Organic chemistry Practical organic chemistry introduction Organic chemistry


- Slides: 28

Organic chemistry Practical organic chemistry

introduction Organic chemistry is all around us. The reactions and interactions of organic • molecules allow us to see smell, fight, and fear. Organic chemistry provides the molecules that feed us, treat our illnesses, protect our crops, and clean our clothes. Historically, the term organic chemistry (carbon chemistry)mean the • chemistry of compounds found in living organisms. The term “organic” substances isolated from plants and animals • There are more than 37 million known organic compounds. • Each of these compounds has its own physical properties • Chemists have learned that organic compounds • can be classified into groups according to their structural features •

FUNCTIONAL GROUP It is an atom or group of atom in the molecule that determine the chemical reactivity

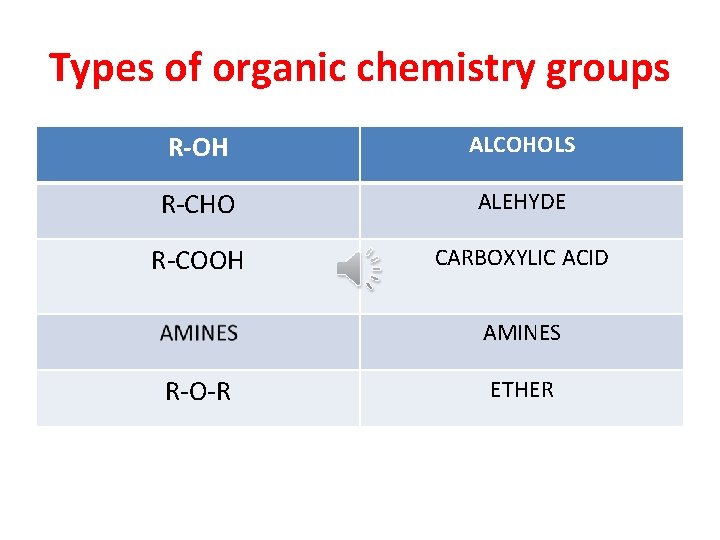
Types of organic chemistry groups R-OH ALCOHOLS R-CHO ALEHYDE R-COOH CARBOXYLIC ACID AMINES R-O-R ETHER

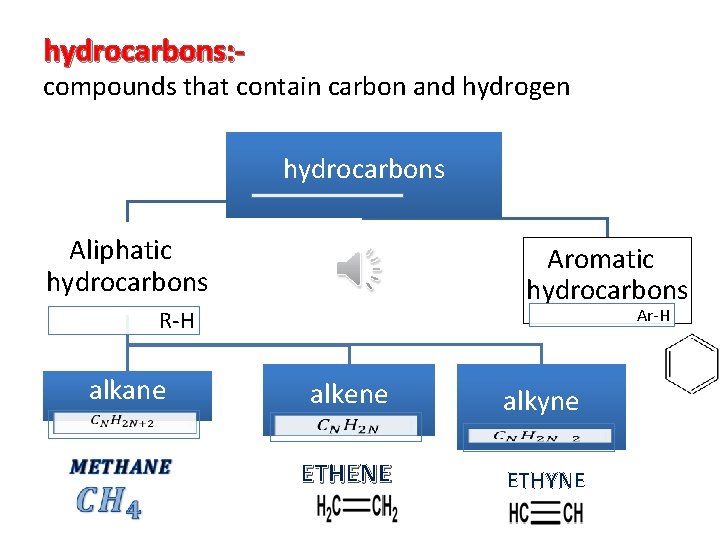
hydrocarbons: - compounds that contain carbon and hydrogen hydrocarbons Aliphatic hydrocarbons Aromatic hydrocarbons Ar-H R-H alkane alkene alkyne ETHENE ETHYNE

