Experiment 2 Qualitative Test for Elements in Organic










- Slides: 22

Experiment 2 Qualitative Test for Elements in Organic Compounds

Organic Compounds • Contains the element Carbon • Carbon’s ability to form strong covalent bond to other carbon is the single property of carbon atom that accounts for the existence of the study of organic chemistry. • It can also form strong covalent bonds with other elements such as H, O, N, S, etc.

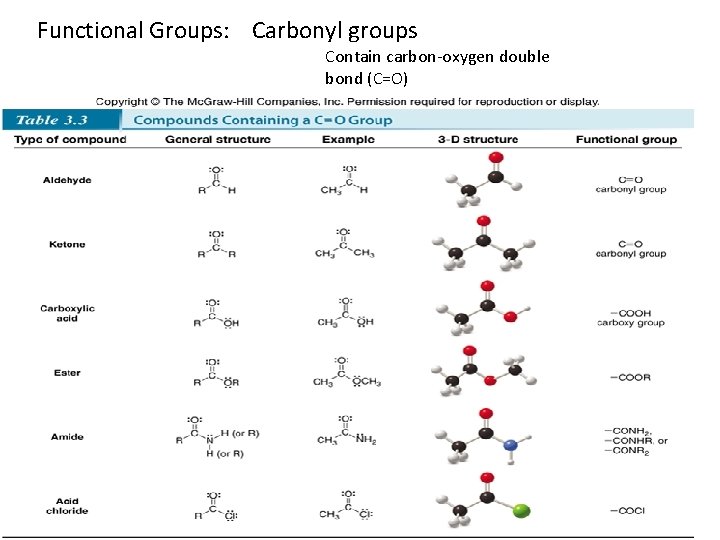
• Functional groups – Is an atom or a group of atoms with characteristic chemical and physical properties. – Can contain heteroatom(s) and/or multiple bonds – Determines a molecule’s shape, properties, and the type of reactions it undergoes – There are three types of most common functional groups • Hydrocarbon • Single bond to a heteroatom • Compounds containing C=O group *The symbol “R” is used to represent any carbon chains or rings

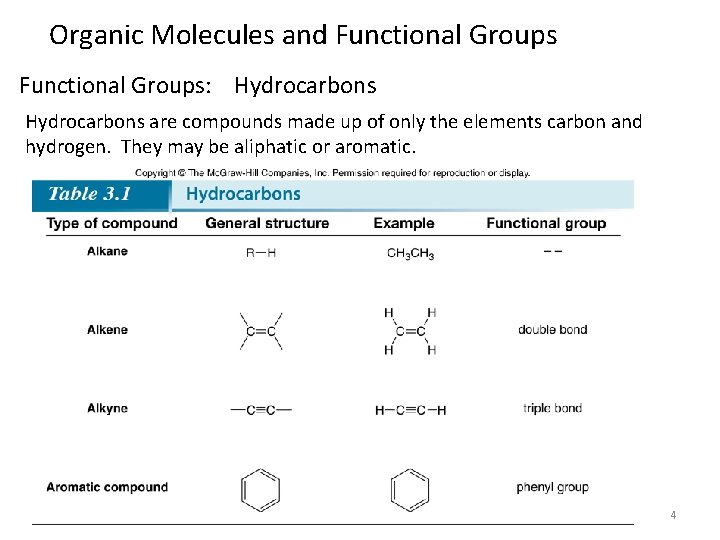
Organic Molecules and Functional Groups: Hydrocarbons are compounds made up of only the elements carbon and hydrogen. They may be aliphatic or aromatic. 4

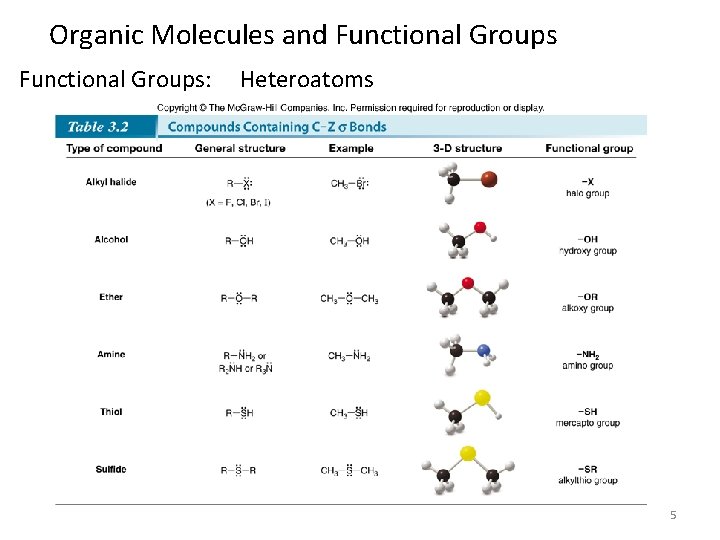
Organic Molecules and Functional Groups: Heteroatoms 5

