Organic Chemistry I Aliphatic Compounds Organic Chemistry Organic
























- Slides: 152

Organic Chemistry I Aliphatic Compounds

Organic Chemistry • Organic chemistry is the chemistry of compounds containing carbon. – In this chapter we will discuss the structural features of organic molecules, nomenclature, and a few important chemical reactions.

Hydrocarbons • The simplest organic compounds are hydrocarbons, compounds containing only carbon and hydrogen. – The three main groups of hydrocarbons are: saturated hydrocarbons, hydrocarbons with only single bonds between the carbon atoms. unsaturated hydrocarbons, hydrocarbons that contain double or triple bonds between carbon atoms. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 3

Hydrocarbons • The simplest organic compounds are hydrocarbons, compounds containing only carbon and hydrogen. – The three main groups of hydrocarbons are: aromatic hydrocarbons, hydrocarbons that contain a benzene ring (a six-membered ring of carbon atoms with alternating single and double carbon-carbon bonds described by resonance formulas). Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 4

Hydrocarbons • The simplest organic compounds are hydrocarbons, compounds containing only carbon and hydrogen. – The saturated and unsaturated hydrocarbons are often referred to as the aliphatic hydrocarbons. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 5

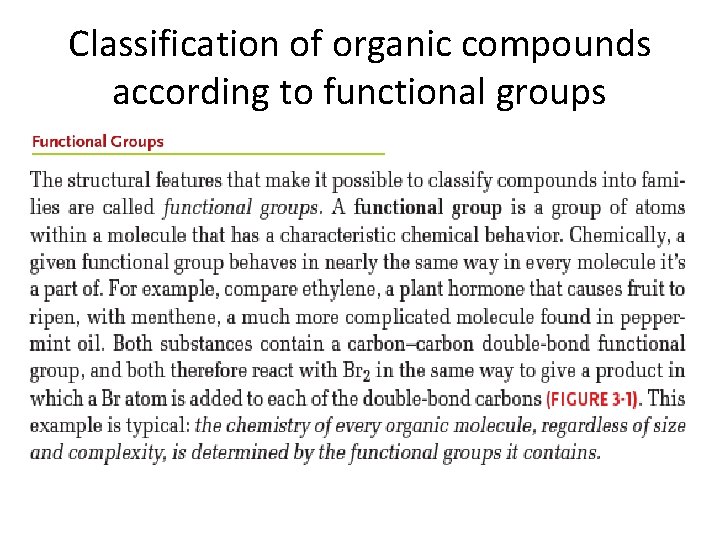
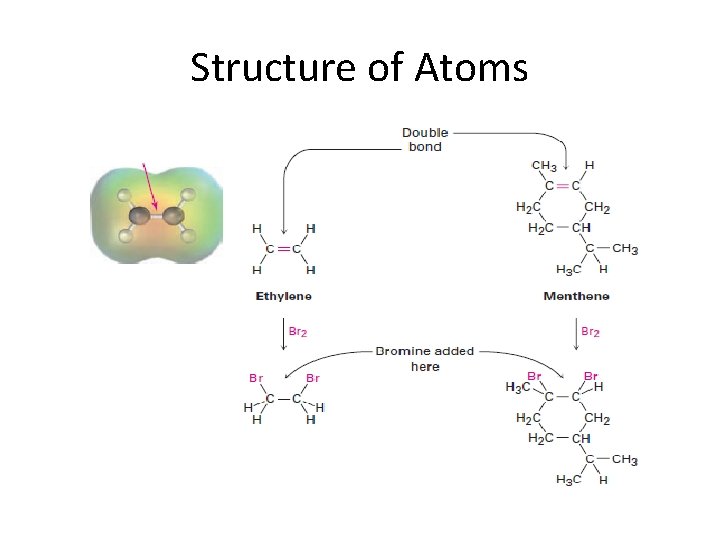
Classification of organic compounds according to functional groups

Structure of Atoms




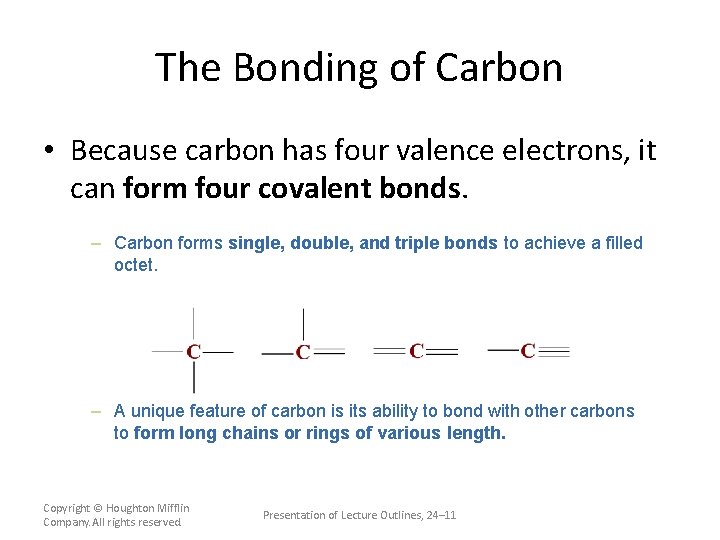
The Bonding of Carbon • Because carbon has four valence electrons, it can form four covalent bonds. – Carbon forms single, double, and triple bonds to achieve a filled octet. – A unique feature of carbon is its ability to bond with other carbons to form long chains or rings of various length. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 11

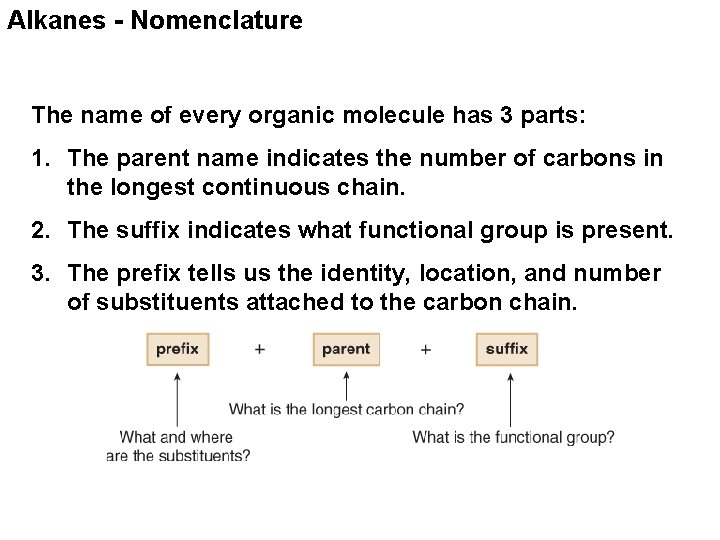
Alkanes - Nomenclature The name of every organic molecule has 3 parts: 1. The parent name indicates the number of carbons in the longest continuous chain. 2. The suffix indicates what functional group is present. 3. The prefix tells us the identity, location, and number of substituents attached to the carbon chain.

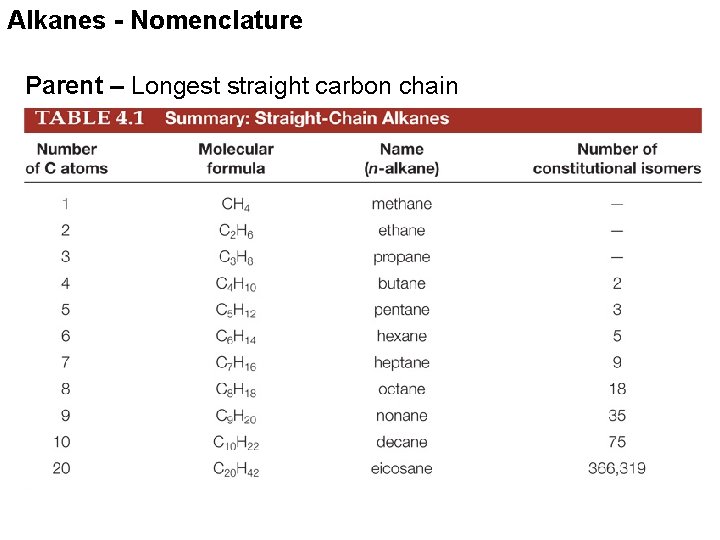
Alkanes - Nomenclature Parent – Longest straight carbon chain

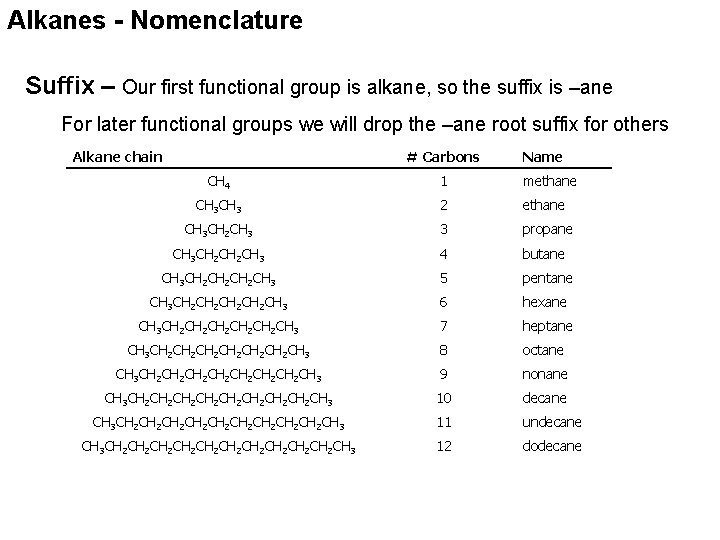
Alkanes - Nomenclature Suffix – Our first functional group is alkane, so the suffix is –ane For later functional groups we will drop the –ane root suffix for others Alkane chain # Carbons Name CH 4 1 methane CH 3 2 ethane CH 3 CH 2 CH 3 3 propane CH 3 CH 2 CH 3 4 butane CH 3 CH 2 CH 2 CH 3 5 pentane CH 3 CH 2 CH 2 CH 3 6 hexane CH 3 CH 2 CH 2 CH 2 CH 3 7 heptane CH 3 CH 2 CH 2 CH 2 CH 3 8 octane CH 3 CH 2 CH 2 CH 3 9 nonane CH 3 CH 2 CH 2 CH 3 10 decane CH 3 CH 2 CH 2 CH 2 CH 3 11 undecane CH 3 CH 2 CH 2 CH 2 CH 3 12 dodecane

Alkanes - Nomenclature Prefix – Our substituents will be branches in the alkane structure A branch is another alkane minus one hydrogen – an alkyl group Example – if CH 3 - is a branch on a longer chain: CH 3 - is CH 4 minus 1 hydrogen Since it is a side chain it will replace the –ane suffix with –yl CH 3 - is a methyl group We can also abbreviate this group as Me-

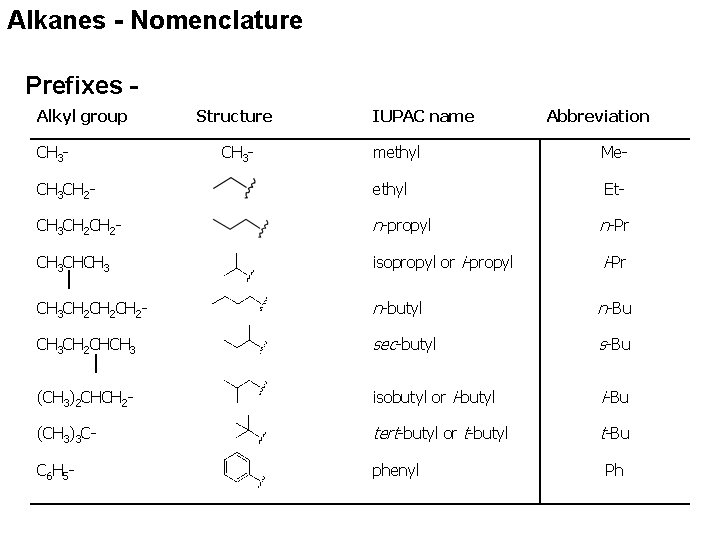
Alkanes - Nomenclature Prefixes Alkyl group CH 3 - Structure CH 3 - IUPAC name Abbreviation methyl Me- CH 3 CH 2 - ethyl Et- CH 3 CH 2 - n-propyl n-Pr CH 3 CHCH 3 isopropyl or i-propyl i-Pr CH 3 CH 2 CH 2 - n-butyl n-Bu CH 3 CH 2 CHCH 3 sec-butyl s-Bu (CH 3)2 CHCH 2 - isobutyl or i-butyl i-Bu (CH 3)3 C- tert-butyl or t-butyl t-Bu C 6 H 5 - phenyl Ph

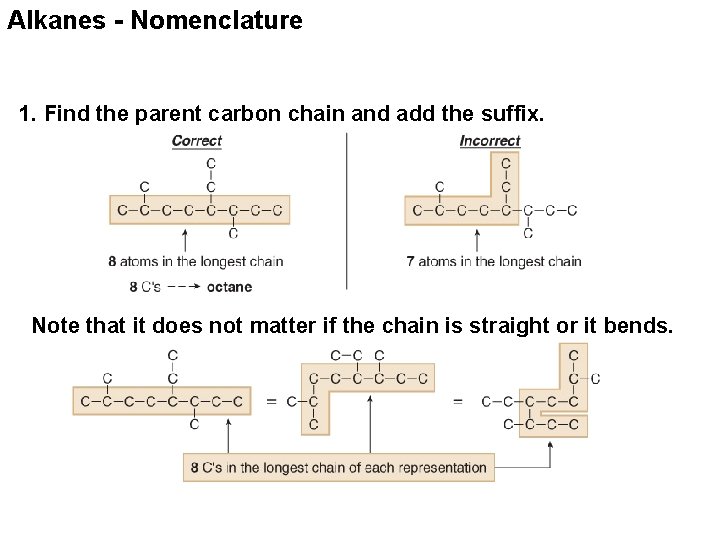
Alkanes - Nomenclature 1. Find the parent carbon chain and add the suffix. Note that it does not matter if the chain is straight or it bends.

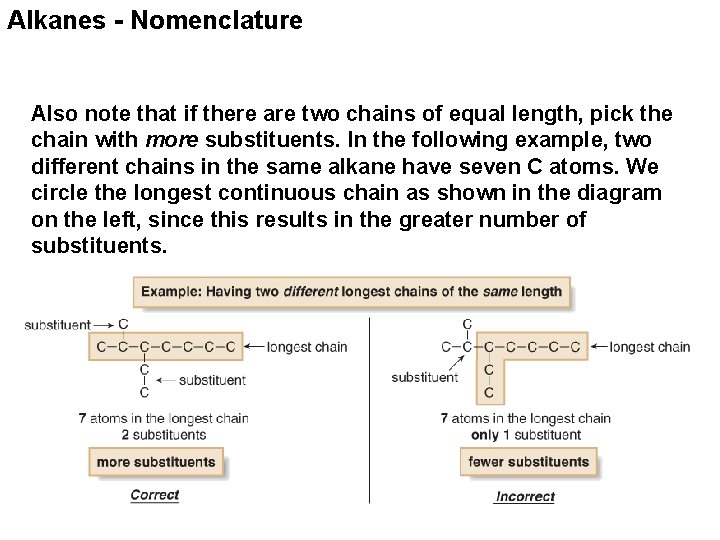
Alkanes - Nomenclature Also note that if there are two chains of equal length, pick the chain with more substituents. In the following example, two different chains in the same alkane have seven C atoms. We circle the longest continuous chain as shown in the diagram on the left, since this results in the greater number of substituents.

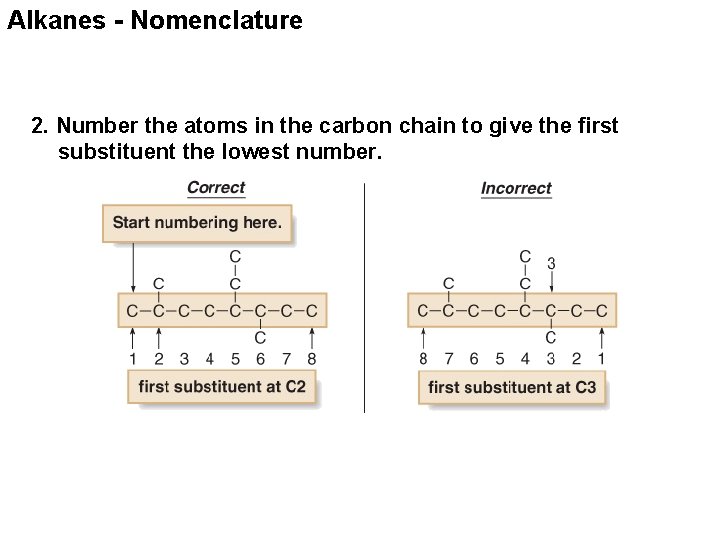
Alkanes - Nomenclature 2. Number the atoms in the carbon chain to give the first substituent the lowest number.

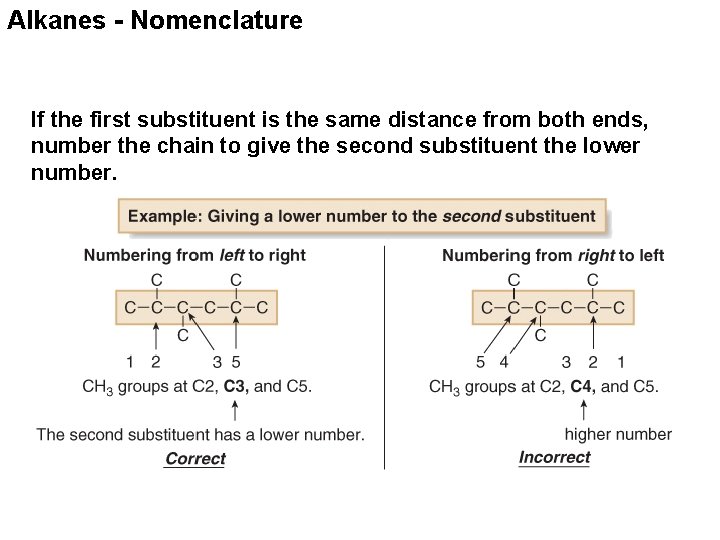
Alkanes - Nomenclature If the first substituent is the same distance from both ends, number the chain to give the second substituent the lower number.

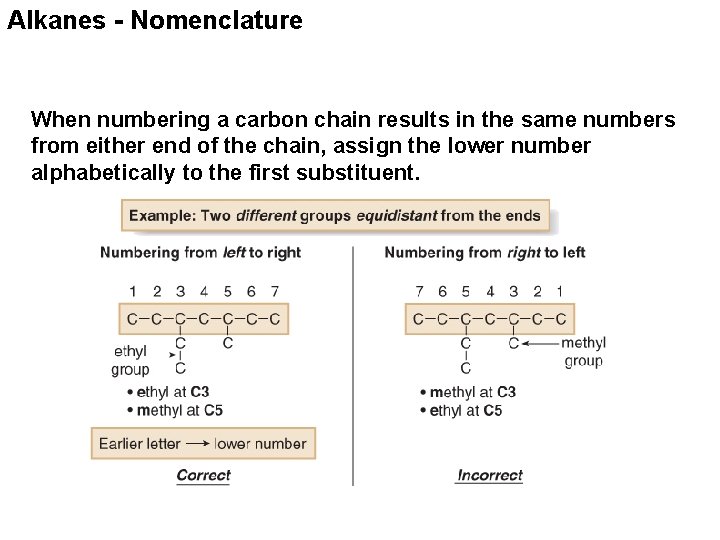
Alkanes - Nomenclature When numbering a carbon chain results in the same numbers from either end of the chain, assign the lower number alphabetically to the first substituent.