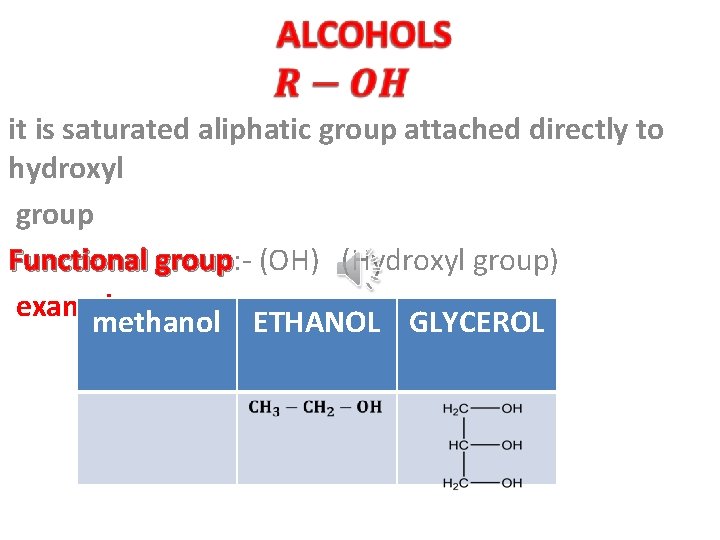
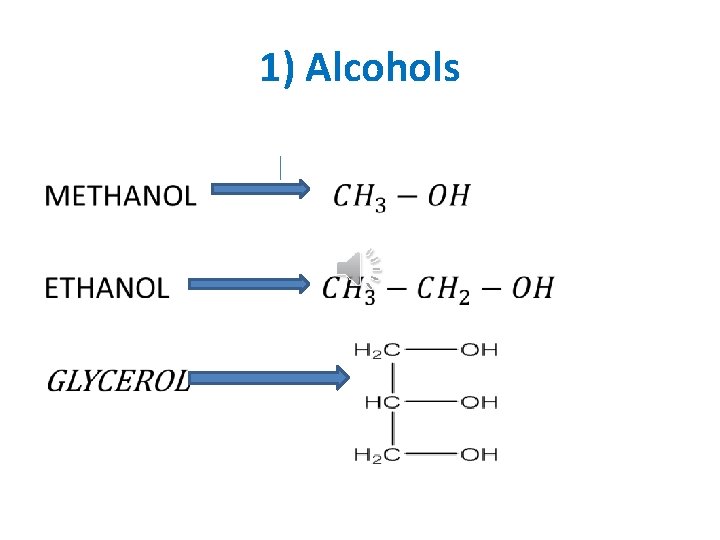
it is saturated aliphatic group attached directly to hydroxyl group Functional group: - (OH) (Hydroxyl group) examples methanol ETHANOL GLYCEROL

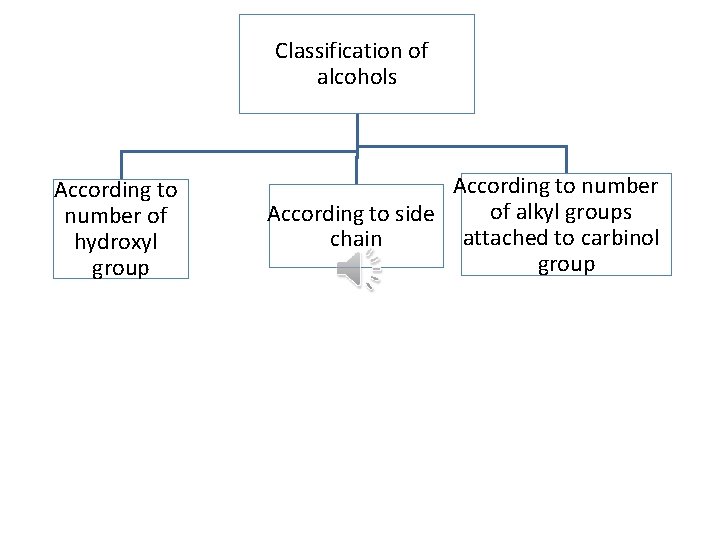
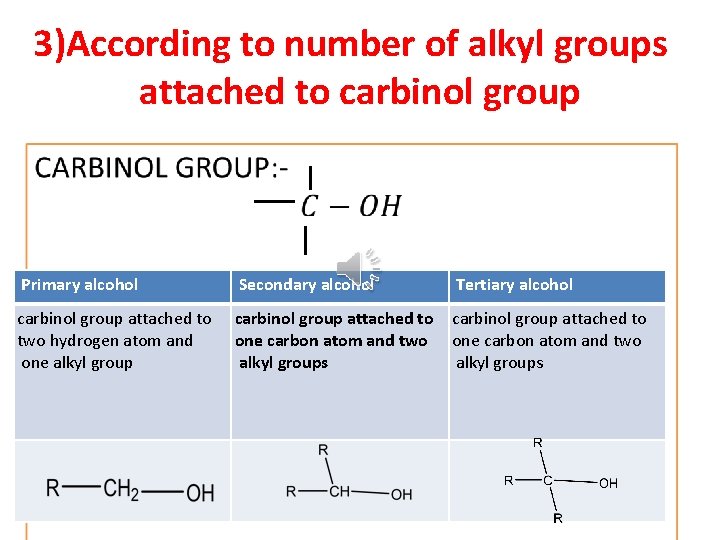
Classification of alcohols According to number of hydroxyl group According to number of alkyl groups According to side attached to carbinol chain group

