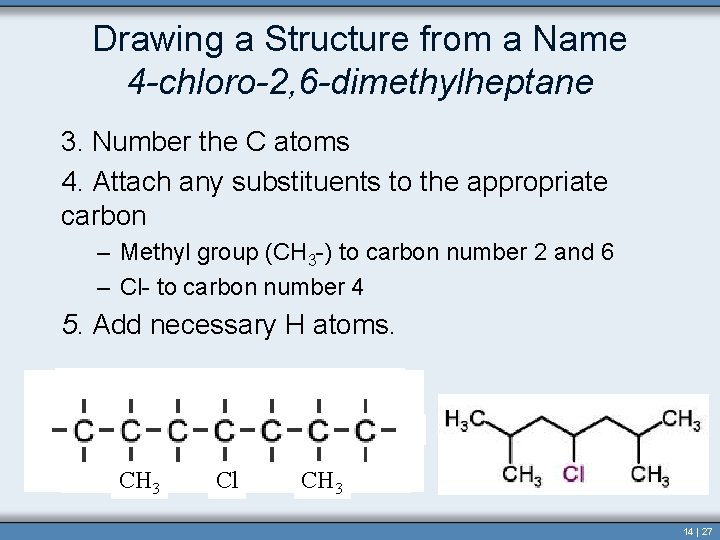
Drawing a Structure from a Name 4 -chloro-2, 6 -dimethylheptane 1. Identify and draw the parent chain 2. Add bonds to each carbon so that each has four bonds. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 26

Drawing a Structure from a Name 4 -chloro-2, 6 -dimethylheptane 3. Number the C atoms 4. Attach any substituents to the appropriate carbon – Methyl group (CH 3 -) to carbon number 2 and 6 – Cl- to carbon number 4 5. Add necessary H atoms. CH 3 Cl CH 3 14 | 27
 Importance of organic compounds
Importance of organic compounds Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Founder of organic chemistry
Founder of organic chemistry Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Define organic chemistry
Define organic chemistry Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Pericyclic
Pericyclic Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Ario practice problems
Ario practice problems Iupac nomenclature
Iupac nomenclature Organic chemistry lab report sample
Organic chemistry lab report sample Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Organic chemistry grade 10
Organic chemistry grade 10 Cyclo organic chemistry
Cyclo organic chemistry Organic chemistry wade
Organic chemistry wade Eth meth prop but pent
Eth meth prop but pent Alkane cracking
Alkane cracking Meth eth prop
Meth eth prop Organic chemistry myanmar
Organic chemistry myanmar Propagation organic chemistry
Propagation organic chemistry Gc organic chemistry
Gc organic chemistry Hono organic chemistry
Hono organic chemistry Geminal and vicinal
Geminal and vicinal

















