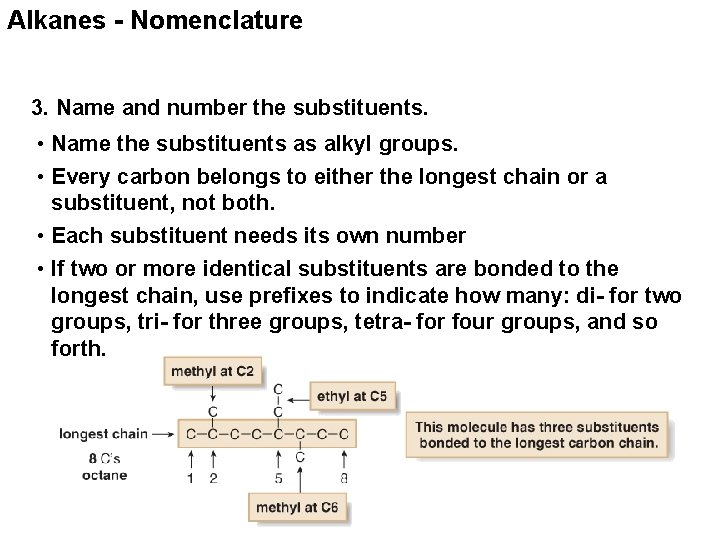
Alkanes - Nomenclature 3. Name and number the substituents. • Name the substituents as alkyl groups. • Every carbon belongs to either the longest chain or a substituent, not both. • Each substituent needs its own number • If two or more identical substituents are bonded to the longest chain, use prefixes to indicate how many: di- for two groups, tri- for three groups, tetra- for four groups, and so forth.

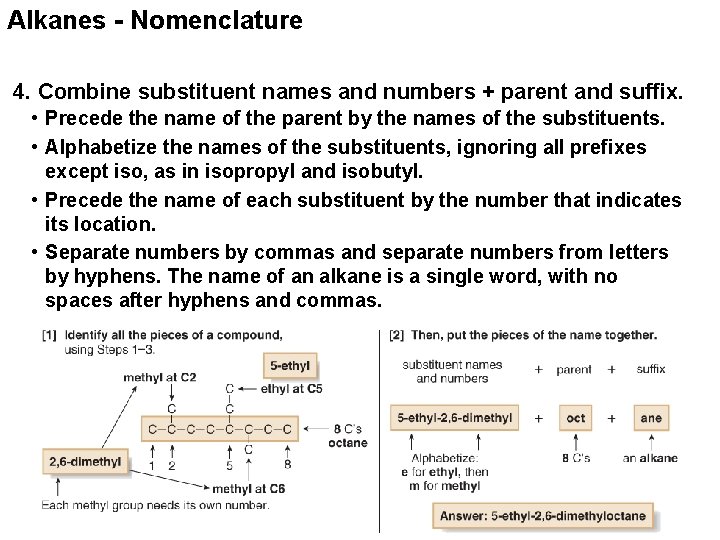
Alkanes - Nomenclature 4. Combine substituent names and numbers + parent and suffix. • Precede the name of the parent by the names of the substituents. • Alphabetize the names of the substituents, ignoring all prefixes except iso, as in isopropyl and isobutyl. • Precede the name of each substituent by the number that indicates its location. • Separate numbers by commas and separate numbers from letters by hyphens. The name of an alkane is a single word, with no spaces after hyphens and commas.

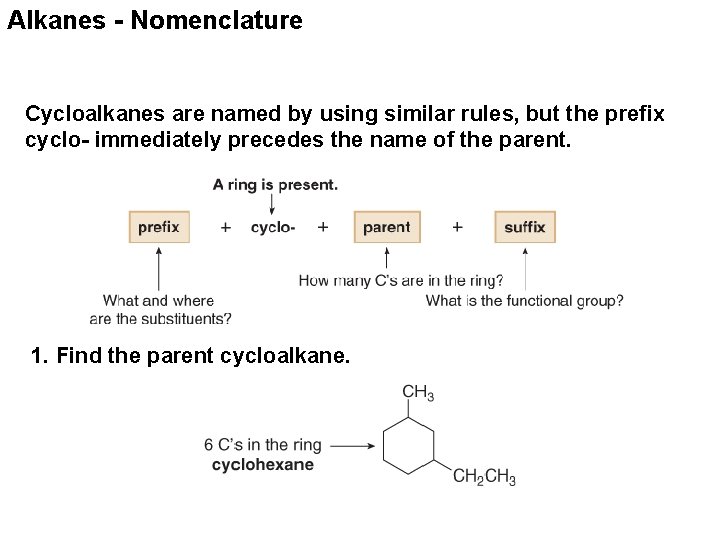
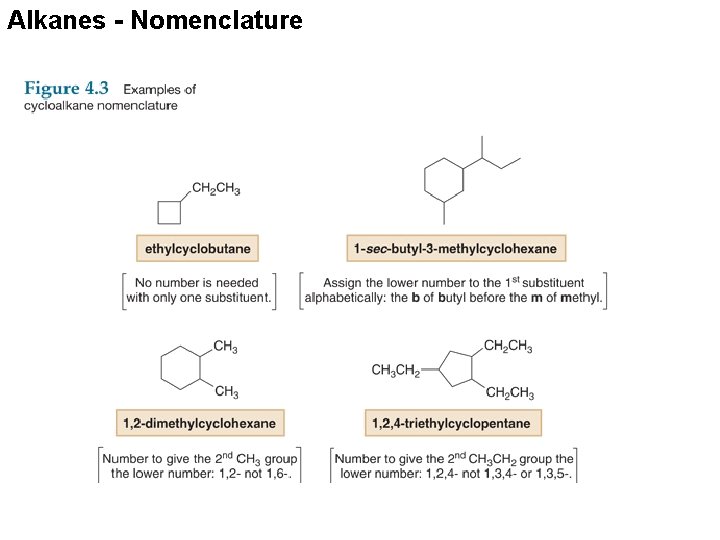
Alkanes - Nomenclature Cycloalkanes are named by using similar rules, but the prefix cyclo- immediately precedes the name of the parent. 1. Find the parent cycloalkane.

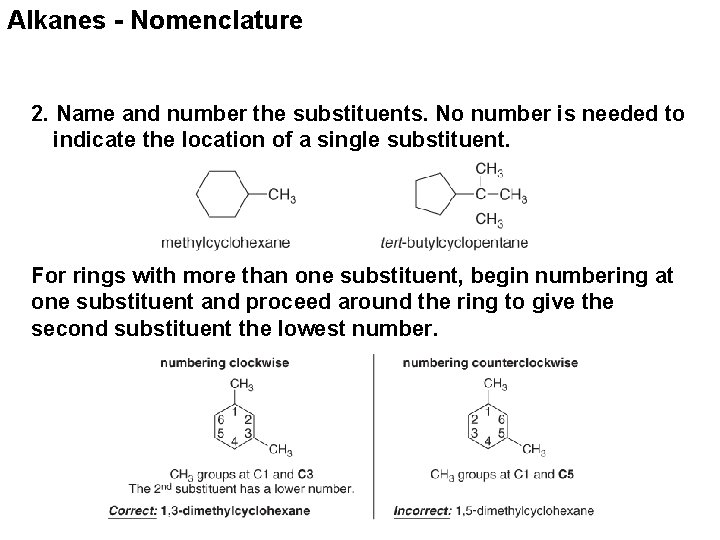
Alkanes - Nomenclature 2. Name and number the substituents. No number is needed to indicate the location of a single substituent. For rings with more than one substituent, begin numbering at one substituent and proceed around the ring to give the second substituent the lowest number.

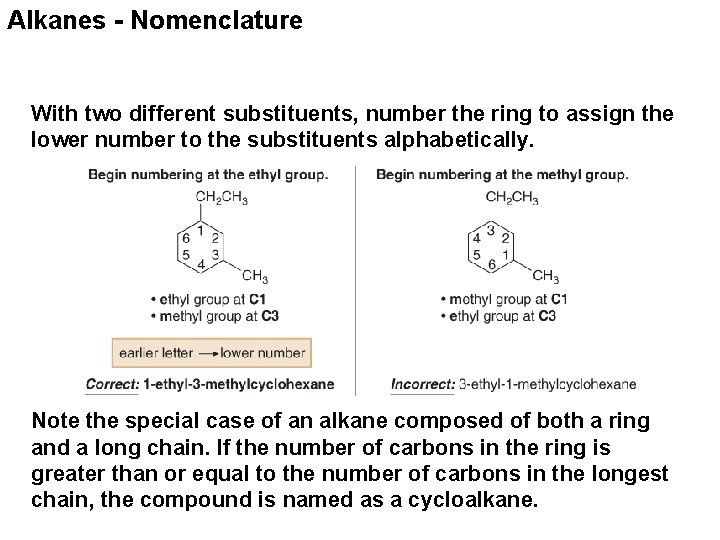
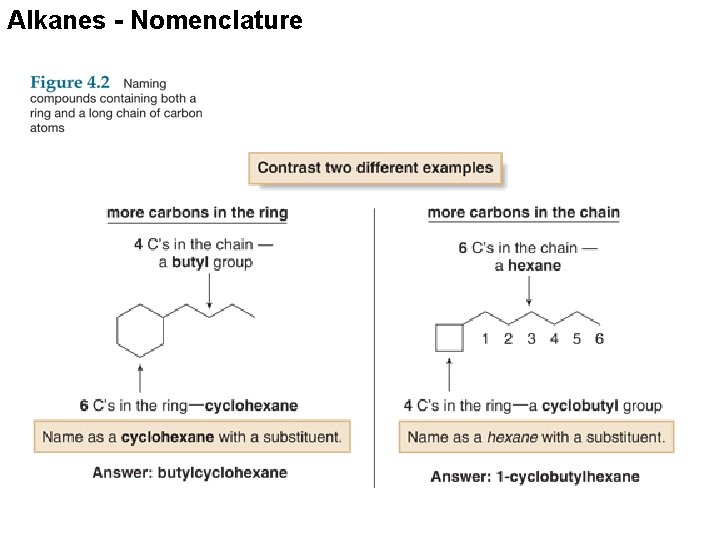
Alkanes - Nomenclature With two different substituents, number the ring to assign the lower number to the substituents alphabetically. Note the special case of an alkane composed of both a ring and a long chain. If the number of carbons in the ring is greater than or equal to the number of carbons in the longest chain, the compound is named as a cycloalkane.

Alkanes - Nomenclature

Alkanes - Nomenclature

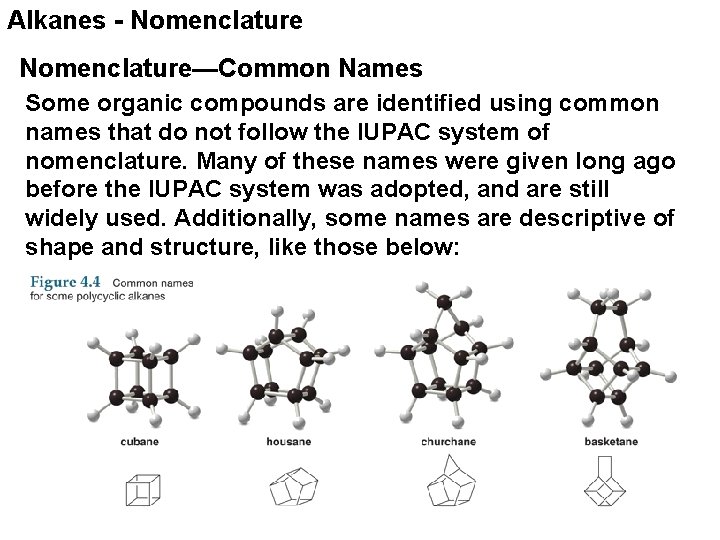
Alkanes - Nomenclature—Common Names Some organic compounds are identified using common names that do not follow the IUPAC system of nomenclature. Many of these names were given long ago before the IUPAC system was adopted, and are still widely used. Additionally, some names are descriptive of shape and structure, like those below:

Other Functional Groups - Nomenclature The IUPAC rules for all other functional groups will differ only by the following: 1. Suffix will change to reflect functional group 2. Some functional groups have priority over others 3. We actually cover the functional groups in 210/212 in order of this priority (except Amines, Ch 22): For now: Alcohol > Alkyne > Alkene > alkane=alkyl halide 4. The longest chain must contain the suffix functional group – even if not the longest chain overall 5. Numbering gives this functional group the lowest number – even if there are other groups that would be lower 6. If an alkene has stereochemistry, it must be specified in the prefix

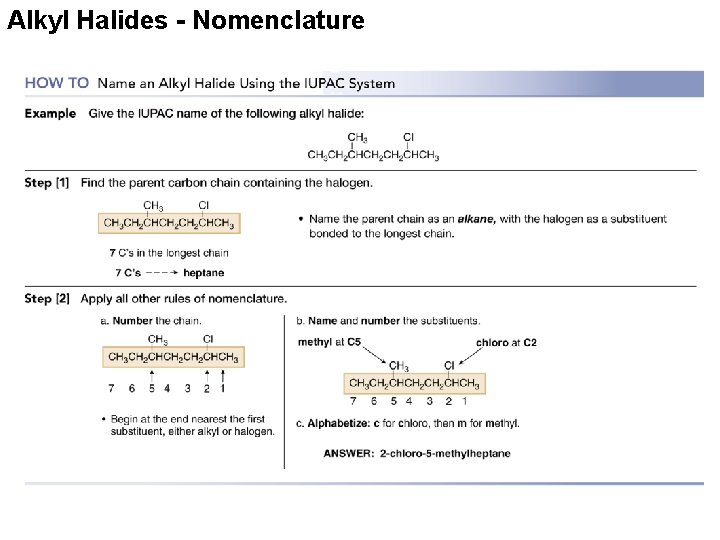
Alkyl Halides - Nomenclature

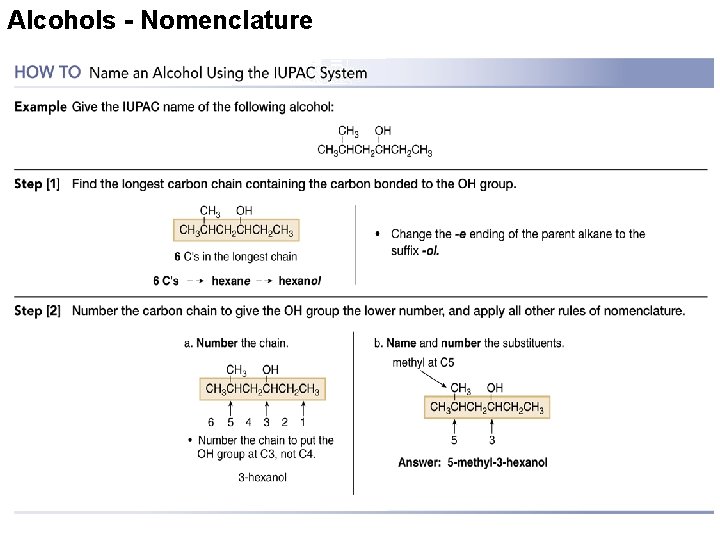
Alcohols - Nomenclature

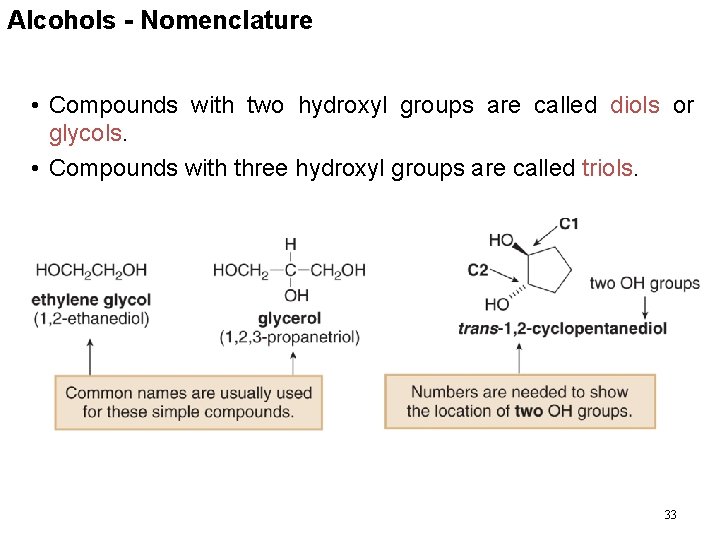
Alcohols - Nomenclature • Compounds with two hydroxyl groups are called diols or glycols. • Compounds with three hydroxyl groups are called triols. 33

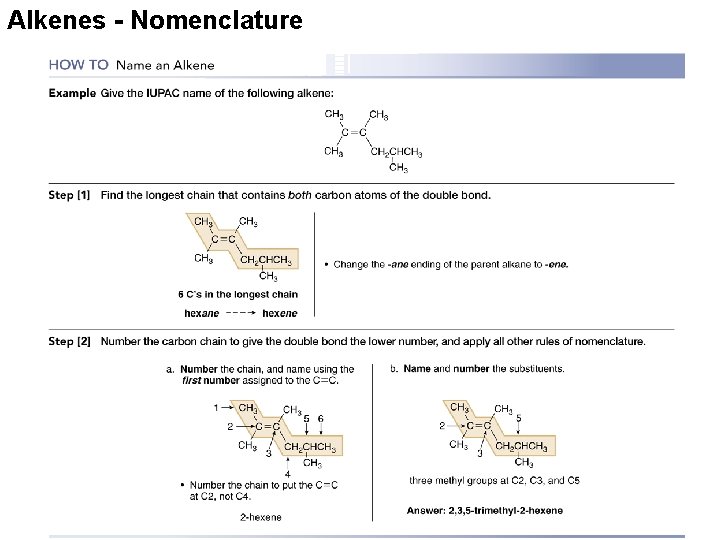
Alkenes - Nomenclature

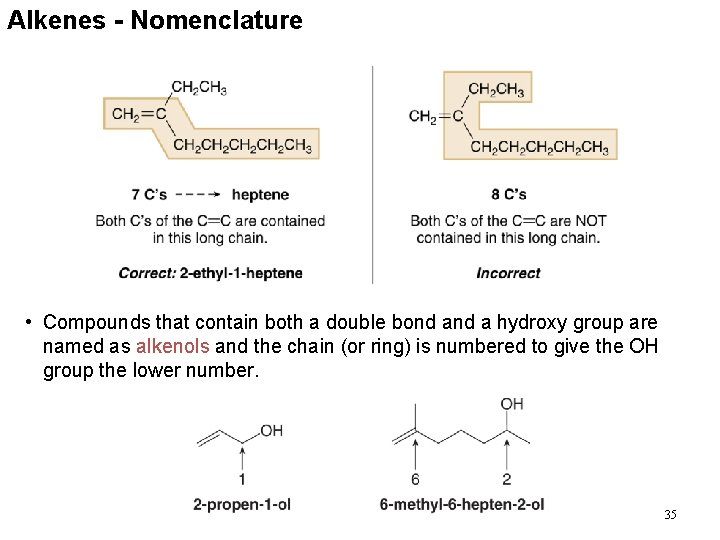
Alkenes - Nomenclature • Compounds that contain both a double bond a hydroxy group are named as alkenols and the chain (or ring) is numbered to give the OH group the lower number. 35

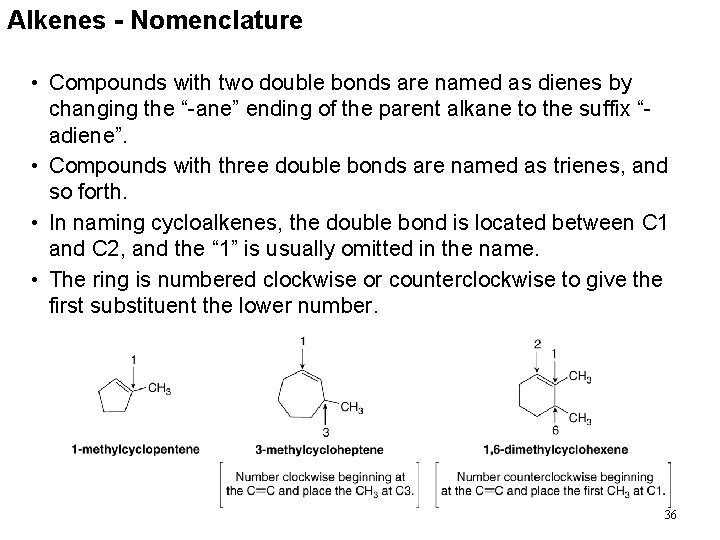
Alkenes - Nomenclature • Compounds with two double bonds are named as dienes by changing the “-ane” ending of the parent alkane to the suffix “adiene”. • Compounds with three double bonds are named as trienes, and so forth. • In naming cycloalkenes, the double bond is located between C 1 and C 2, and the “ 1” is usually omitted in the name. • The ring is numbered clockwise or counterclockwise to give the first substituent the lower number. 36