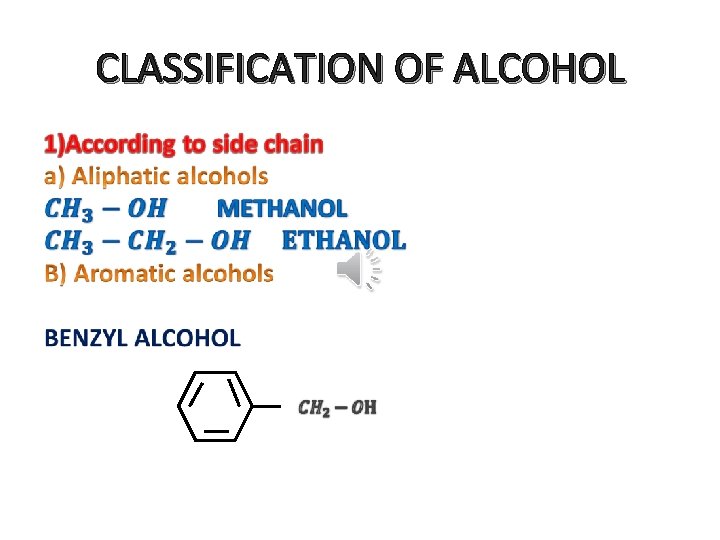
CLASSIFICATION OF ALCOHOL •

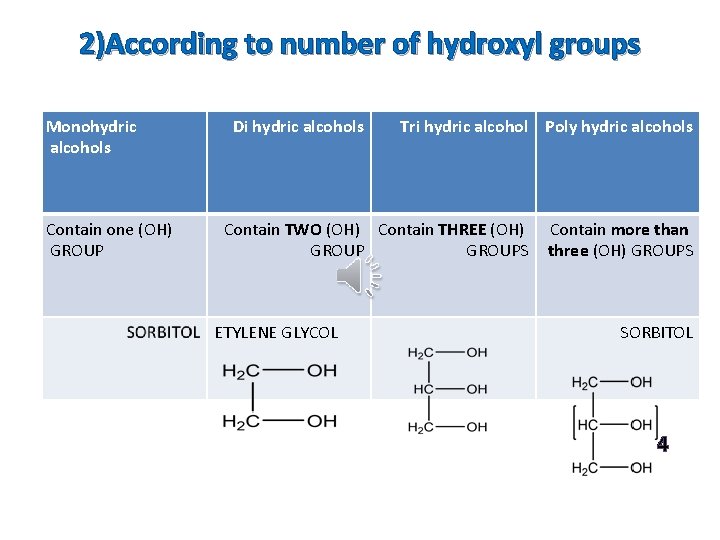
2)According to number of hydroxyl groups Monohydric alcohols Contain one (OH) GROUP Di hydric alcohols Tri hydric alcohol Poly hydric alcohols Contain TWO (OH) Contain THREE (OH) GROUPS ETYLENE GLYCOL Contain more than three (OH) GROUPS SORBITOL 4

3)According to number of alkyl groups attached to carbinol group • Primary alcohol Secondary alcohol Tertiary alcohol carbinol group attached to two hydrogen atom and one alkyl group carbinol group attached to one carbon atom and two alkyl groups