Functional Groups: Carbonyl groups Contain carbon-oxygen double bond (C=O) 6

Experiment • • • Test for Carbon and Hydrogen Test for Nitrogen Test for Halogens Test for Oxygen Test for Sulfur

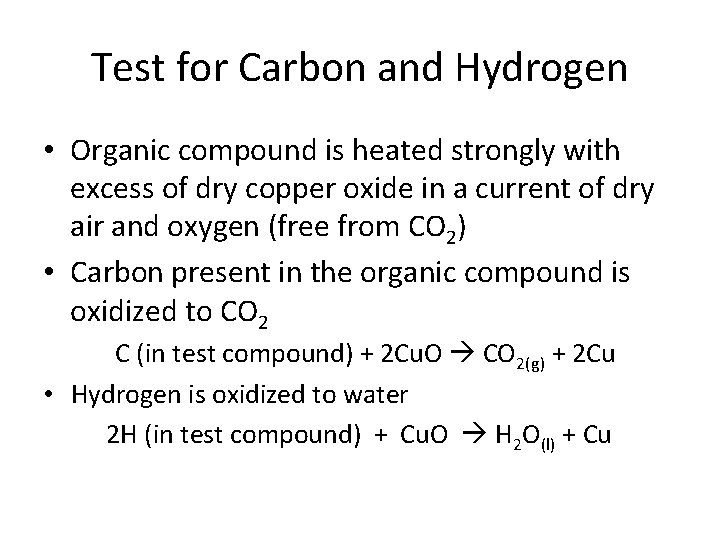
Test for Carbon and Hydrogen • Organic compound is heated strongly with excess of dry copper oxide in a current of dry air and oxygen (free from CO 2) • Carbon present in the organic compound is oxidized to CO 2 C (in test compound) + 2 Cu. O CO 2(g) + 2 Cu • Hydrogen is oxidized to water 2 H (in test compound) + Cu. O H 2 O(l) + Cu

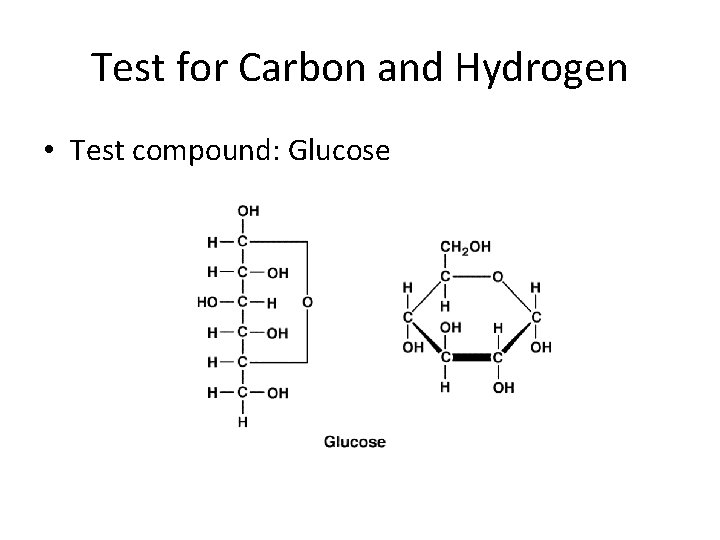
Test for Carbon and Hydrogen • Test compound: Glucose

Test for Carbon and Hydrogen • Positive Result: A white precipitate forms at the test tube with lime water and as well as presence of droplets of water in the tube Ca(OH)2(aq) + CO 2(g) Ca. CO 3(s) + H 2 O(l) (lime water)