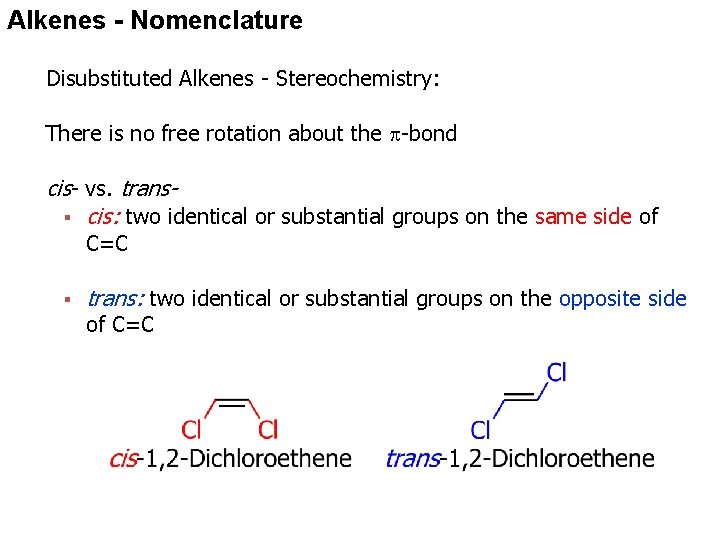
Alkenes - Nomenclature Disubstituted Alkenes - Stereochemistry: There is no free rotation about the -bond cis- vs. trans§ cis: two identical or substantial groups on the same side of C=C § trans: two identical or substantial groups on the opposite side of C=C

Alkenes - Nomenclature

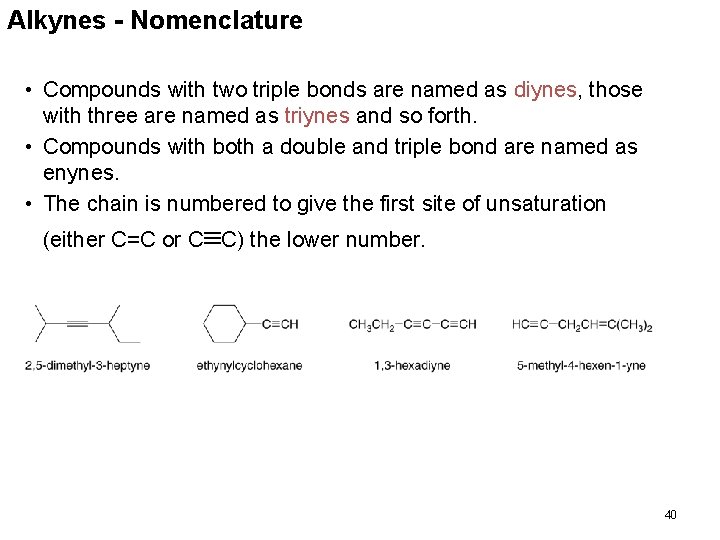
Alkynes - Nomenclature • Alkynes are named in the same general way that alkenes are named. • In the IUPAC system, change the –ane ending of the parent alkane name to the suffix –yne. • Choose the longest continuous chain that contains both atoms of the triple bond and number the chain to give the triple bond the lower number. 39

Alkynes - Nomenclature • Compounds with two triple bonds are named as diynes, those with three are named as triynes and so forth. • Compounds with both a double and triple bond are named as enynes. • The chain is numbered to give the first site of unsaturation (either C=C or C C) the lower number. 40

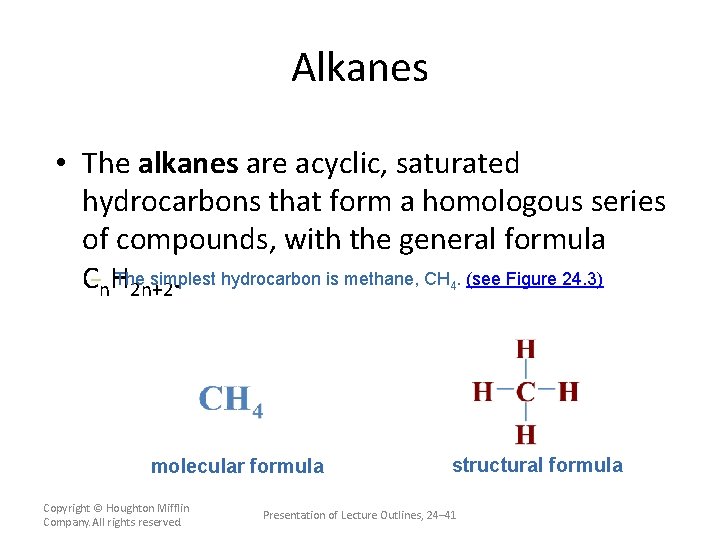
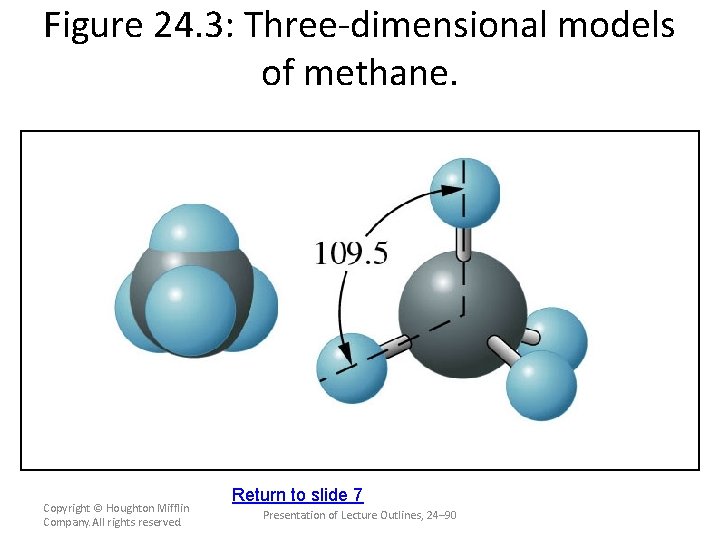
Alkanes • The alkanes are acyclic, saturated hydrocarbons that form a homologous series of compounds, with the general formula The simplest hydrocarbon is methane, CH. (see Figure 24. 3) C–n. H 2 n+2. 4 molecular formula Copyright © Houghton Mifflin Company. All rights reserved. structural formula Presentation of Lecture Outlines, 24– 41

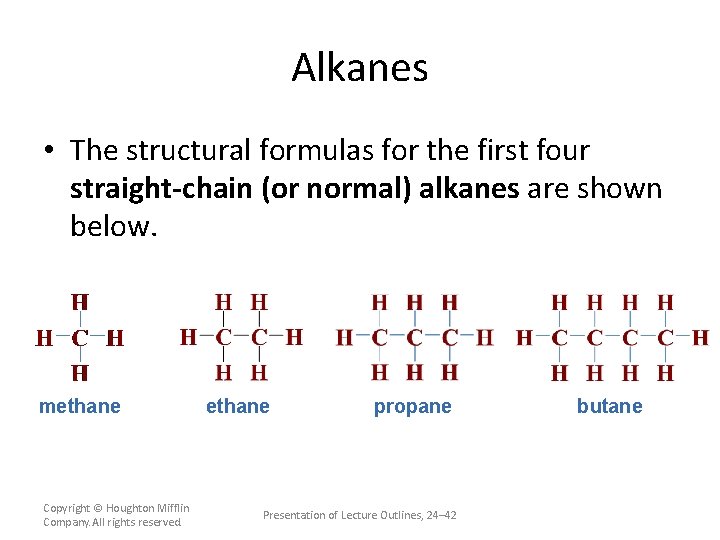
Alkanes • The structural formulas for the first four straight-chain (or normal) alkanes are shown below. methane Copyright © Houghton Mifflin Company. All rights reserved. ethane propane Presentation of Lecture Outlines, 24– 42 butane

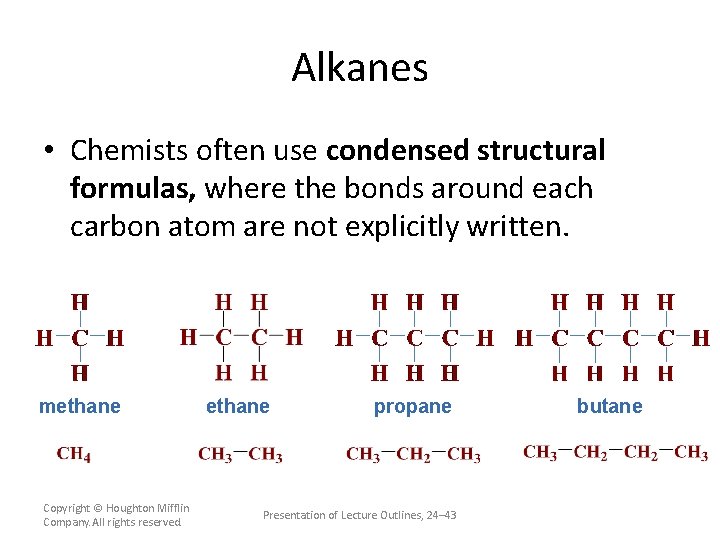
Alkanes • Chemists often use condensed structural formulas, where the bonds around each carbon atom are not explicitly written. methane Copyright © Houghton Mifflin Company. All rights reserved. ethane propane Presentation of Lecture Outlines, 24– 43 butane

Alkanes • The alkanes constitute a homologous series of compounds in which one compound differs from a preceding one by a fixed group of atoms, in this case, a –CH 2– group. – Members of a homologous series have similar chemical properties, and their physical properties change throughout the series in a regular way. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 44

Alkanes • The alkanes constitute a homologous series of compounds in which one compound differs from a preceding one by a fixed group of atoms, in this case, a –CH 2– group. – Table 24. 1 lists the melting points and boiling points of the first ten alkanes. – Note that the melting and boiling points increase with an increase in the number of carbon atoms. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 45

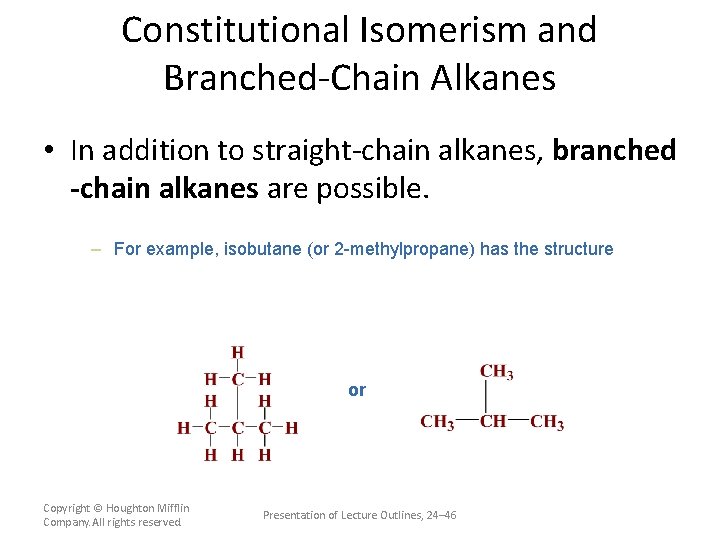
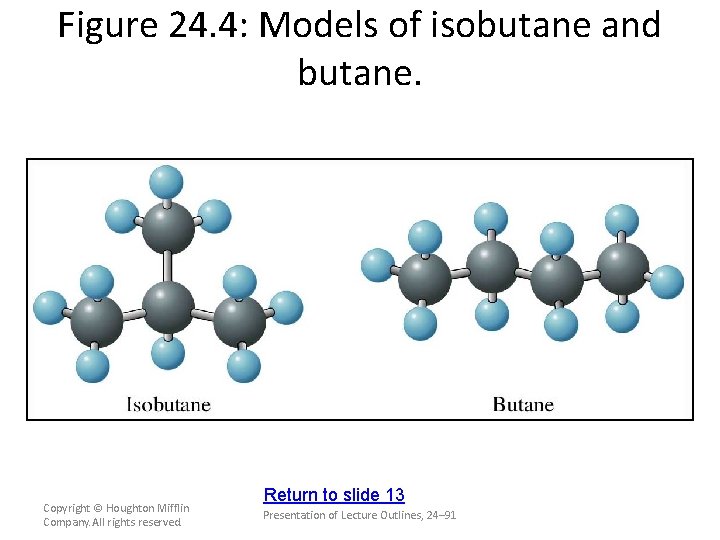
Constitutional Isomerism and Branched-Chain Alkanes • In addition to straight-chain alkanes, branched -chain alkanes are possible. – For example, isobutane (or 2 -methylpropane) has the structure or Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 46

Constitutional Isomerism and Branched-Chain Alkanes • In addition to straight-chain alkanes, branched -chain alkanes are possible. – Note that isobutane, C 4 H 10, has the same molecular formula as normal butane. – Butane and isobutane are constitutional (or structural) isomers, compounds with the same molecular formula but different structural formulas. (see Figure 24. 4) Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 47

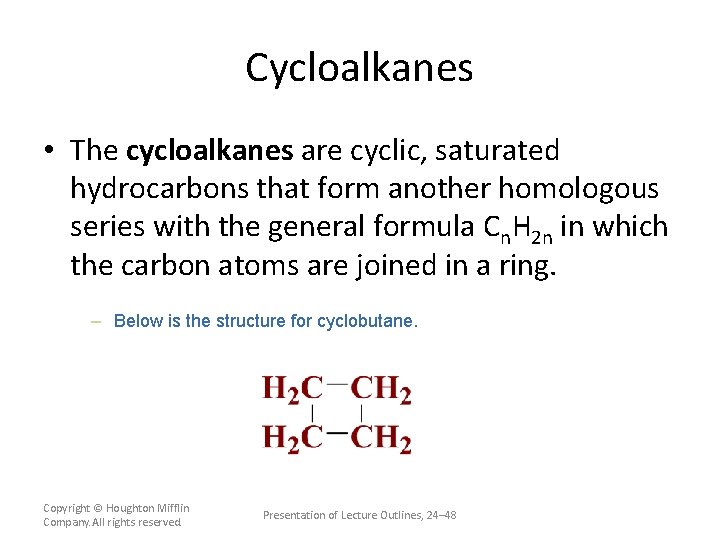
Cycloalkanes • The cycloalkanes are cyclic, saturated hydrocarbons that form another homologous series with the general formula Cn. H 2 n in which the carbon atoms are joined in a ring. – Below is the structure for cyclobutane. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 48

Cycloalkanes • The cycloalkanes are cyclic, saturated hydrocarbons that form another homologous series with the general formula Cn. H 2 n in which the carbon atoms are joined in a ring. – Figure 24. 6 gives the names and structural formulas for the first four cycloalkanes. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 49

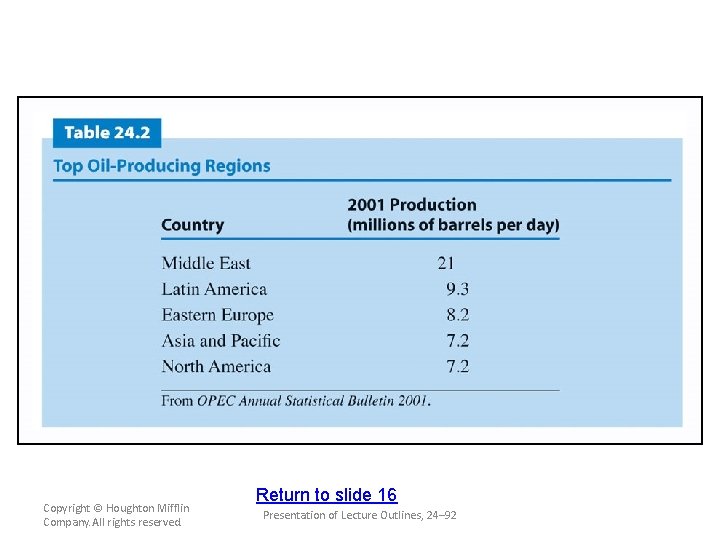
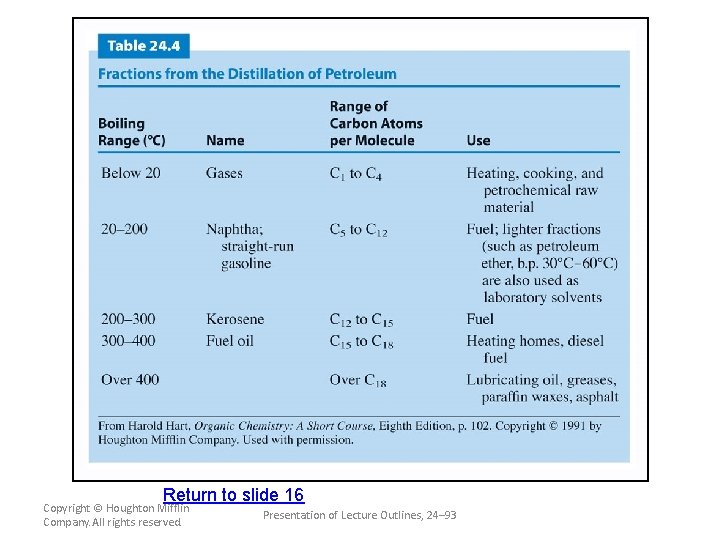
Sources and Uses of Alkanes and Cycloalkanes • Fossil fuels are the principal source of all types of organic compounds. – Crude oil is a mixture of alkanes, cycloalkanes, and aromatic hydrocarbons. (see Table 24. 2) – Because fossil fuels are mixtures of hydrocarbons, it is usually necessary to separate these mixtures by distillation. (see Table 24. 4) – The alkanes serve as the starting point for most plastics and pharmaceuticals. (see Figure 24. 7) Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 50

Reactions of Alkanes With Oxygen • All hydrocarbons burn in an excess of O 2 to produce carbon dioxide, water, and heat. – For example, a propane gas grill uses the reaction Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 51

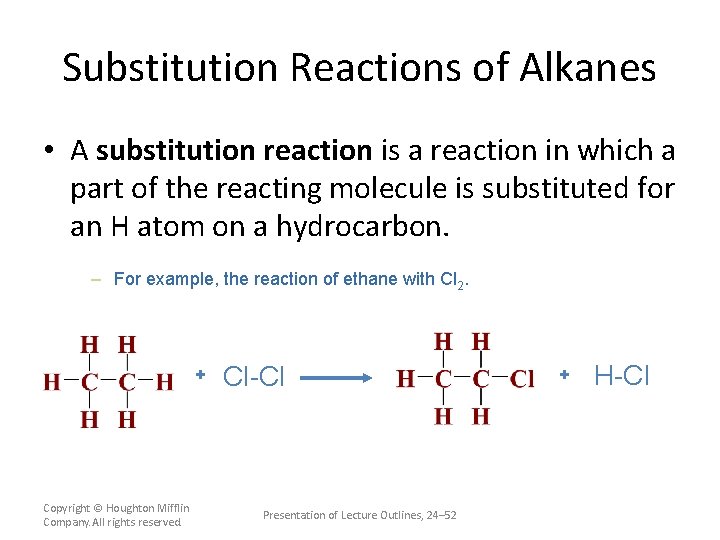
Substitution Reactions of Alkanes • A substitution reaction is a reaction in which a part of the reacting molecule is substituted for an H atom on a hydrocarbon. – For example, the reaction of ethane with Cl 2. + Copyright © Houghton Mifflin Company. All rights reserved. Cl-Cl Presentation of Lecture Outlines, 24– 52 + H-Cl