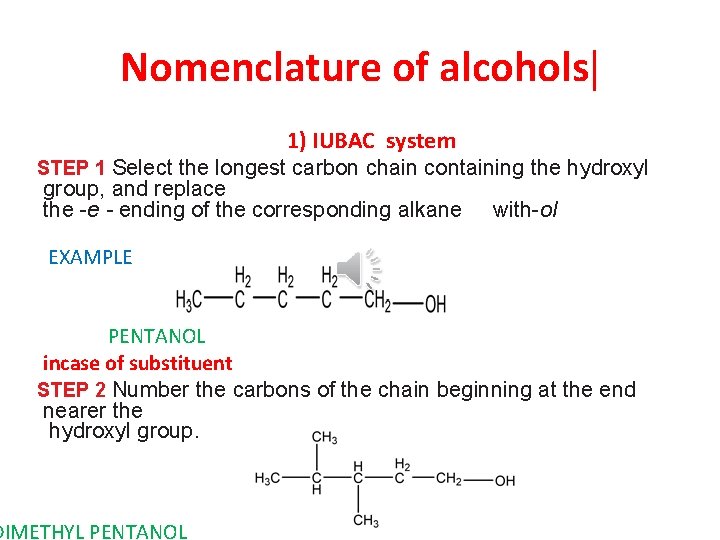
Nomenclature of alcohols| 1) IUBAC system STEP 1 Select the longest carbon chain containing the hydroxyl group, and replace the -e - ending of the corresponding alkane with-ol EXAMPLE PENTANOL incase of substituent STEP 2 Number the carbons of the chain beginning at the end nearer the hydroxyl group. DIMETHYL PENTANOL

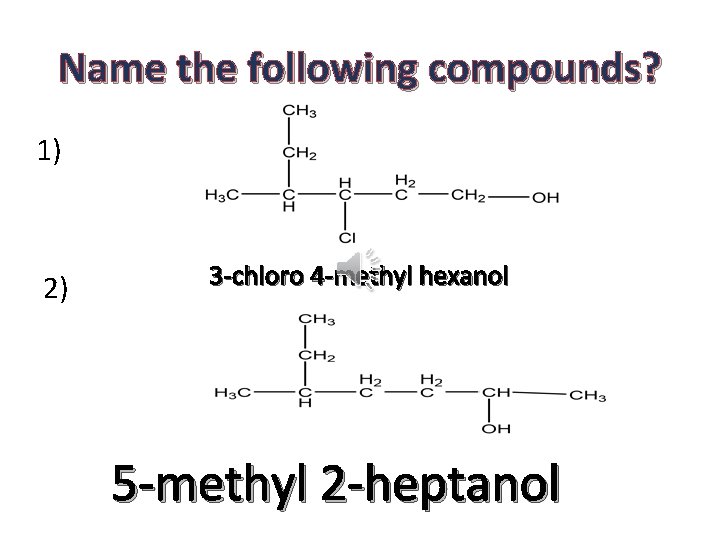
Name the following compounds? 1) 2) 3 -chloro 4 -methyl hexanol 5 -methyl 2 -heptanol

Practical organic chemistry Alcohols

1) Alcohols •

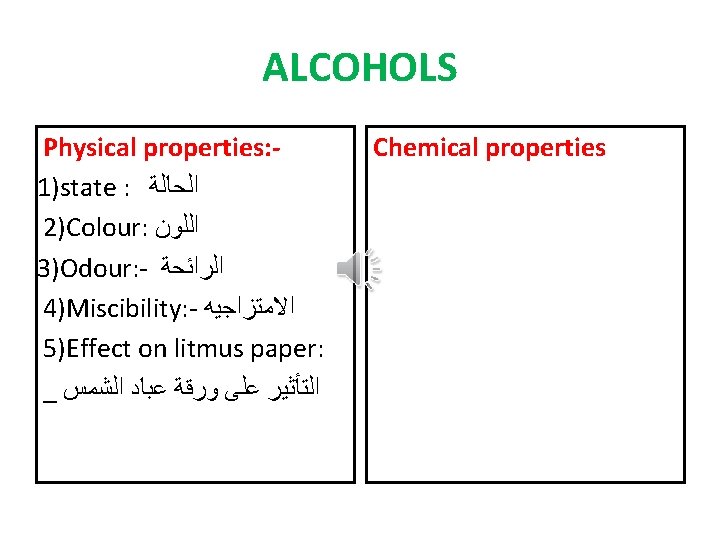
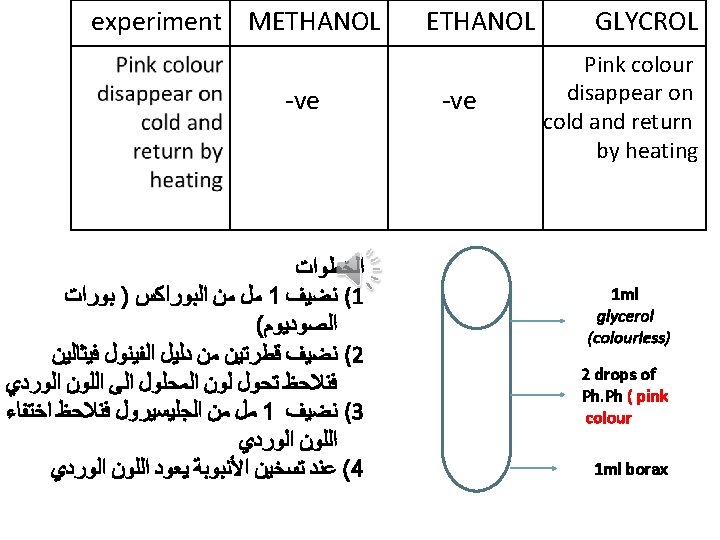
ALCOHOLS Physical properties: 1)state : ﺍﻟﺤﺎﻟﺔ 2)Colour: ﺍﻟﻠﻮﻥ 3)Odour: - ﺍﻟﺮﺍﺋﺤﺔ 4)Miscibility: - ﺍﻻﻣﺘﺰﺍﺟﻴﻪ 5)Effect on litmus paper: _ ﺍﻟﺘﺄﺜﻴﺮ ﻋﻠﻰ ﻭﺭﻗﺔ ﻋﺒﺎﺩ ﺍﻟﺸﻤﺲ Chemical properties