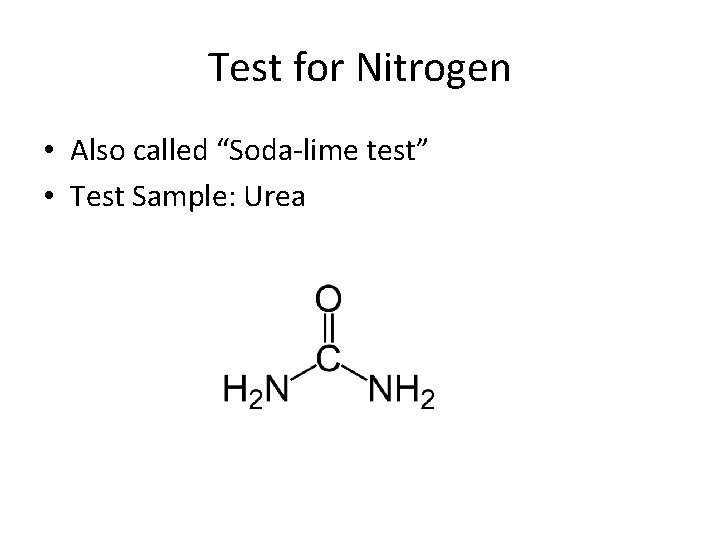
Test for Nitrogen • Also called “Soda-lime test” • Test Sample: Urea

Test for Nitrogen • A pinch of organic compound is heated strongly in a dry test tube with soda lime (Na. OH + Ca. O) • Positive Result: A smell of ammonia indicates presence of nitrogen + Na. OH(s) + Ca. O(s) 2 NH 3(g) + Na 2 CO 3

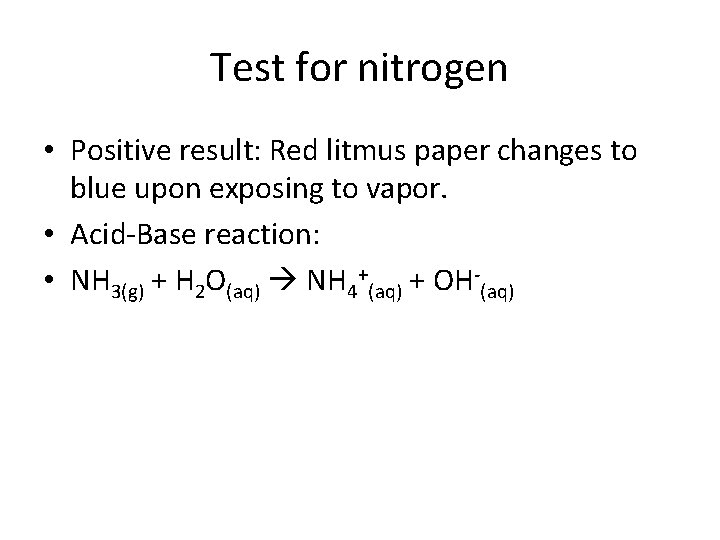
Test for nitrogen • Positive result: Red litmus paper changes to blue upon exposing to vapor. • Acid-Base reaction: • NH 3(g) + H 2 O(aq) NH 4+(aq) + OH-(aq)

Test for nitrogen • Limitation: – Soda lime test is not a reliable confirmatory test for the presence of nitrogen since some nitrogen containing compound such as nitro (-NO 2), or azo compounds (-N=N-)

Test for Halogens • Beilstein Test – A clean and bent copper wire is heated in a non luminous flame until it ceases to impart green flame. It is then dip in organic halide. – Positive result: a visible blue-green flame indicating the formation of volatile cupric halide. – The test is used to confirm the presence of halogens in a certain compound

Test for Halogens • Silver Nitrate Test – Monochloroacetic acid is reacted with silver nitrate in the presence of hot distilled water and nitric acid. – Positive result: formation of white precipitate indicating the formation of Ag. Cl(s)

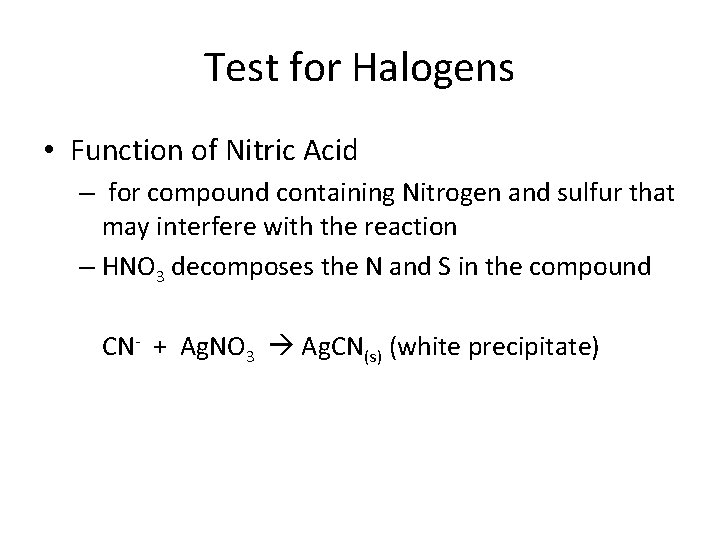
Test for Halogens • Function of Nitric Acid – for compound containing Nitrogen and sulfur that may interfere with the reaction – HNO 3 decomposes the N and S in the compound CN- + Ag. NO 3 Ag. CN(s) (white precipitate)