Alkenes and Alkynes • The alkenes and alkynes are unsaturated hydrocarbons (cyclic or acyclic) that contain carbon-carbon double or triple bonds. Under proper conditions, molecular hydrogen can be added to an alkene or an alkyne to produce a saturated compound in a process called catalytic hydrogenation. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 53

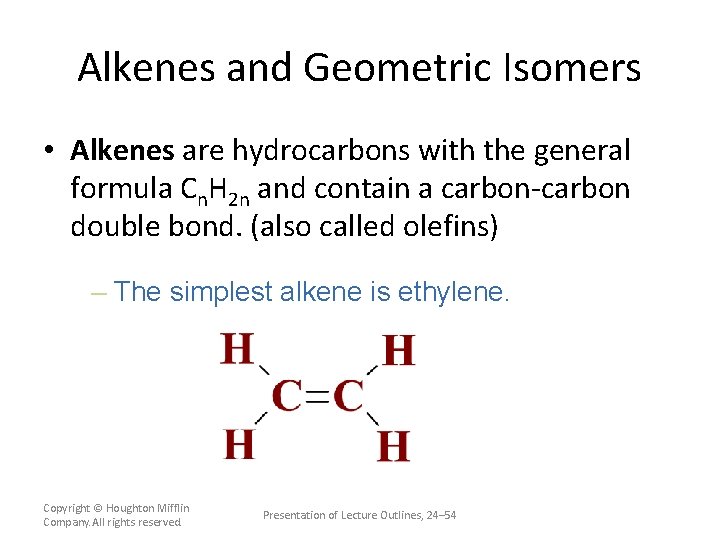
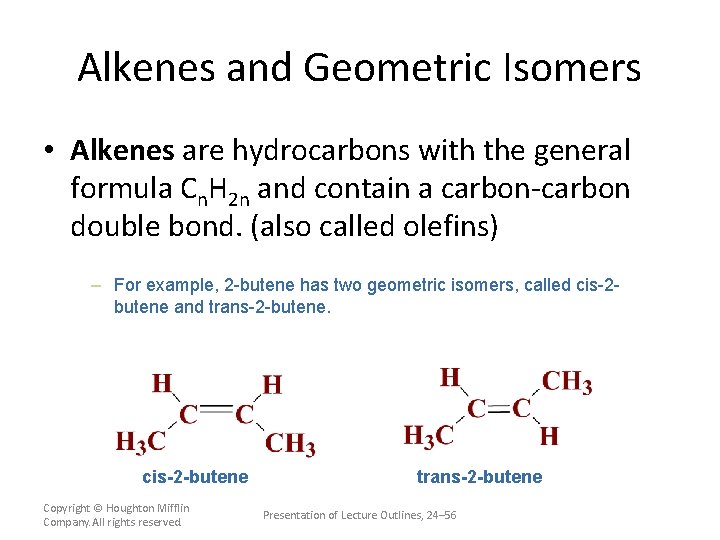
Alkenes and Geometric Isomers • Alkenes are hydrocarbons with the general formula Cn. H 2 n and contain a carbon-carbon double bond. (also called olefins) – The simplest alkene is ethylene. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 54

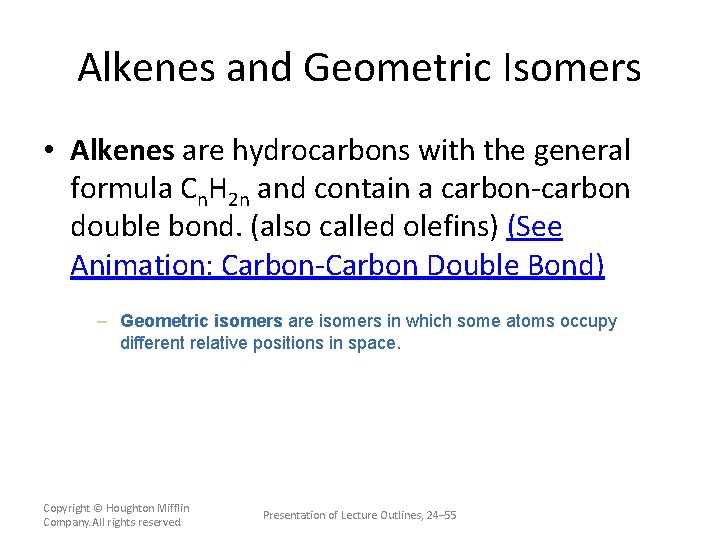
Alkenes and Geometric Isomers • Alkenes are hydrocarbons with the general formula Cn. H 2 n and contain a carbon-carbon double bond. (also called olefins) (See Animation: Carbon-Carbon Double Bond) – Geometric isomers are isomers in which some atoms occupy different relative positions in space. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 55

Alkenes and Geometric Isomers • Alkenes are hydrocarbons with the general formula Cn. H 2 n and contain a carbon-carbon double bond. (also called olefins) – For example, 2 -butene has two geometric isomers, called cis-2 butene and trans-2 -butene. cis-2 -butene Copyright © Houghton Mifflin Company. All rights reserved. trans-2 -butene Presentation of Lecture Outlines, 24– 56

Oxidation Reactions of Alkenes • Because alkenes are hydrocarbons, they undergo complete combustion reactions with oxygen. – Unsaturated hydrocarbons can also be partially oxidized under relatively mild conditions. – For example, when aqueous potassium permanganate is added to an alkene (or alkyne), the purple color of KMn. O 4 fades as a brown precipitate of Mn. O 2 forms. (see Figure 24. 9) Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 57

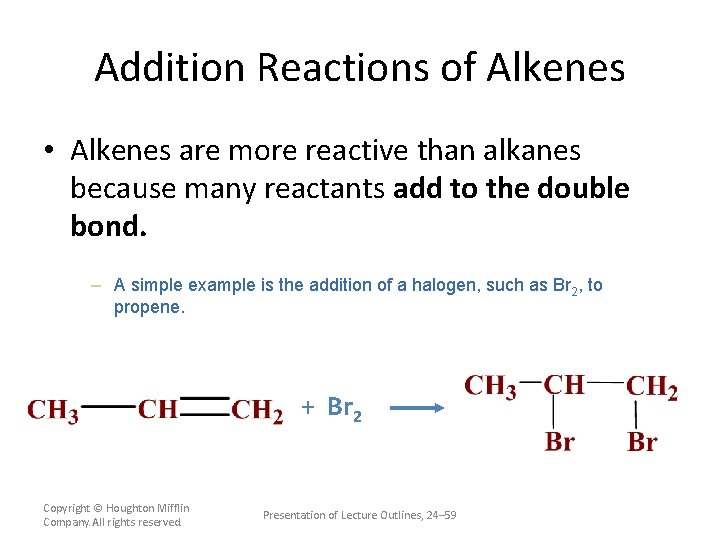
Addition Reactions of Alkenes • Alkenes are more reactive than alkanes because many reactants add to the double bond. – An addition reaction is a reaction in which parts of a reactant are added to each carbon atom of a carbon-carbon double bond which converts to a carbon-carbon single bond. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 58

Addition Reactions of Alkenes • Alkenes are more reactive than alkanes because many reactants add to the double bond. – A simple example is the addition of a halogen, such as Br 2, to propene. + Br 2 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 59

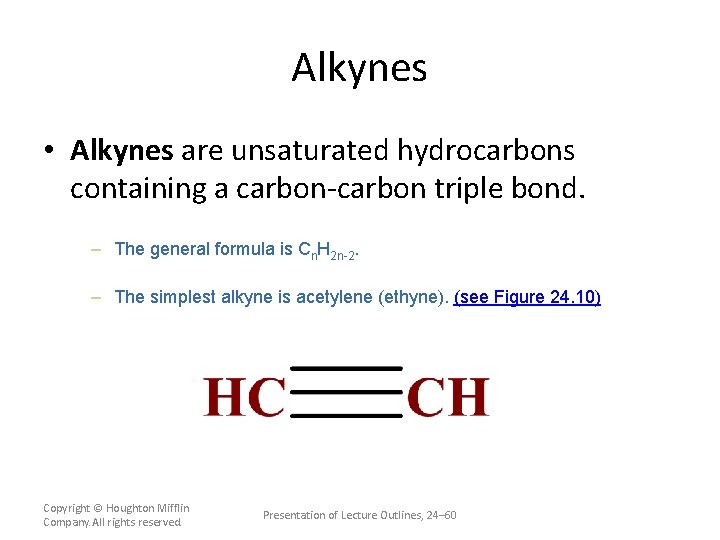
Alkynes • Alkynes are unsaturated hydrocarbons containing a carbon-carbon triple bond. – The general formula is Cn. H 2 n-2. – The simplest alkyne is acetylene (ethyne). (see Figure 24. 10) Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 60

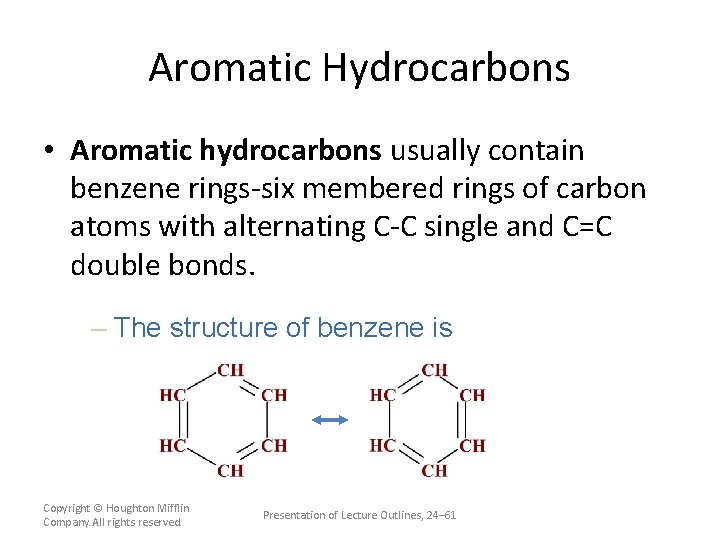
Aromatic Hydrocarbons • Aromatic hydrocarbons usually contain benzene rings-six membered rings of carbon atoms with alternating C-C single and C=C double bonds. – The structure of benzene is Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 61

Aromatic Hydrocarbons • Aromatic hydrocarbons usually contain benzene rings-six membered rings of carbon atoms with alternating C-C single and C=C double bonds. – Figure 24. 11 illustrates the delocalization of the electrons in benzene. – Aromatic compounds are found everywhere from pain relievers to flavoring agents. (see Figure 24. 13) Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 62

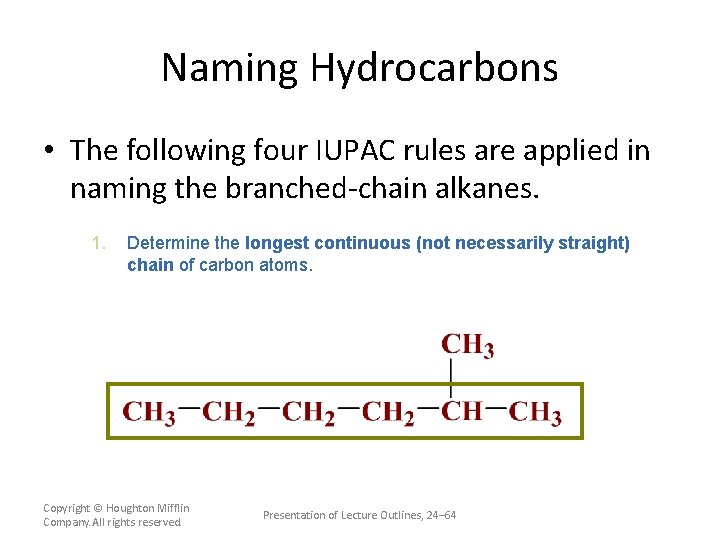
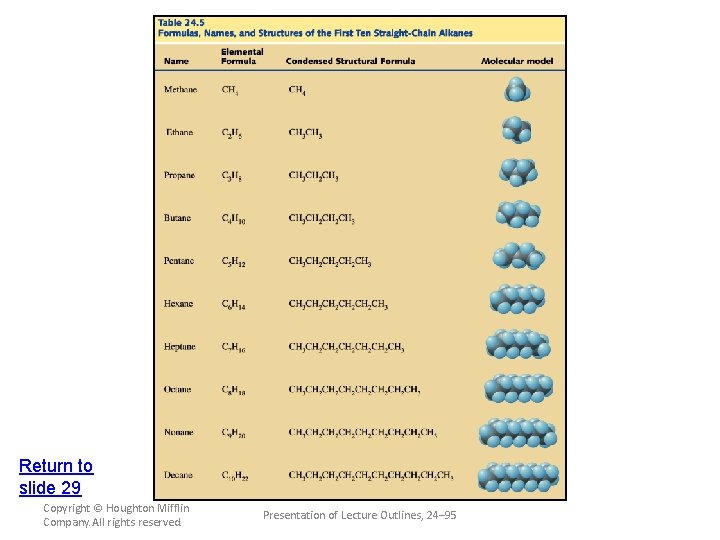
Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 1. Determine the longest continuous (not necessarily straight) chain of carbon atoms. The base name corresponds to the number of carbon atoms in the longest chain. (see Table 24. 5) The full name for the alkane will include the names of any branches. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 63

Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 1. Determine the longest continuous (not necessarily straight) chain of carbon atoms. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 64

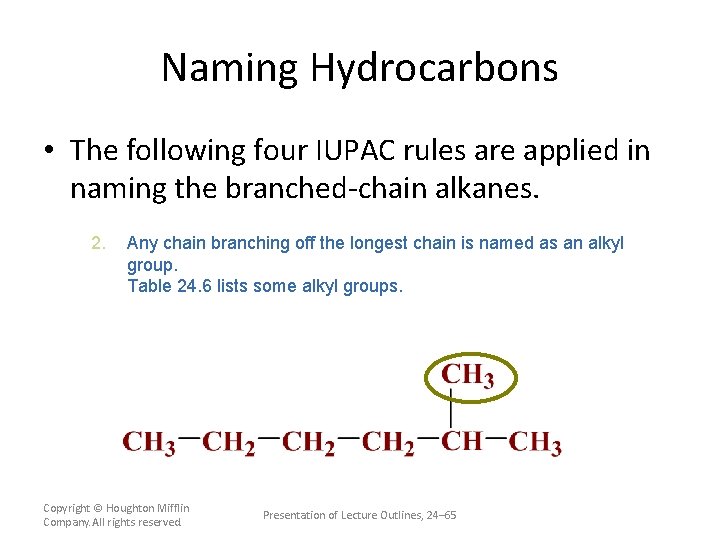
Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 2. Any chain branching off the longest chain is named as an alkyl group. Table 24. 6 lists some alkyl groups. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 65

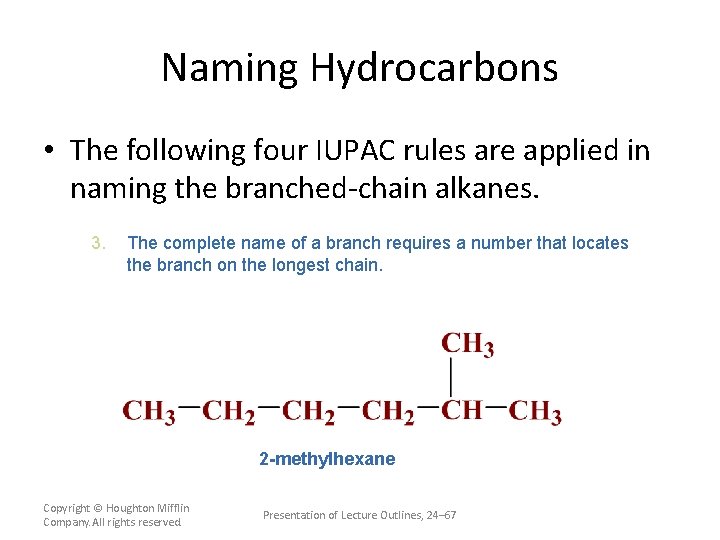
Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 3. The complete name of a branch requires a number that locates the branch on the longest chain. Always number from the end of the longest chain closest to the first branch. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 66

Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 3. The complete name of a branch requires a number that locates the branch on the longest chain. 2 -methylhexane Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 67

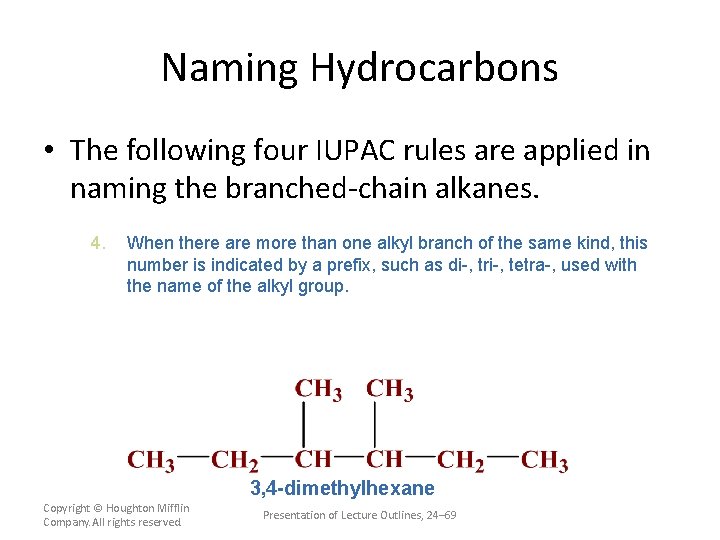
Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 4. When there are more than one alkyl branch of the same kind, this number is indicated by a prefix, such as di-, tri-, tetra-, used with the name of the alkyl group. The position of each group on the longest chain is given by numbers. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 68

Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 4. When there are more than one alkyl branch of the same kind, this number is indicated by a prefix, such as di-, tri-, tetra-, used with the name of the alkyl group. 3, 4 -dimethylhexane Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 69

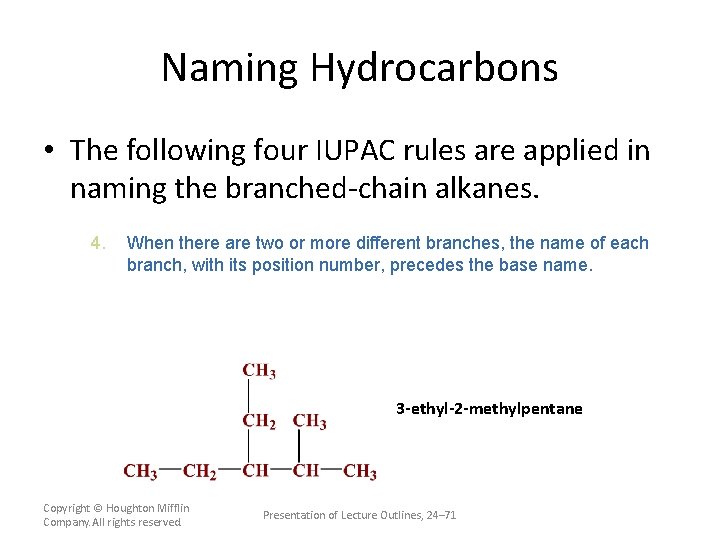
Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 4. When there are two or more different branches, the name of each branch, with its position number, precedes the base name. The branch names are placed in alphabetical order. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 70

Naming Hydrocarbons • The following four IUPAC rules are applied in naming the branched-chain alkanes. 4. When there are two or more different branches, the name of each branch, with its position number, precedes the base name. 3 -ethyl-2 -methylpentane Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 71