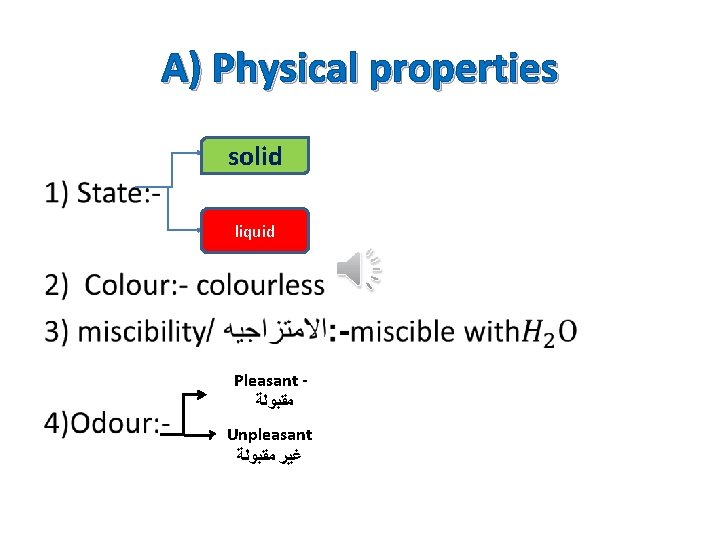
A) Physical properties solid liquid Pleasant ﻣﻘﺒﻮﻟﺔ Unpleasant ﻏﻴﺮ ﻣﻘﺒﻮﻟﺔ •

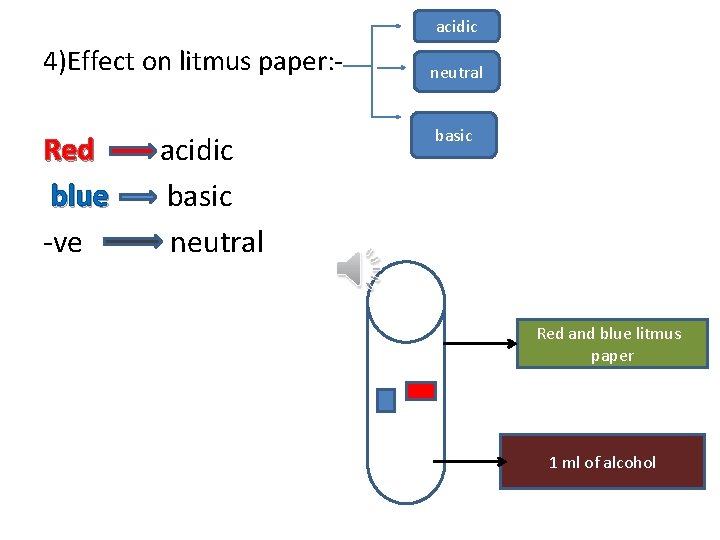
acidic 4)Effect on litmus paper: - Red blue -ve acidic basic neutral basic Red and blue litmus paper 1 ml of alcohol