Test for Oxygen • Ferrox test – Test to determine the presence of oxygen in an organic compound ( aldehydes, ethers, ketones, alcohols, etc. ) – Ferrox reagent: 2 Fe(NH 4)(SO 4)2 + 6 KSCN Fe[Fe(SCN)6] Iron (III) amm. Sulfate Potassium thiocyanate Iron(III) hexathiocyanato ferrate (III)

Test for Oxygen • The prepared ferrox paper is dipped in the test solution (hexane, alcohol, ether). • Positive result: red to reddish-purple solution upon mixing with the test sample. • Ethanol and ether showed positive result while hexane exhibited no change in color of solution

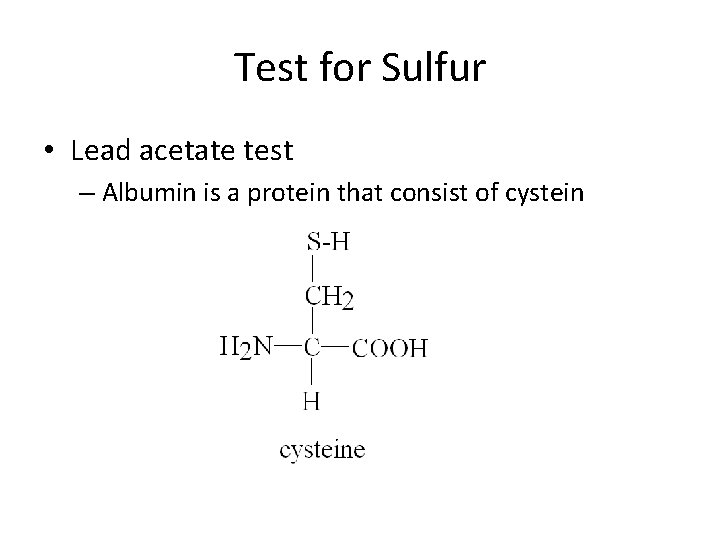
Test for Sulfur • Lead acetate test – Albumin is a protein that consist of cystein

Test for Sulfur • In strongly basic solution, the amino acid cysteine will react to lead ions (Pb 2+) forming black precipitate, Pb. S. • Heating is necessary to be able to break up the polypeptide chains • Positive Result: Black precipitate after heating

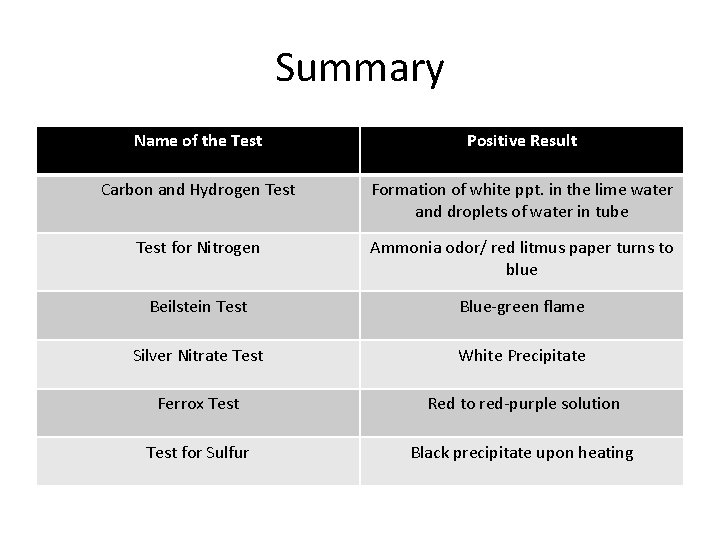
Summary Name of the Test Positive Result Carbon and Hydrogen Test Formation of white ppt. in the lime water and droplets of water in tube Test for Nitrogen Ammonia odor/ red litmus paper turns to blue Beilstein Test Blue-green flame Silver Nitrate Test White Precipitate Ferrox Test Red to red-purple solution Test for Sulfur Black precipitate upon heating