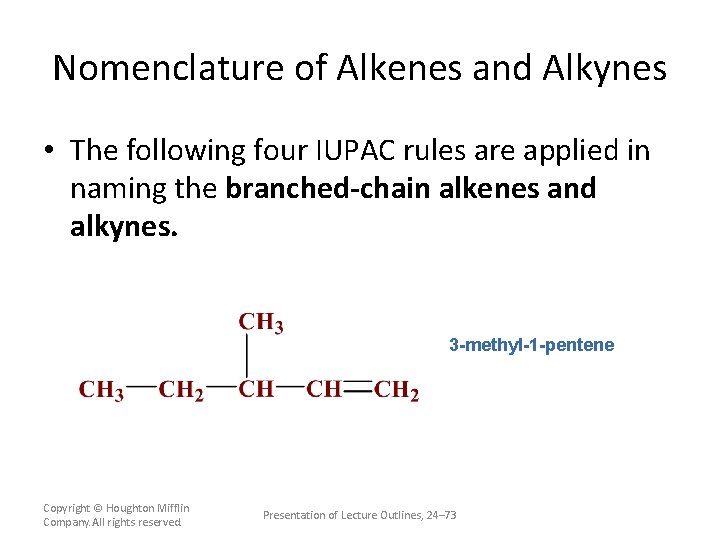
Nomenclature of Alkenes and Alkynes • The following four IUPAC rules are applied in naming the branched-chain alkenes and alkynes. – – The rules are essentially the same as those for alkanes, except that names end in –ene for alkenes and –yne for alkynes. The position of the double (or triple) bond is indicated in the name by bond position number. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 72

Nomenclature of Alkenes and Alkynes • The following four IUPAC rules are applied in naming the branched-chain alkenes and alkynes. 3 -methyl-1 -pentene Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 73

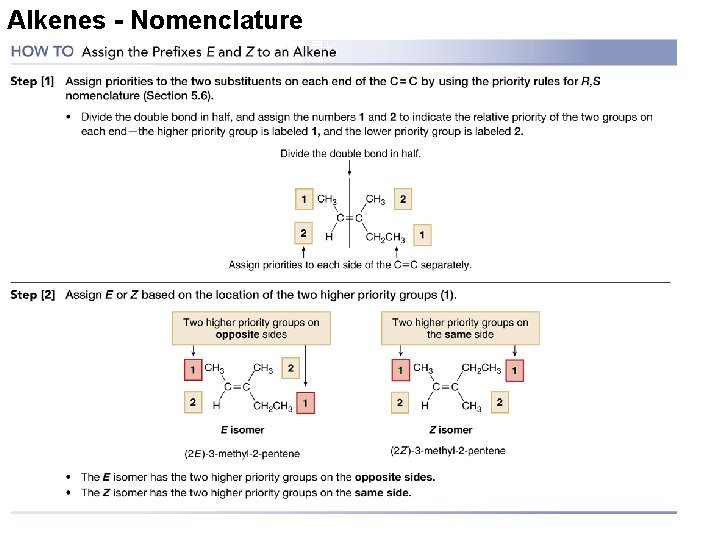
Nomenclature of Alkenes and Alkynes • The following four IUPAC rules are applied in naming the branched-chain alkenes and alkynes. – Recall that alkenes also exhibit cis and trans isomerism and so either cis or trans must be included in the name. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 74

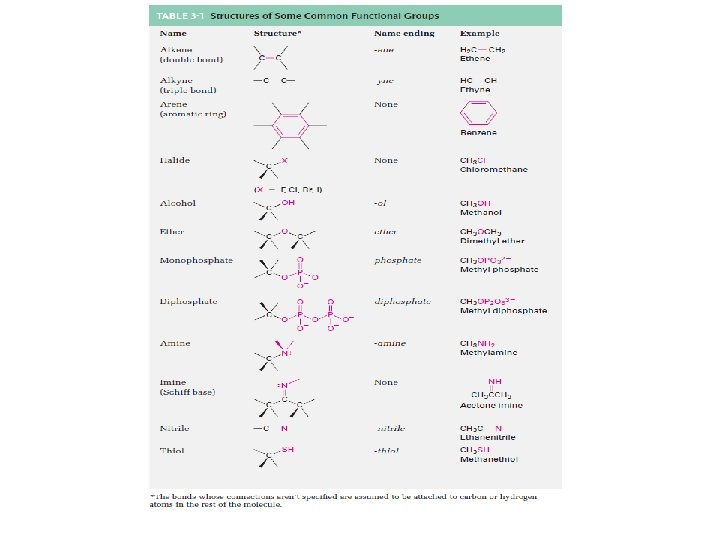
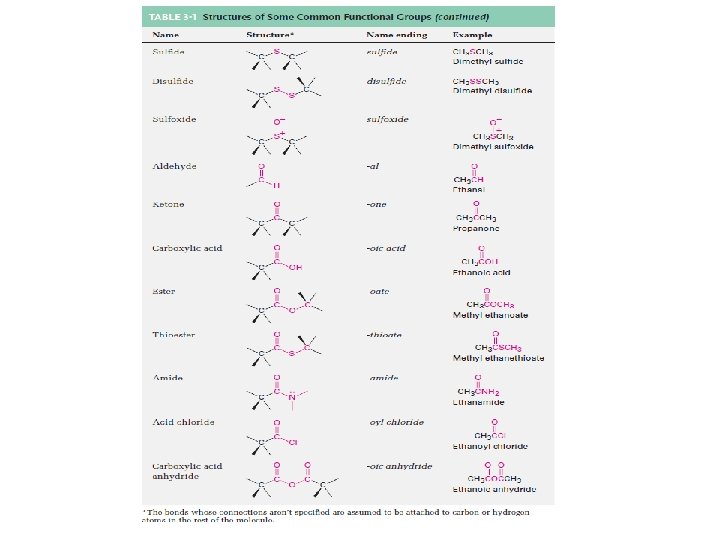
Derivatives of Hydrocarbons • A functional group is a reactive portion of a molecule that undergoes predictable reactions. – Table 24. 7 lists some common organic functional groups. – In the previous sections we discussed the hydrocarbons and their reactions. – All other organic compounds can be considered to be derivatives of hydrocarbons. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 75

Organic Compounds Containing Oxygen • Many of the important functional groups in organic compounds contain oxygen. – Examples are alcohols ethers aldehydes ketones carboxylic acids esters Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 76

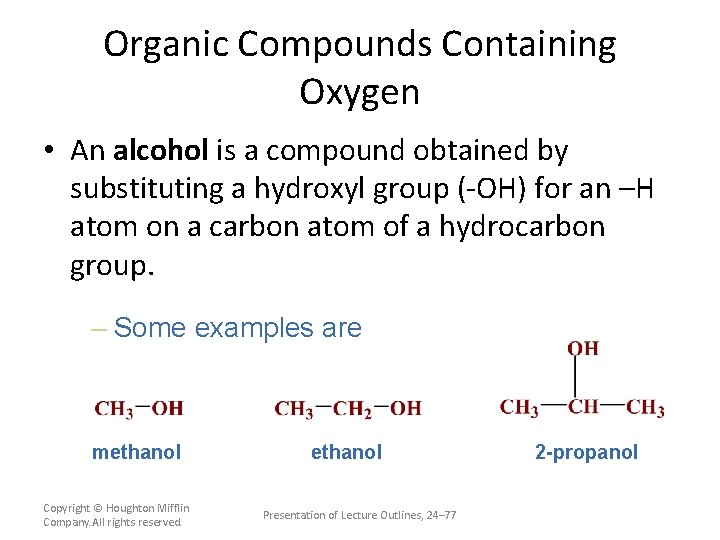
Organic Compounds Containing Oxygen • An alcohol is a compound obtained by substituting a hydroxyl group (-OH) for an –H atom on a carbon atom of a hydrocarbon group. – Some examples are methanol Copyright © Houghton Mifflin Company. All rights reserved. ethanol Presentation of Lecture Outlines, 24– 77 2 -propanol

Organic Compounds Containing Oxygen • An ether is a compound with an oxygen “bridge” between two alkyl groups. – An example is diethyl ether – This is the most common ether, often called simply ether, used as an anesthetic. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 78

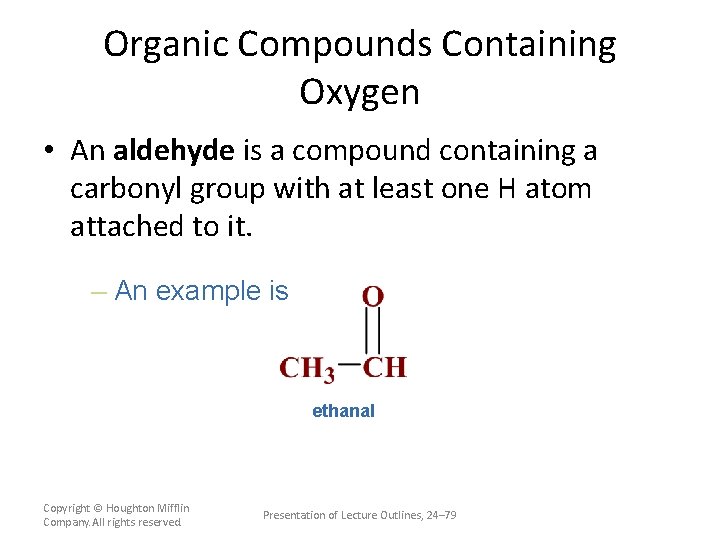
Organic Compounds Containing Oxygen • An aldehyde is a compound containing a carbonyl group with at least one H atom attached to it. – An example is ethanal Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 79

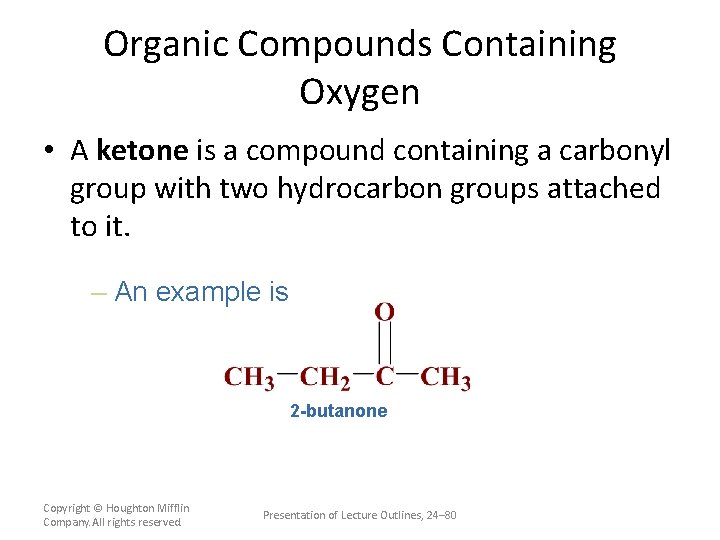
Organic Compounds Containing Oxygen • A ketone is a compound containing a carbonyl group with two hydrocarbon groups attached to it. – An example is 2 -butanone Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 80

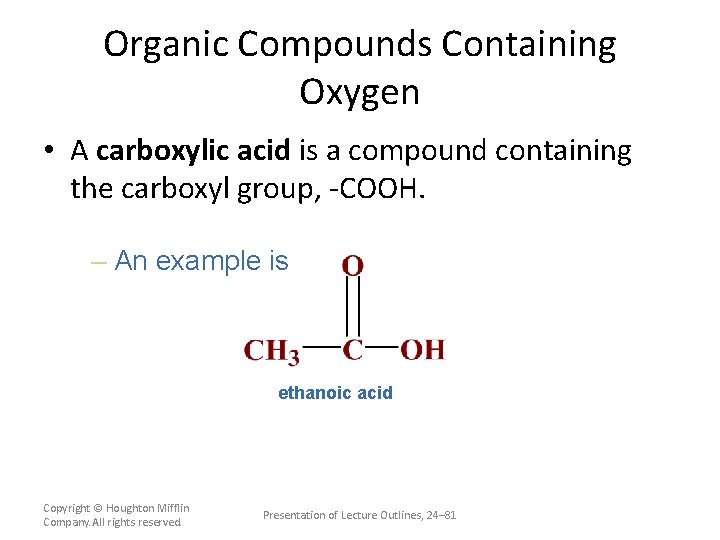
Organic Compounds Containing Oxygen • A carboxylic acid is a compound containing the carboxyl group, -COOH. – An example is ethanoic acid Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 81

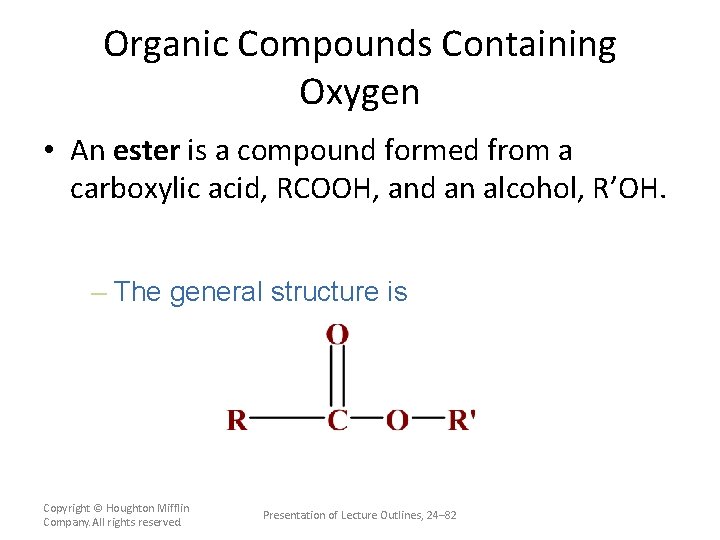
Organic Compounds Containing Oxygen • An ester is a compound formed from a carboxylic acid, RCOOH, and an alcohol, R’OH. – The general structure is Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 82

Organic Compounds Containing Nitrogen • Most organic bases are amines, which are compounds that are structurally derived by replacing one or more hydrogen atoms of ammonia with hydrocarbon groups. primary amine Copyright © Houghton Mifflin Company. All rights reserved. secondary amine Presentation of Lecture Outlines, 24– 83 tertiary amine

Organic Compounds Containing Nitrogen • Most organic bases are amines, which are compounds that are structurally derived by replacing one or more hydrogen atoms of ammonia with hydrocarbon groups. – Table 24. 9 lists some common amines. Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 84

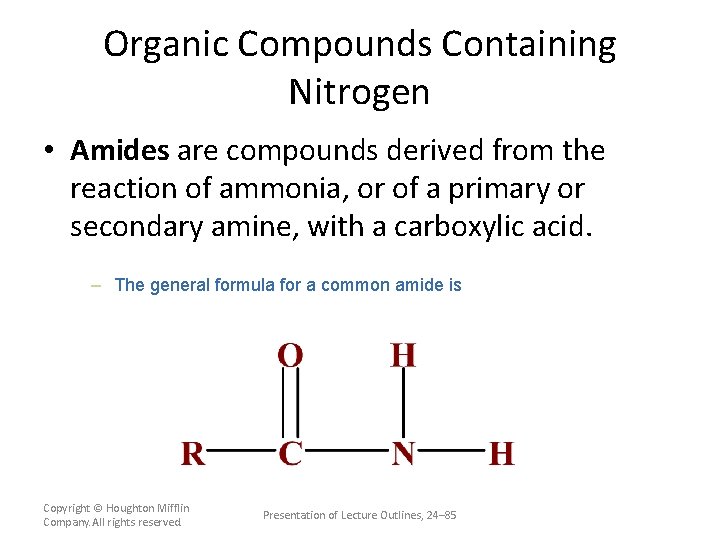
Organic Compounds Containing Nitrogen • Amides are compounds derived from the reaction of ammonia, or of a primary or secondary amine, with a carboxylic acid. – The general formula for a common amide is Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 85

Operational Skills • Writing a condensed structural formula • Predicting cis-trans isomers • Predicting the major product of an addition reaction • Writing the IUPAC name of a hydrocarbon given the structural formula, and vice versa Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 86

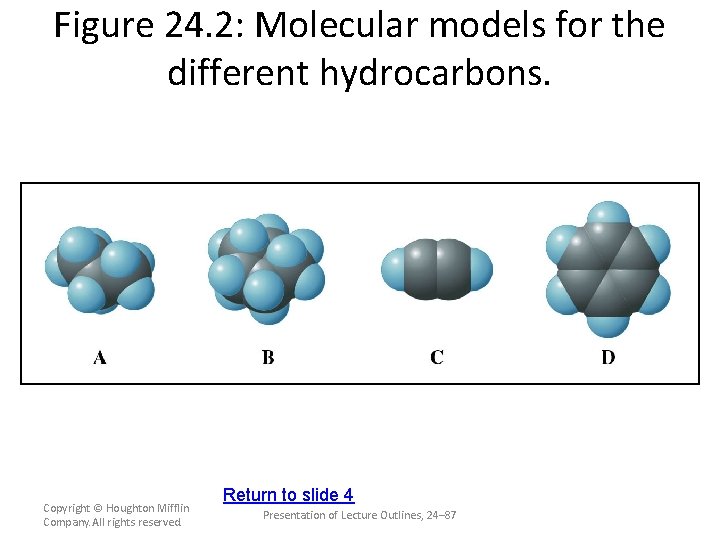
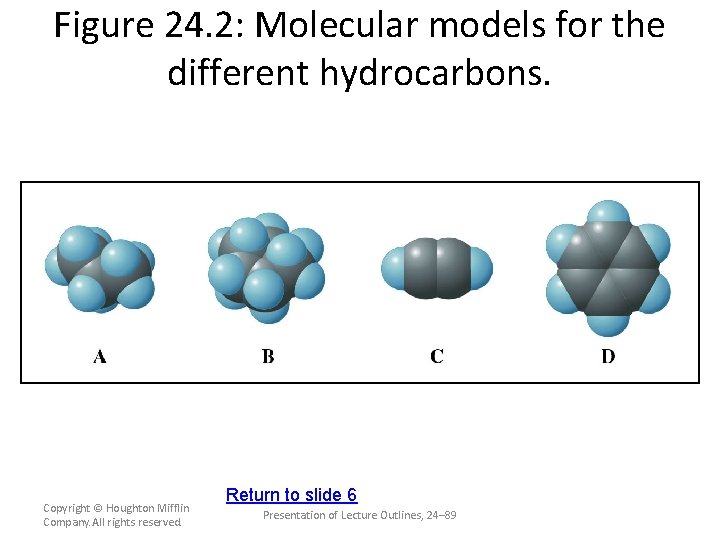
Figure 24. 2: Molecular models for the different hydrocarbons. Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 4 Presentation of Lecture Outlines, 24– 87

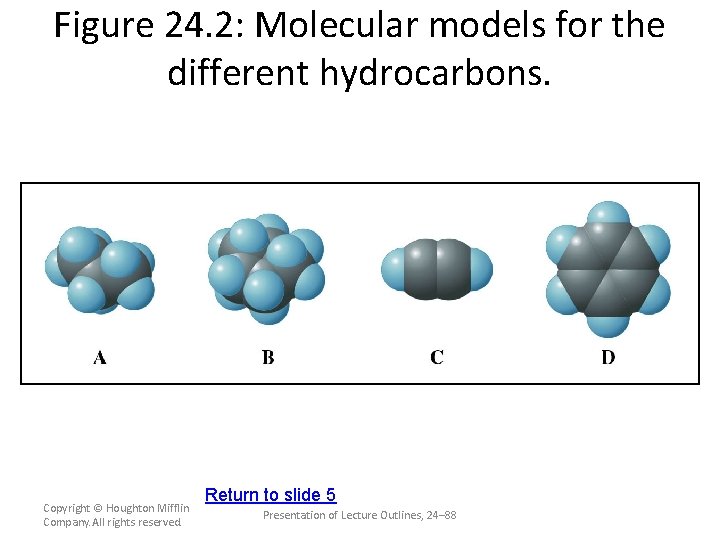
Figure 24. 2: Molecular models for the different hydrocarbons. Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 5 Presentation of Lecture Outlines, 24– 88

Figure 24. 2: Molecular models for the different hydrocarbons. Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 6 Presentation of Lecture Outlines, 24– 89

Figure 24. 3: Three-dimensional models of methane. Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 7 Presentation of Lecture Outlines, 24– 90

Figure 24. 4: Models of isobutane and butane. Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 13 Presentation of Lecture Outlines, 24– 91

Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 16 Presentation of Lecture Outlines, 24– 92

Return to slide 16 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 93

Animation: Carbon-Carbon Double Bond (Click here to open Quick. Time animation) Copyright © Houghton Mifflin Company. All rights reserved. Return to slide 21 Presentation of Lecture Outlines, 24– 94

Return to slide 29 Copyright © Houghton Mifflin Company. All rights reserved. Presentation of Lecture Outlines, 24– 95

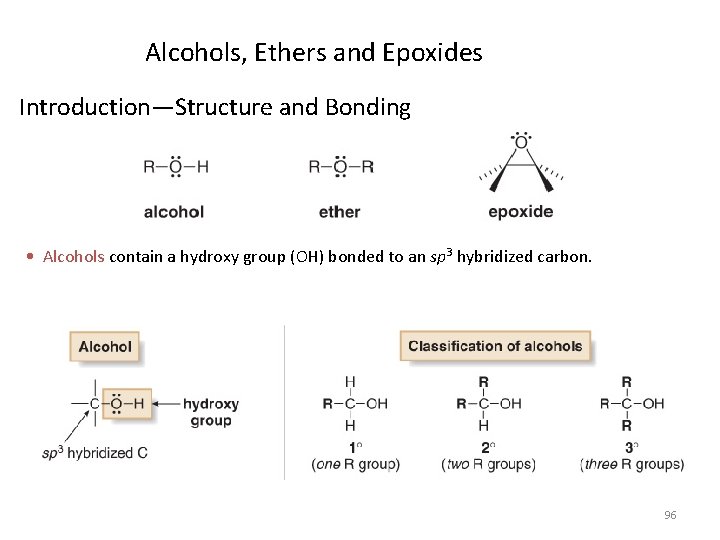
Alcohols, Ethers and Epoxides Introduction—Structure and Bonding • Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 96

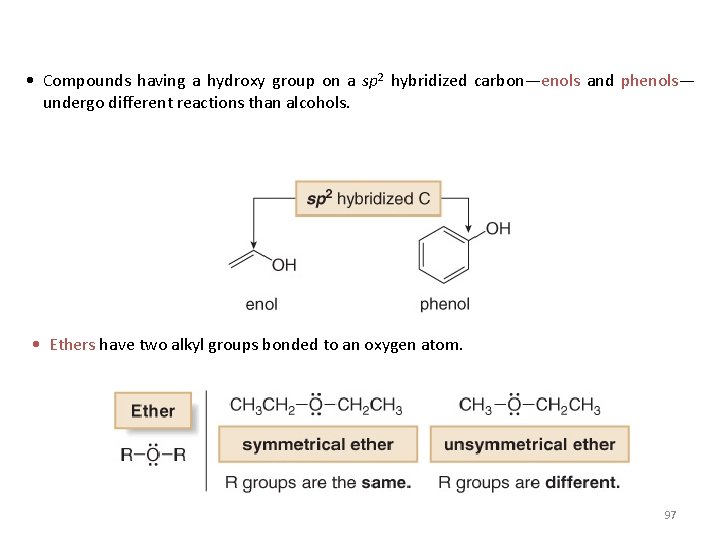
• Compounds having a hydroxy group on a sp 2 hybridized carbon—enols and phenols— undergo different reactions than alcohols. • Ethers have two alkyl groups bonded to an oxygen atom. 97

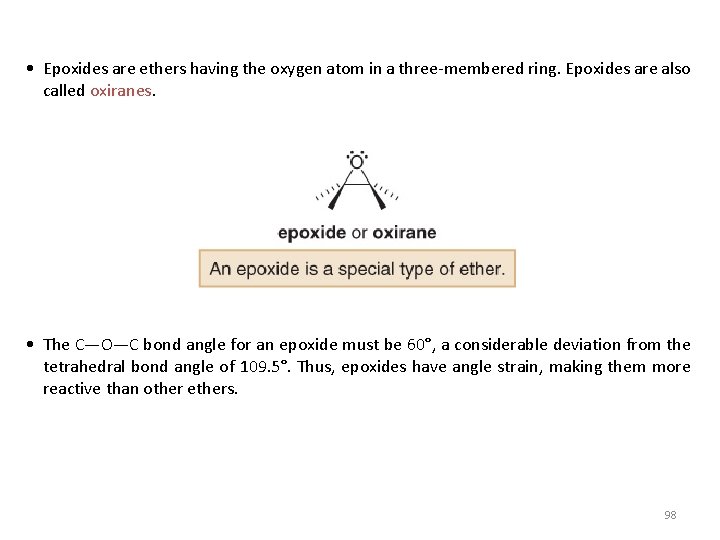
• Epoxides are ethers having the oxygen atom in a three-membered ring. Epoxides are also called oxiranes. • The C—O—C bond angle for an epoxide must be 60°, a considerable deviation from the tetrahedral bond angle of 109. 5°. Thus, epoxides have angle strain, making them more reactive than other ethers. 98

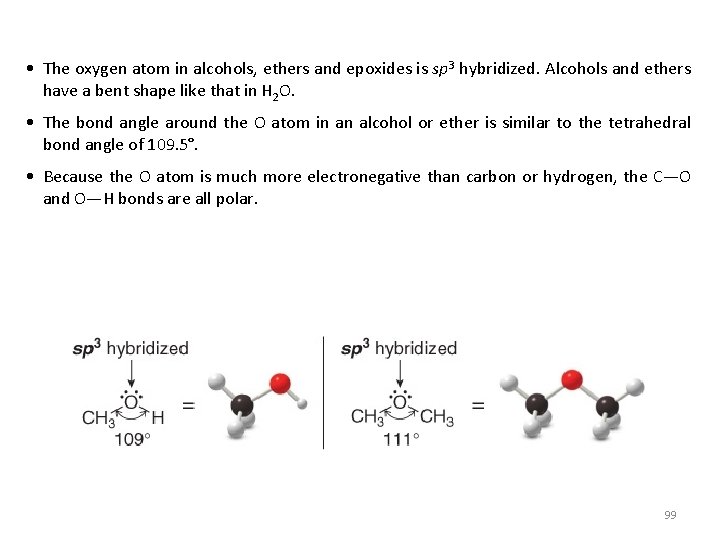
• The oxygen atom in alcohols, ethers and epoxides is sp 3 hybridized. Alcohols and ethers have a bent shape like that in H 2 O. • The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109. 5°. • Because the O atom is much more electronegative than carbon or hydrogen, the C—O and O—H bonds are all polar. 99

100

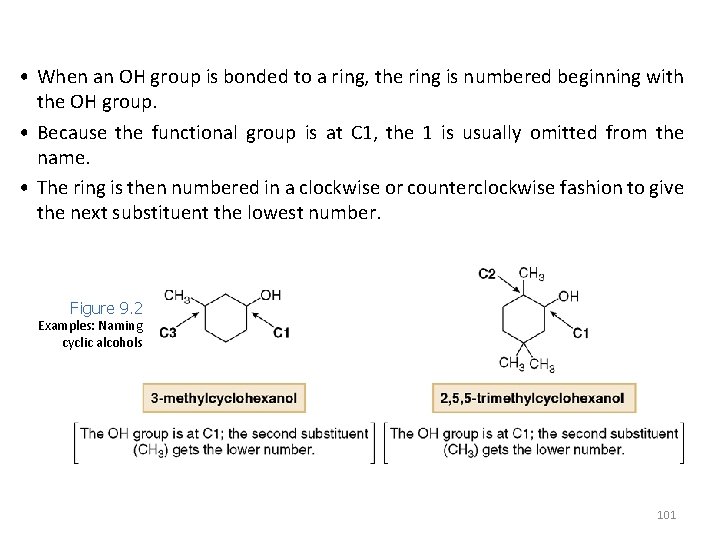
• When an OH group is bonded to a ring, the ring is numbered beginning with the OH group. • Because the functional group is at C 1, the 1 is usually omitted from the name. • The ring is then numbered in a clockwise or counterclockwise fashion to give the next substituent the lowest number. Figure 9. 2 Examples: Naming cyclic alcohols 101

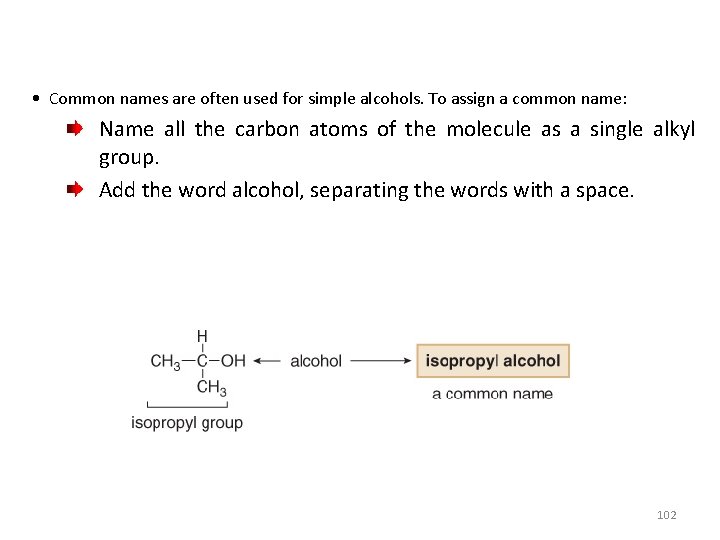
• Common names are often used for simple alcohols. To assign a common name: Name all the carbon atoms of the molecule as a single alkyl group. Add the word alcohol, separating the words with a space. 102

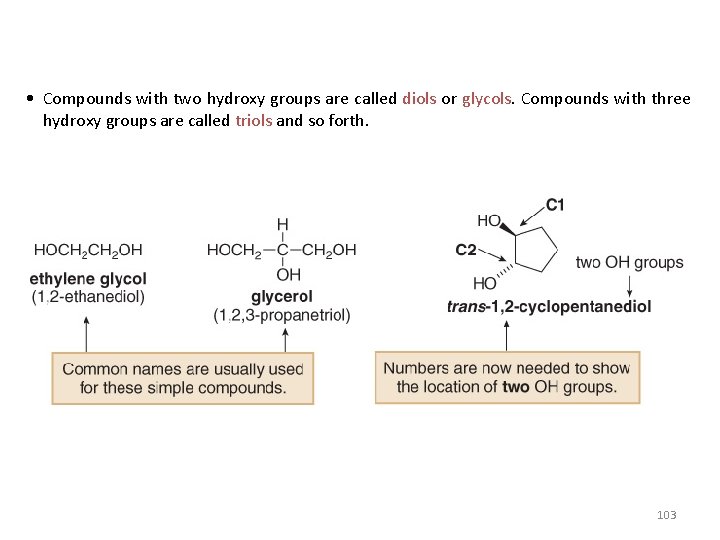
• Compounds with two hydroxy groups are called diols or glycols. Compounds with three hydroxy groups are called triols and so forth. 103

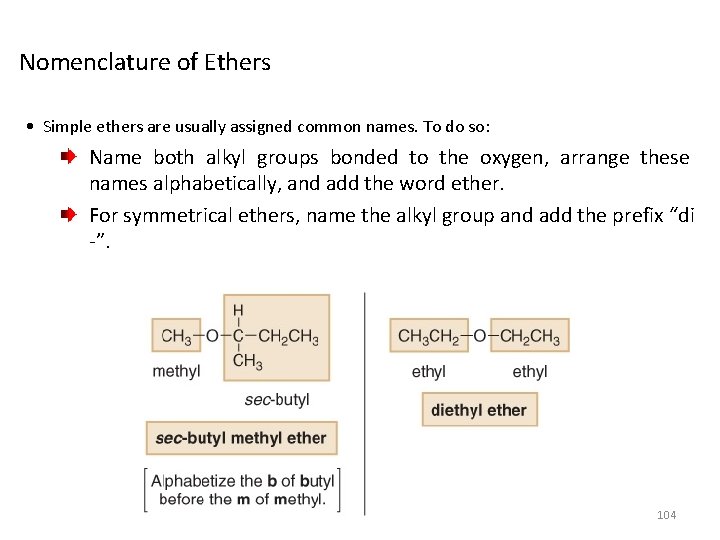
Nomenclature of Ethers • Simple ethers are usually assigned common names. To do so: Name both alkyl groups bonded to the oxygen, arrange these names alphabetically, and add the word ether. For symmetrical ethers, name the alkyl group and add the prefix “di -”. 104

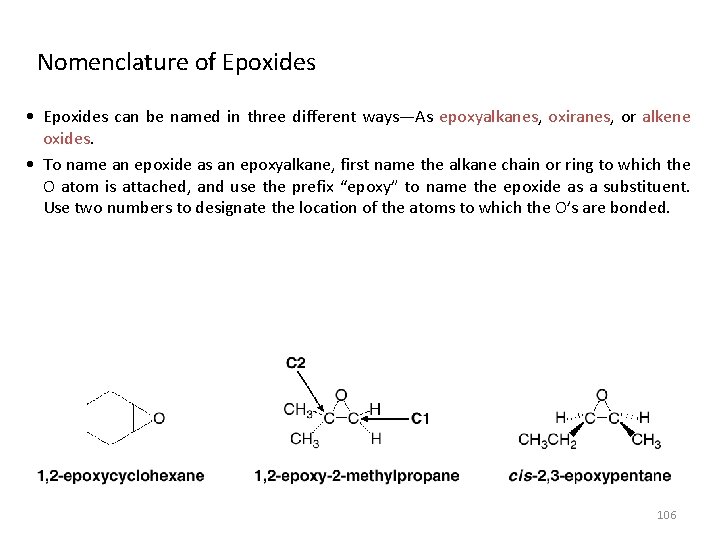
• More complex ethers are named using the IUPAC system. One alkyl group is named as a hydrocarbon chain, and the other is named as part of a substituent bonded to that chain: Name the simpler alkyl group as an alkoxy substituent by changing the –yl ending of the alkyl group to –oxy. Name the remaining alkyl group as an alkane, with the alkoxy group as a substituent bonded to this chain. • Cyclic ethers have an O atom in the ring. A common example is tetrahydrofuran (THF). 105

Nomenclature of Epoxides • Epoxides can be named in three different ways—As epoxyalkanes, oxiranes, or alkene oxides. • To name an epoxide as an epoxyalkane, first name the alkane chain or ring to which the O atom is attached, and use the prefix “epoxy” to name the epoxide as a substituent. Use two numbers to designate the location of the atoms to which the O’s are bonded. 106

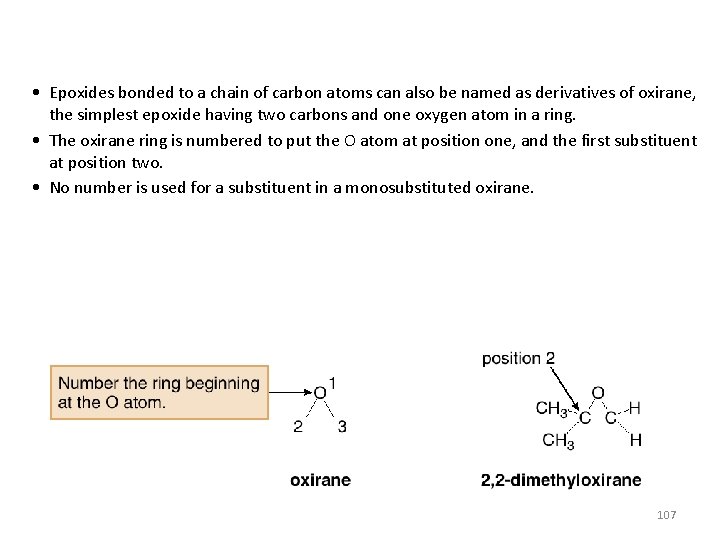
• Epoxides bonded to a chain of carbon atoms can also be named as derivatives of oxirane, the simplest epoxide having two carbons and one oxygen atom in a ring. • The oxirane ring is numbered to put the O atom at position one, and the first substituent at position two. • No number is used for a substituent in a monosubstituted oxirane. 107

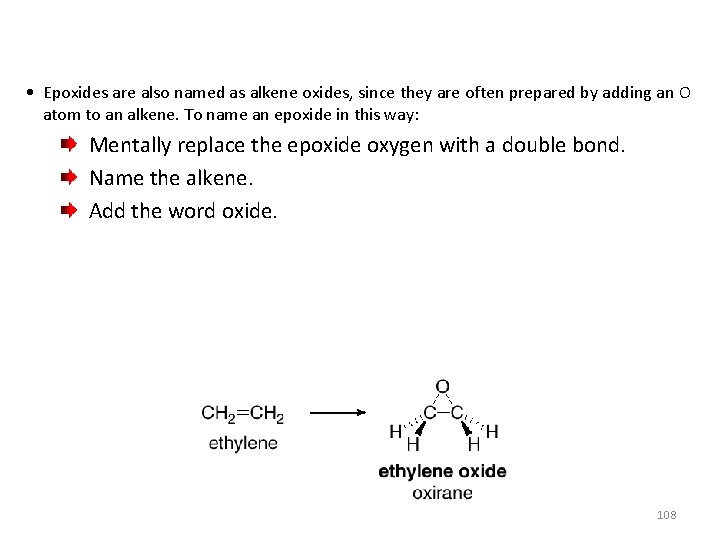
• Epoxides are also named as alkene oxides, since they are often prepared by adding an O atom to an alkene. To name an epoxide in this way: Mentally replace the epoxide oxygen with a double bond. Name the alkene. Add the word oxide. 108

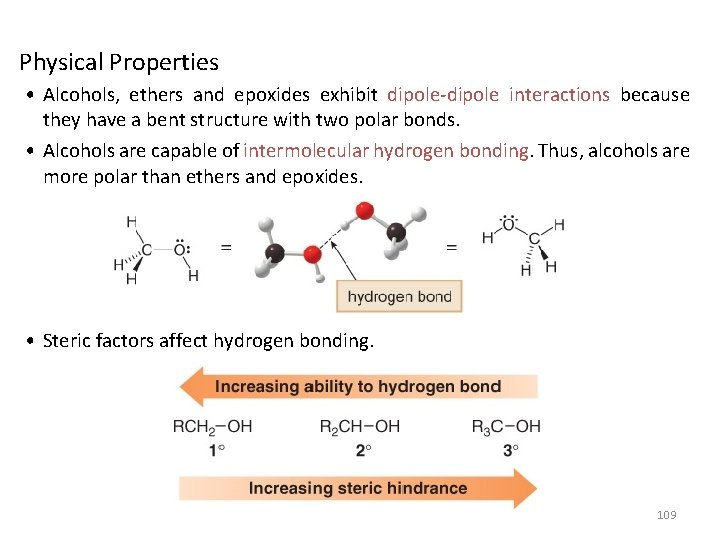
Physical Properties • Alcohols, ethers and epoxides exhibit dipole-dipole interactions because they have a bent structure with two polar bonds. • Alcohols are capable of intermolecular hydrogen bonding. Thus, alcohols are more polar than ethers and epoxides. • Steric factors affect hydrogen bonding. 109