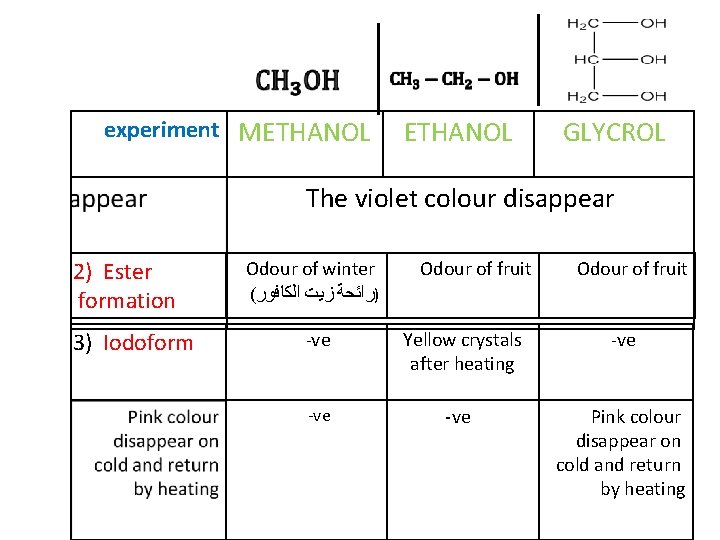
Chemical properties


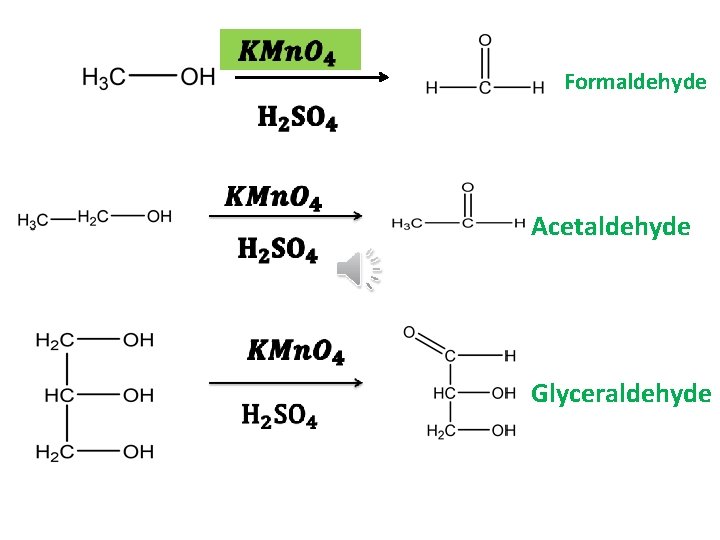
Formaldehyde Acetaldehyde Glyceraldehyde