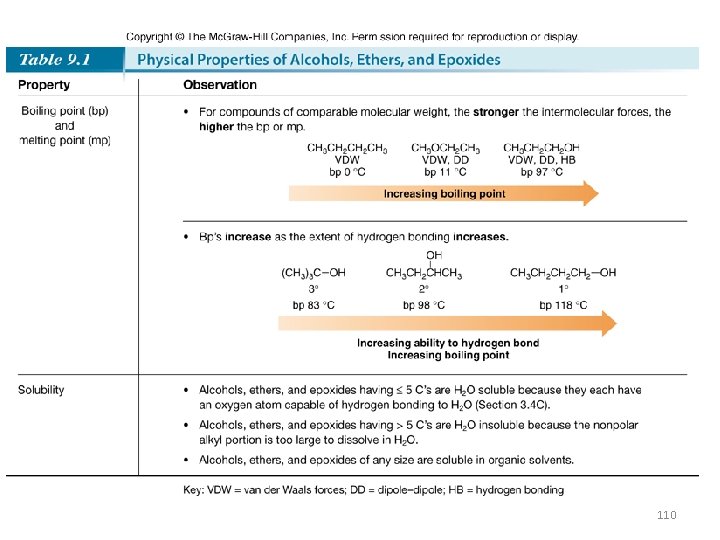
110

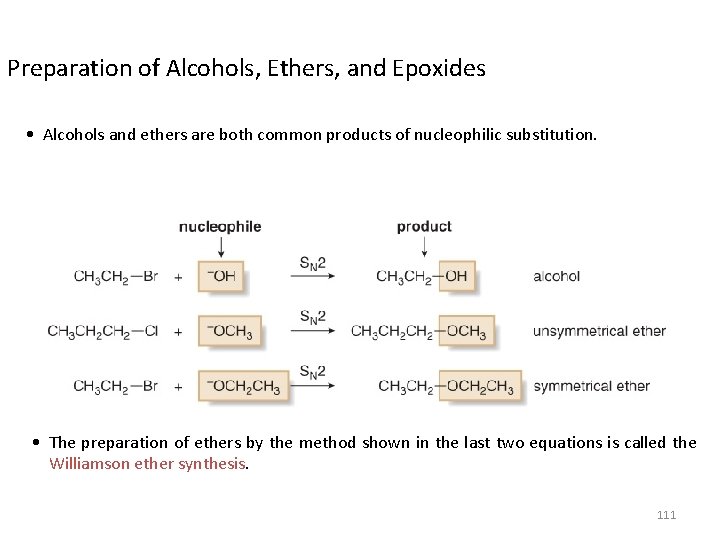
Preparation of Alcohols, Ethers, and Epoxides • Alcohols and ethers are both common products of nucleophilic substitution. • The preparation of ethers by the method shown in the last two equations is called the Williamson ether synthesis. 111

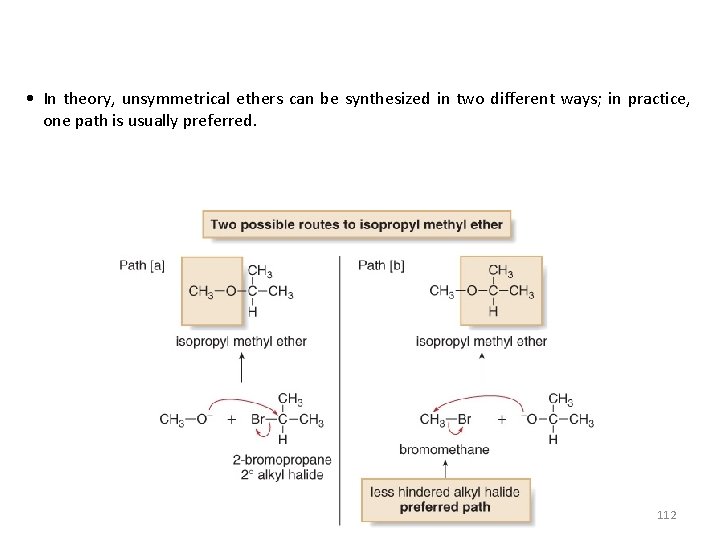
• In theory, unsymmetrical ethers can be synthesized in two different ways; in practice, one path is usually preferred. 112

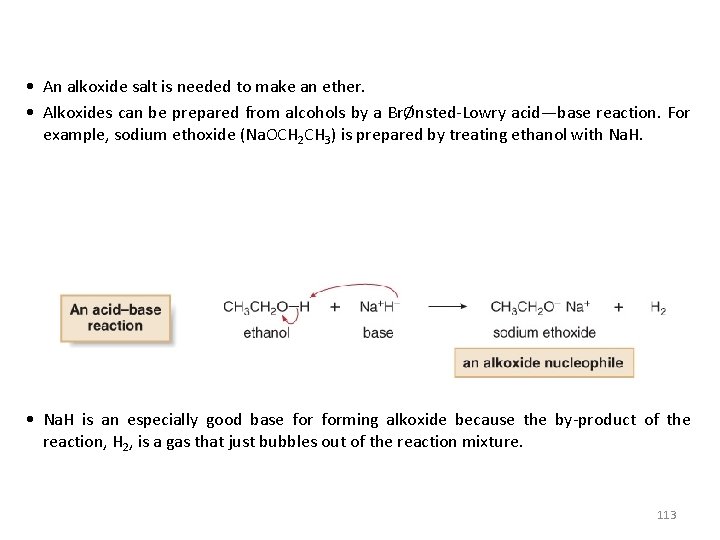
• An alkoxide salt is needed to make an ether. • Alkoxides can be prepared from alcohols by a BrØnsted-Lowry acid—base reaction. For example, sodium ethoxide (Na. OCH 2 CH 3) is prepared by treating ethanol with Na. H. • Na. H is an especially good base forming alkoxide because the by-product of the reaction, H 2, is a gas that just bubbles out of the reaction mixture. 113

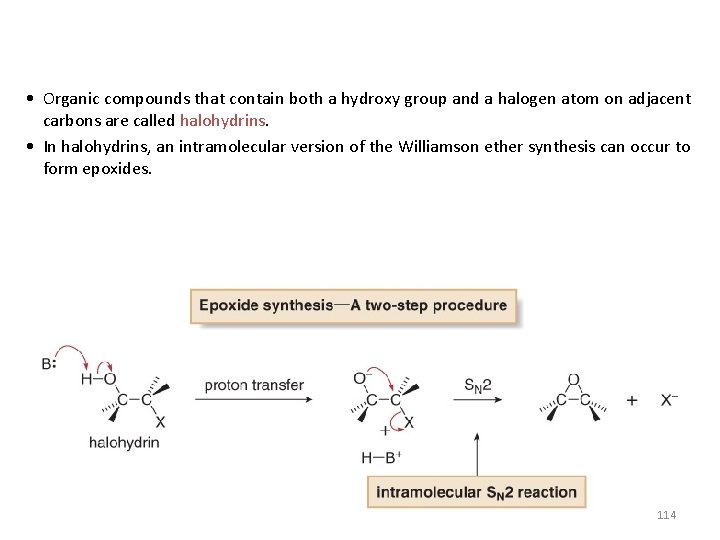
• Organic compounds that contain both a hydroxy group and a halogen atom on adjacent carbons are called halohydrins. • In halohydrins, an intramolecular version of the Williamson ether synthesis can occur to form epoxides. 114

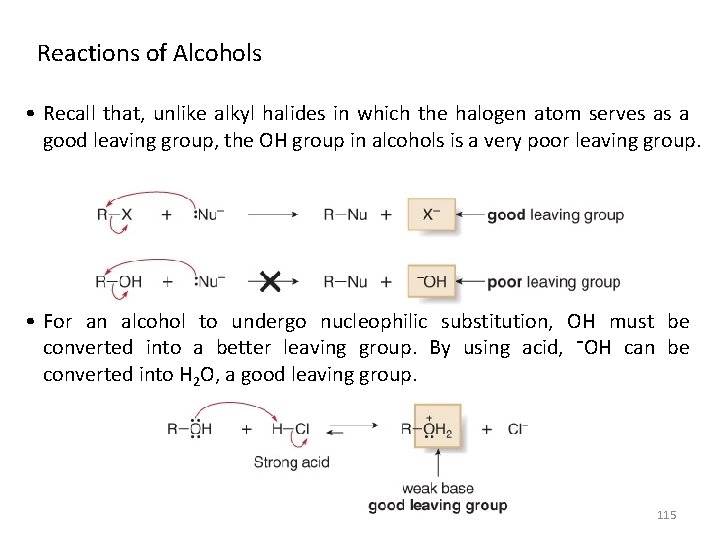
Reactions of Alcohols • Recall that, unlike alkyl halides in which the halogen atom serves as a good leaving group, the OH group in alcohols is a very poor leaving group. • For an alcohol to undergo nucleophilic substitution, OH must be converted into a better leaving group. By using acid, ¯OH can be converted into H 2 O, a good leaving group. 115

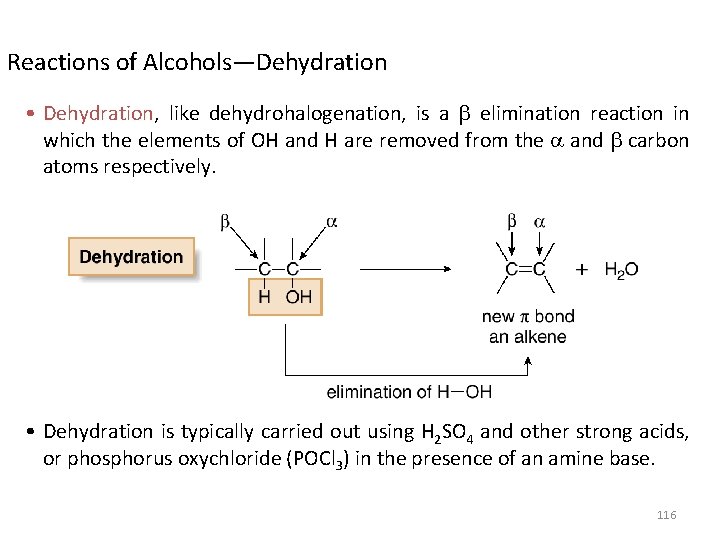
Reactions of Alcohols—Dehydration • Dehydration, like dehydrohalogenation, is a elimination reaction in which the elements of OH and H are removed from the and carbon atoms respectively. • Dehydration is typically carried out using H 2 SO 4 and other strong acids, or phosphorus oxychloride (POCl 3) in the presence of an amine base. 116

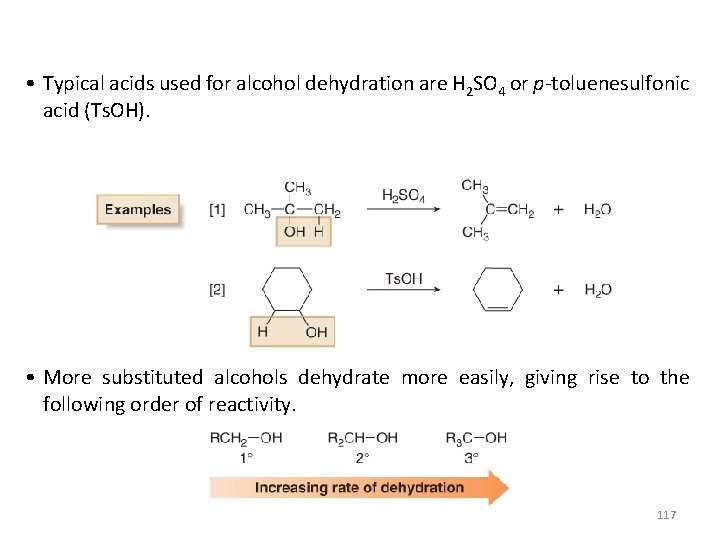
• Typical acids used for alcohol dehydration are H 2 SO 4 or p-toluenesulfonic acid (Ts. OH). • More substituted alcohols dehydrate more easily, giving rise to the following order of reactivity. 117

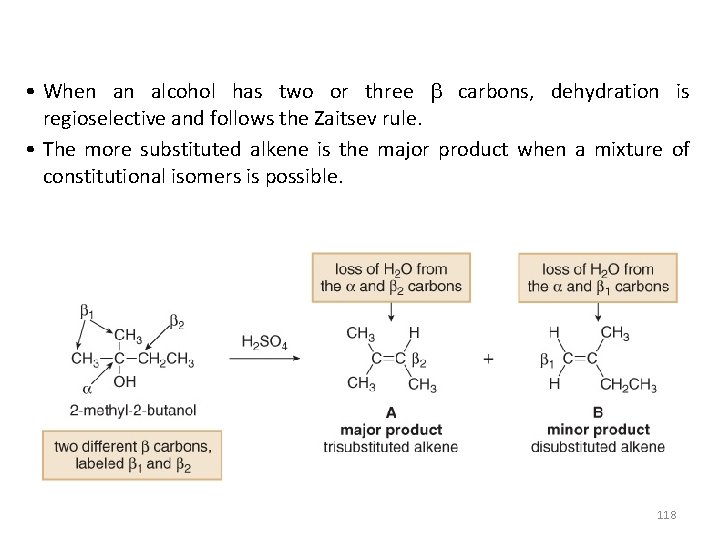
• When an alcohol has two or three carbons, dehydration is regioselective and follows the Zaitsev rule. • The more substituted alkene is the major product when a mixture of constitutional isomers is possible. 118

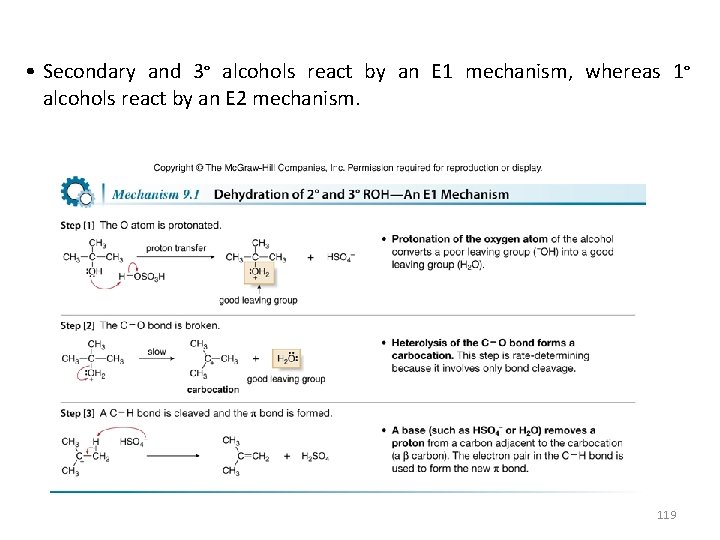
• Secondary and 3° alcohols react by an E 1 mechanism, whereas 1° alcohols react by an E 2 mechanism. 119

• The E 1 dehydration of 20 and 30 alcohols with acid gives clean elimination products without any by-products formed from an SN 1 reaction. • Clean elimination takes place because the reaction mixture contains no good nucleophile to react with the intermediate carbocation, so no competing SN 1 reaction occurs. • This makes the E 1 dehydration of alcohols much more synthetically useful than the E 1 dehydrohalogenation of alkyl halides. 120

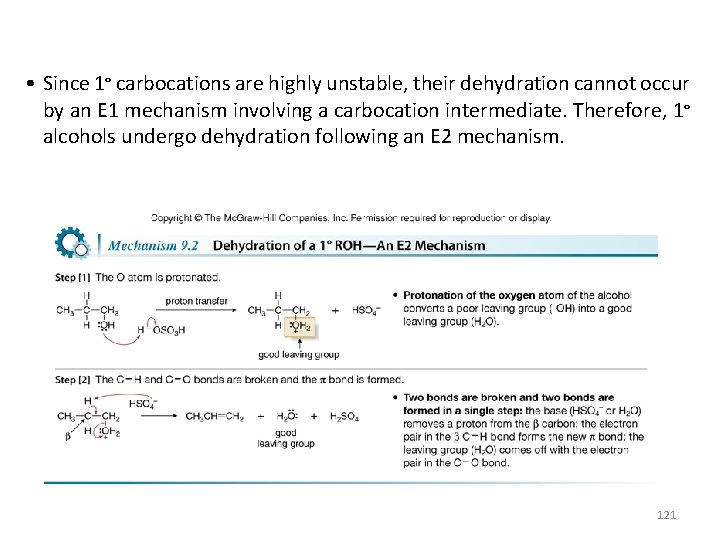
• Since 1° carbocations are highly unstable, their dehydration cannot occur by an E 1 mechanism involving a carbocation intermediate. Therefore, 1° alcohols undergo dehydration following an E 2 mechanism. 121

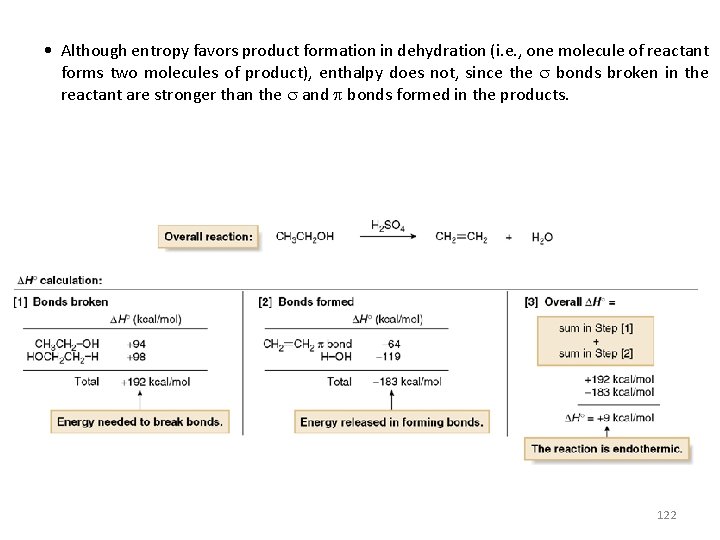
• Although entropy favors product formation in dehydration (i. e. , one molecule of reactant forms two molecules of product), enthalpy does not, since the bonds broken in the reactant are stronger than the and bonds formed in the products. 122

• According to Le Châtelier’s principle, a system at equilibrium will react to counteract any disturbance to the equilibrium. One consequence of this is that removing a product from a reaction mixture as it is formed drives the equilibrium to the right, forming more product. Thus, the alkene, which usually has a lower boiling point than the starting alcohol, can be removed by distillation as it is formed, thus driving the equilibrium to the right to favor production of more product. 123

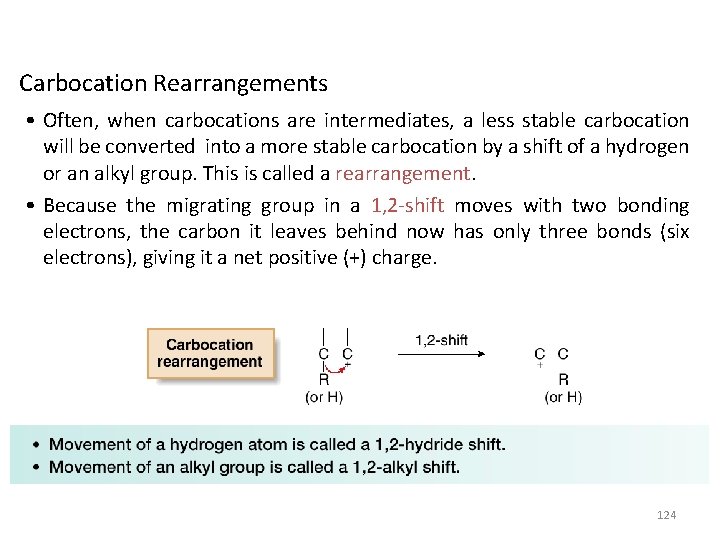
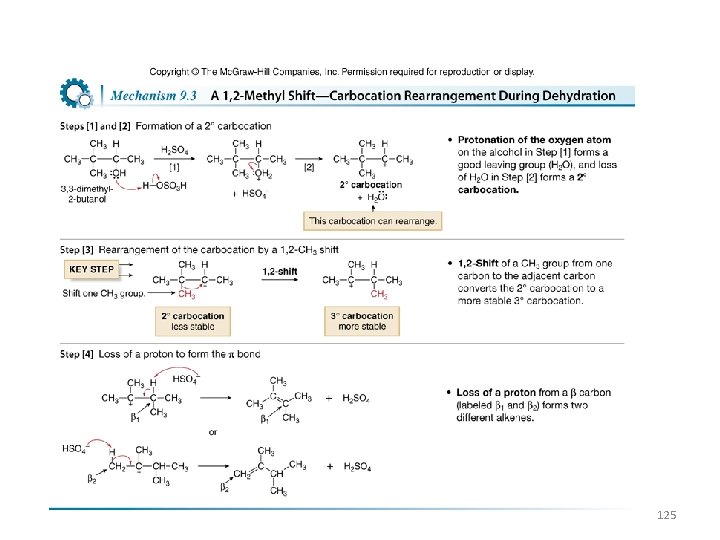
Carbocation Rearrangements • Often, when carbocations are intermediates, a less stable carbocation will be converted into a more stable carbocation by a shift of a hydrogen or an alkyl group. This is called a rearrangement. • Because the migrating group in a 1, 2 -shift moves with two bonding electrons, the carbon it leaves behind now has only three bonds (six electrons), giving it a net positive (+) charge. 124