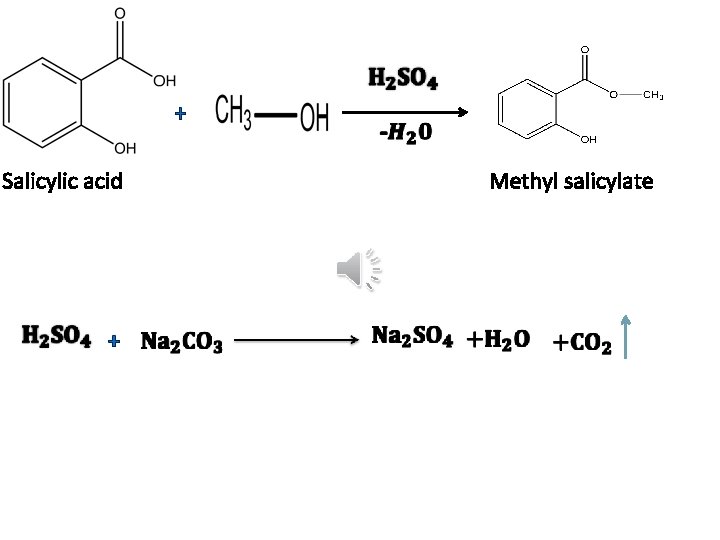
+ Salicylic acid + Methyl salicylate


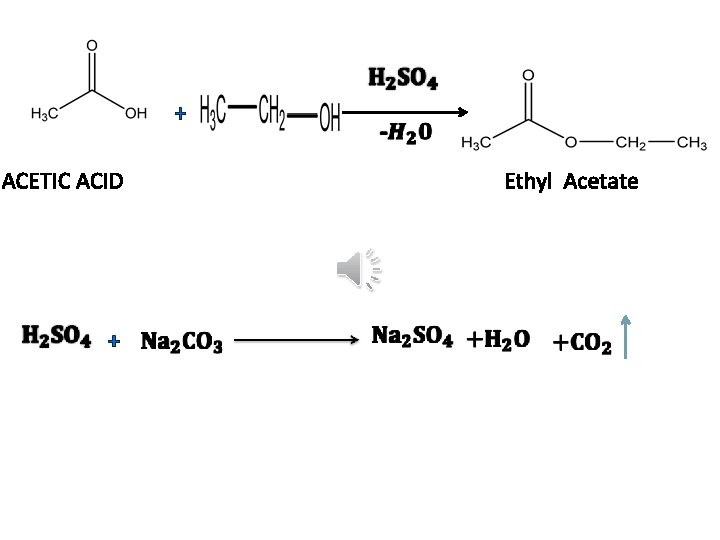
+ ACETIC ACID + Ethyl Acetate


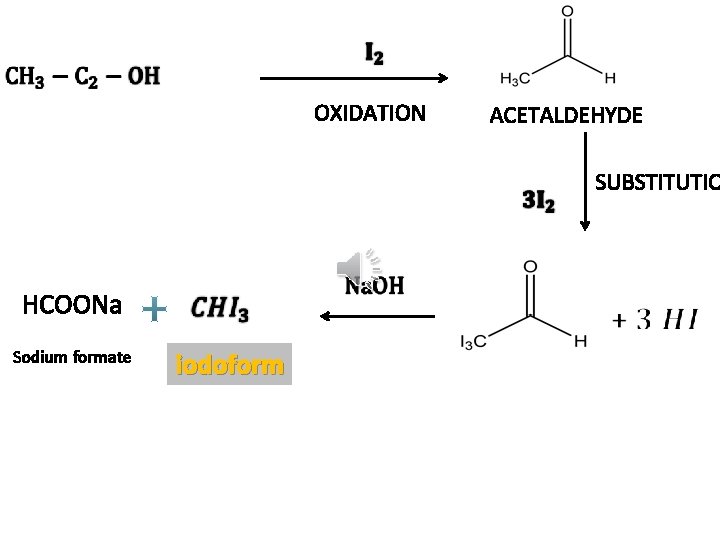
OXIDATION ACETALDEHYDE SUBSTITUTIO HCOONa Sodium formate iodoform


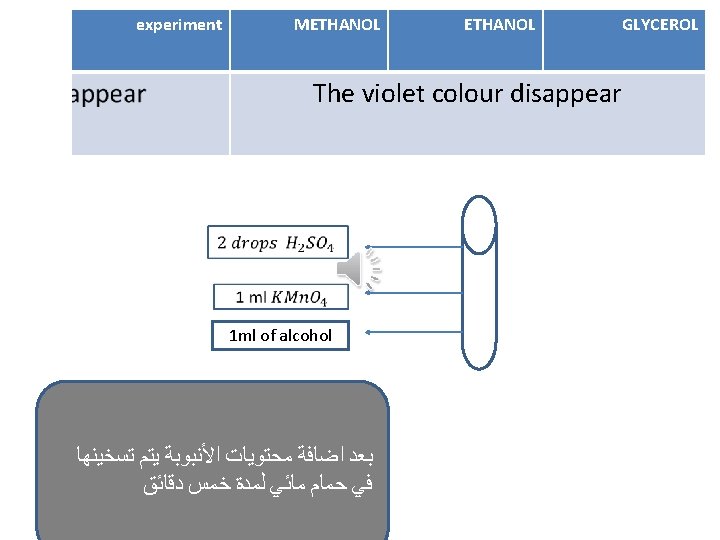
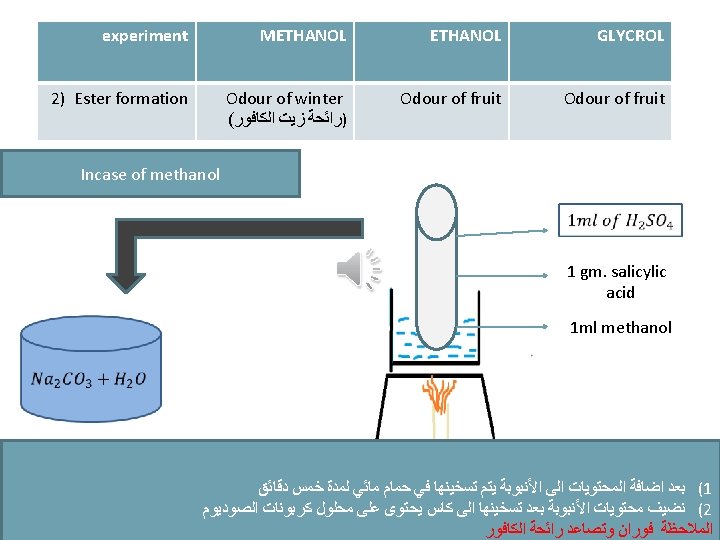
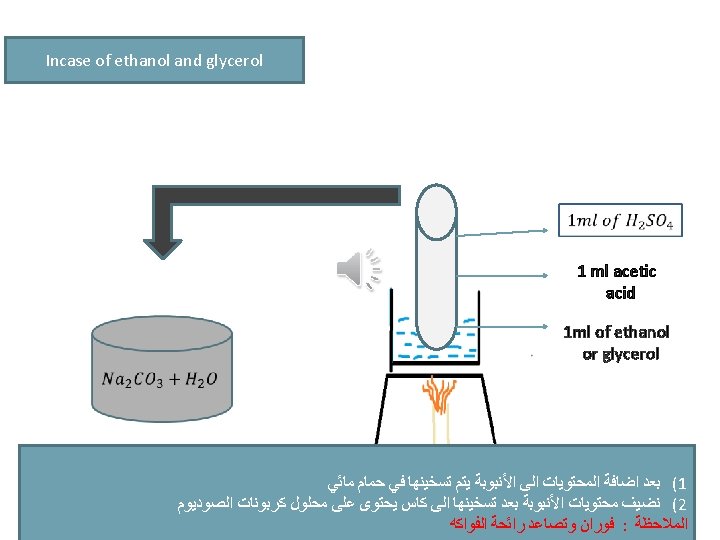
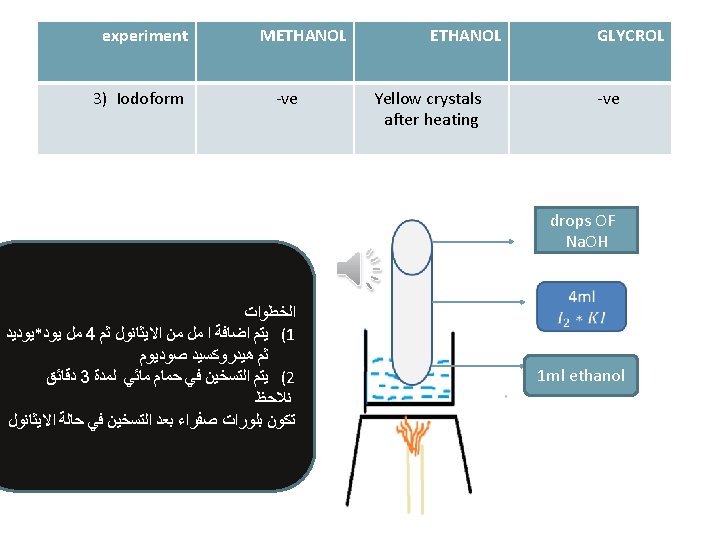
experiment METHANOL GLYCROL The violet colour disappear 2) Ester formation 3) Iodoform Odour of winter ( )ﺭﺍﺋﺤﺔ ﺯﻳﺖ ﺍﻟﻜﺎﻓﻮﺭ Odour of fruit -ve Yellow crystals after heating -ve -ve Pink colour disappear on cold and return by heating
 Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Introduction to organic chemistry
Introduction to organic chemistry Ocr chemistry b
Ocr chemistry b Vce chemistry practical investigation
Vce chemistry practical investigation Ccea gce chemistry practical support
Ccea gce chemistry practical support Angelo kinicki management: a practical introduction
Angelo kinicki management: a practical introduction Read management: a practical introduction online
Read management: a practical introduction online Introduction in practical research 2
Introduction in practical research 2 Management a practical introduction
Management a practical introduction Management a practical introduction 3e
Management a practical introduction 3e Management a practical introduction
Management a practical introduction Numbering carbon chains
Numbering carbon chains Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry But-1-ene to but-2-ene
But-1-ene to but-2-ene Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control David klein organic chemistry
David klein organic chemistry Is alkane an organic compound
Is alkane an organic compound Ario practice problems
Ario practice problems Chemistry nomenclature
Chemistry nomenclature Objective lab report example
Objective lab report example Alkane organic chemistry
Alkane organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis


















