125

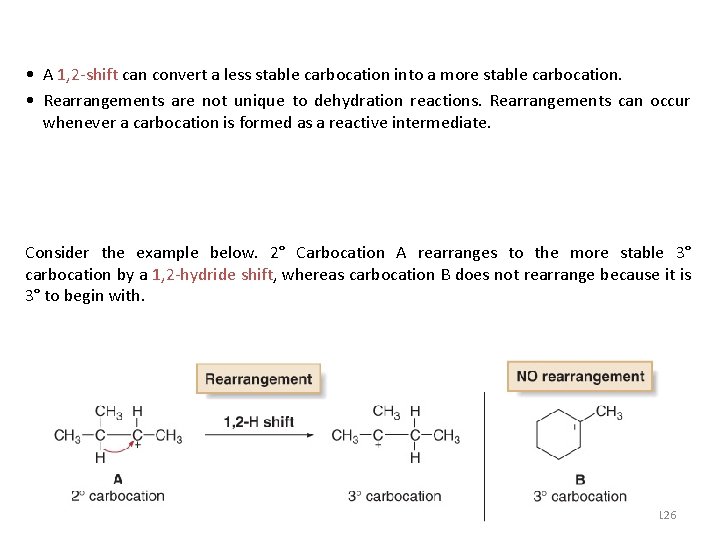
• A 1, 2 -shift can convert a less stable carbocation into a more stable carbocation. • Rearrangements are not unique to dehydration reactions. Rearrangements can occur whenever a carbocation is formed as a reactive intermediate. Consider the example below. 2° Carbocation A rearranges to the more stable 3° carbocation by a 1, 2 -hydride shift, whereas carbocation B does not rearrange because it is 3° to begin with. 126

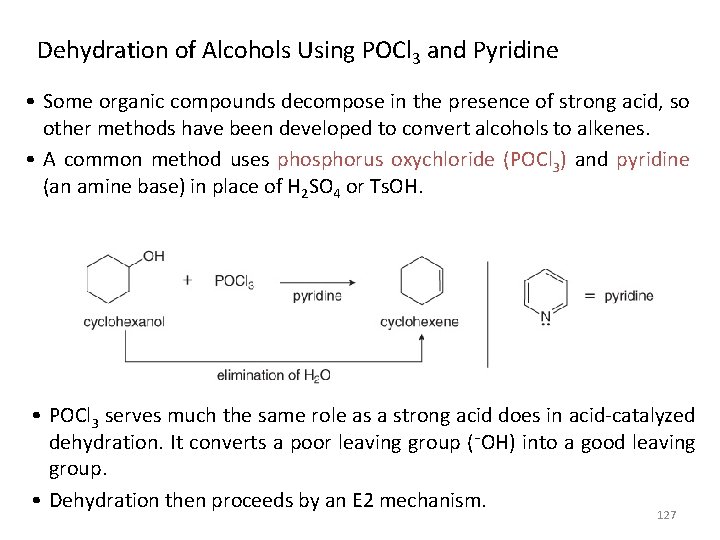
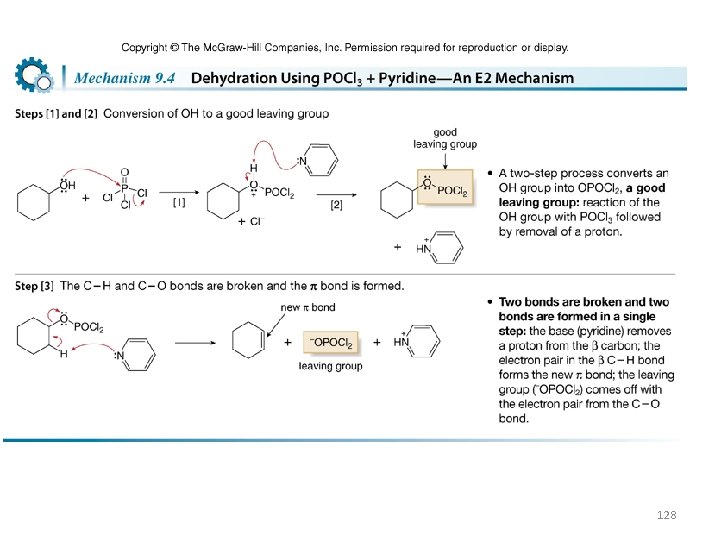
Dehydration of Alcohols Using POCl 3 and Pyridine • Some organic compounds decompose in the presence of strong acid, so other methods have been developed to convert alcohols to alkenes. • A common method uses phosphorus oxychloride (POCl 3) and pyridine (an amine base) in place of H 2 SO 4 or Ts. OH. • POCl 3 serves much the same role as a strong acid does in acid-catalyzed dehydration. It converts a poor leaving group (¯OH) into a good leaving group. • Dehydration then proceeds by an E 2 mechanism. 127

128

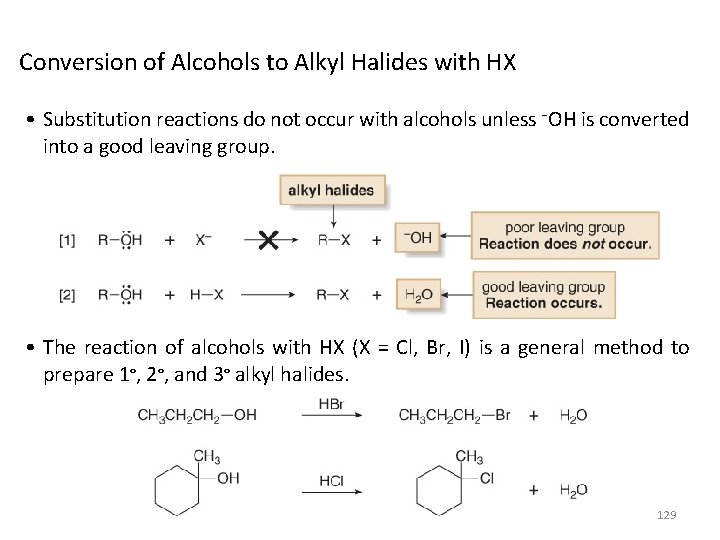
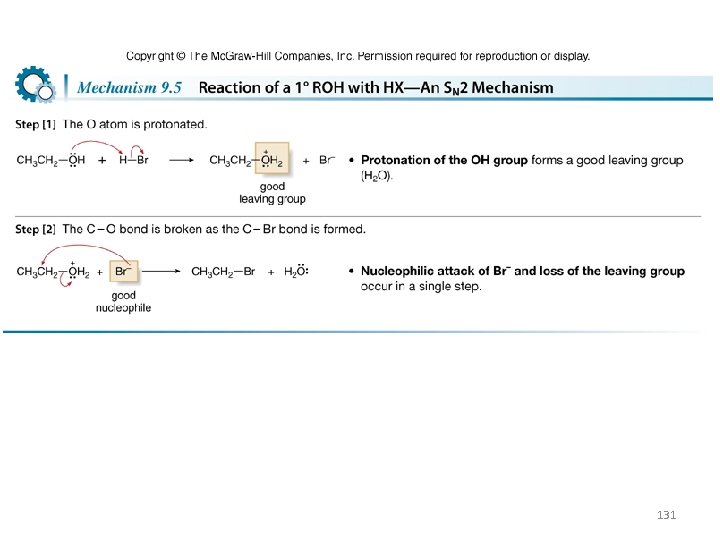
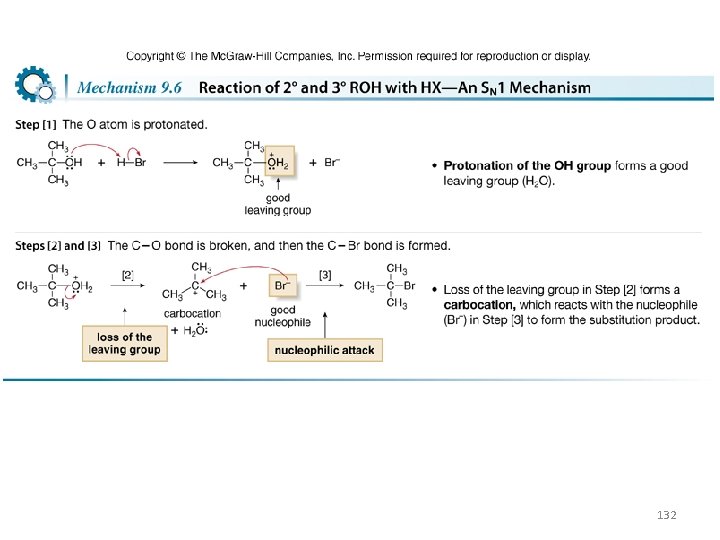
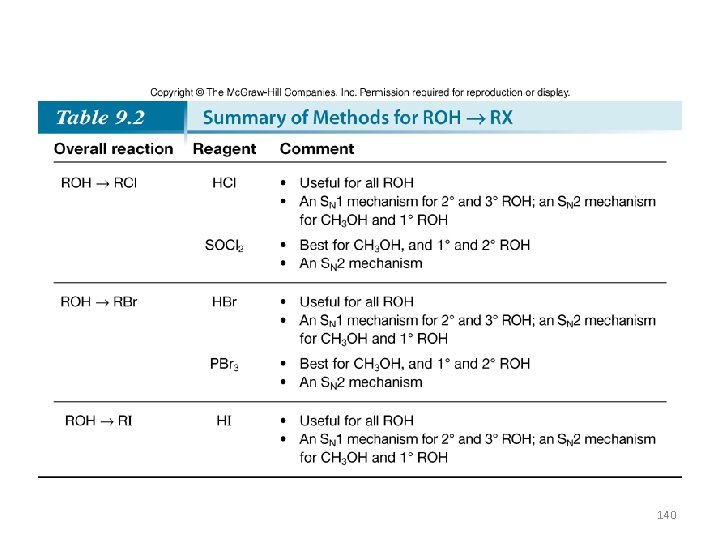
Conversion of Alcohols to Alkyl Halides with HX • Substitution reactions do not occur with alcohols unless ¯OH is converted into a good leaving group. • The reaction of alcohols with HX (X = Cl, Br, I) is a general method to prepare 1°, 2°, and 3° alkyl halides. 129

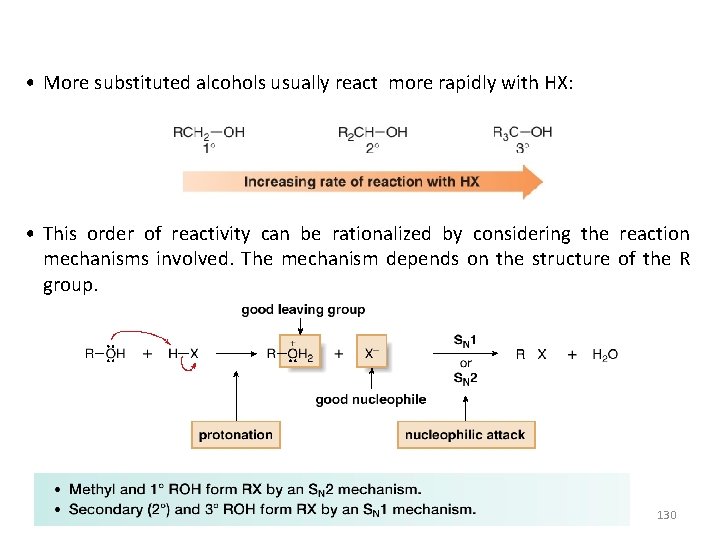
• More substituted alcohols usually react more rapidly with HX: • This order of reactivity can be rationalized by considering the reaction mechanisms involved. The mechanism depends on the structure of the R group. 130

131

132

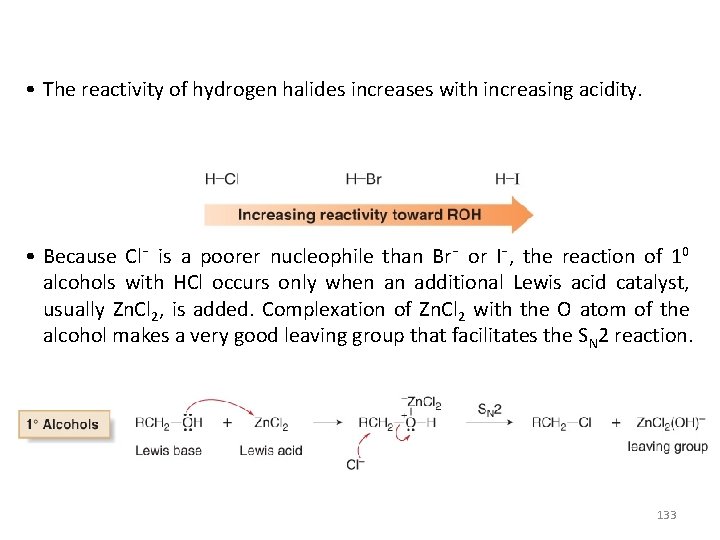
• The reactivity of hydrogen halides increases with increasing acidity. • Because Cl¯ is a poorer nucleophile than Br¯ or I¯, the reaction of 10 alcohols with HCl occurs only when an additional Lewis acid catalyst, usually Zn. Cl 2, is added. Complexation of Zn. Cl 2 with the O atom of the alcohol makes a very good leaving group that facilitates the SN 2 reaction. 133

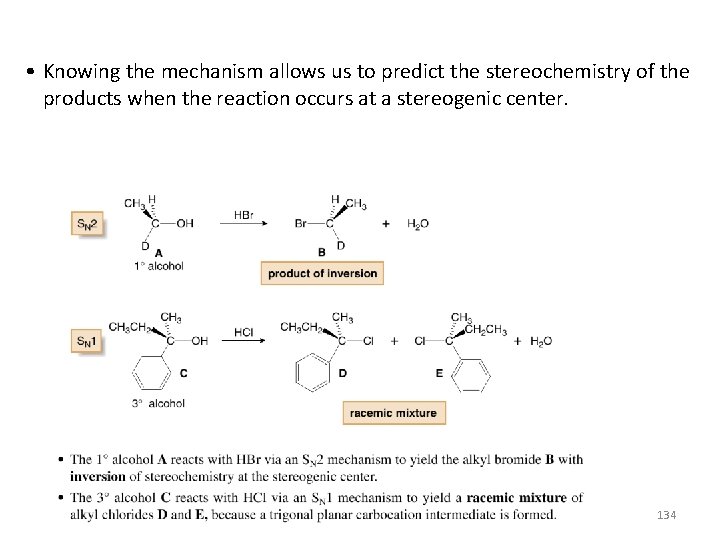
• Knowing the mechanism allows us to predict the stereochemistry of the products when the reaction occurs at a stereogenic center. 134

Conversion of Alcohols to Alkyl Halides with SOCl 2 and PBr 3 • • Primary and 2° alcohols can be converted to alkyl halides using SOCl 2 and PBr 3. SOCl 2 (thionyl chloride) converts alcohols into alkyl chlorides. PBr 3 (phosphorus tribromide) converts alcohols into alkyl bromides. Both reagents convert ¯OH into a good leaving group in situ—that is, directly in the reaction mixture—as well as provide the nucleophile, either Cl¯ or Br¯, to displace the leaving group. 135

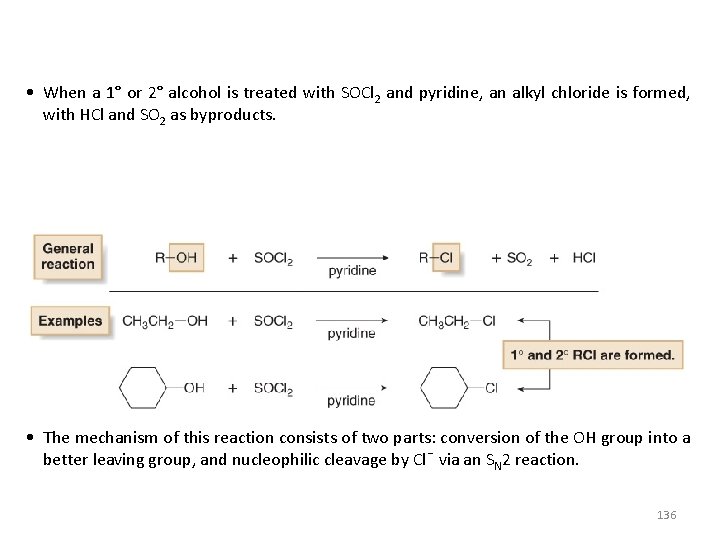
• When a 1° or 2° alcohol is treated with SOCl 2 and pyridine, an alkyl chloride is formed, with HCl and SO 2 as byproducts. • The mechanism of this reaction consists of two parts: conversion of the OH group into a better leaving group, and nucleophilic cleavage by Cl¯ via an SN 2 reaction. 136

137

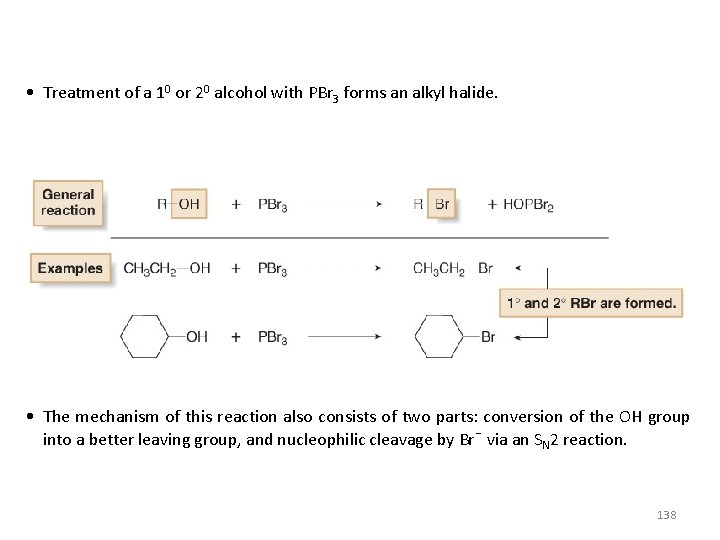
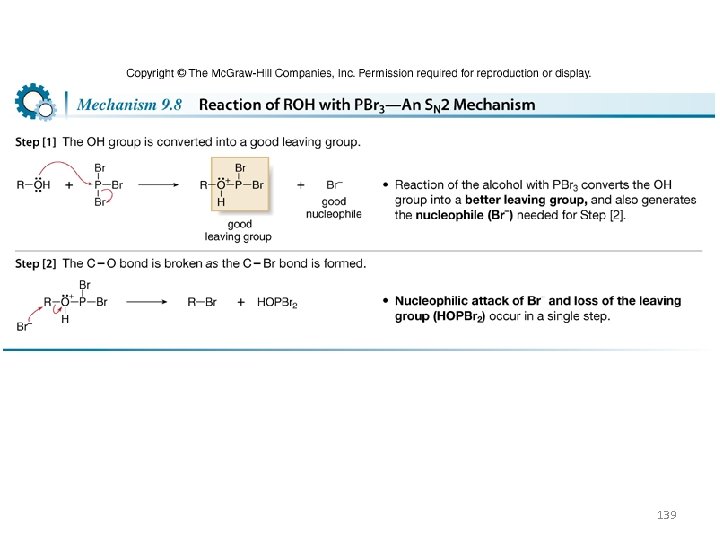
• Treatment of a 10 or 20 alcohol with PBr 3 forms an alkyl halide. • The mechanism of this reaction also consists of two parts: conversion of the OH group into a better leaving group, and nucleophilic cleavage by Br¯ via an SN 2 reaction. 138

139

140

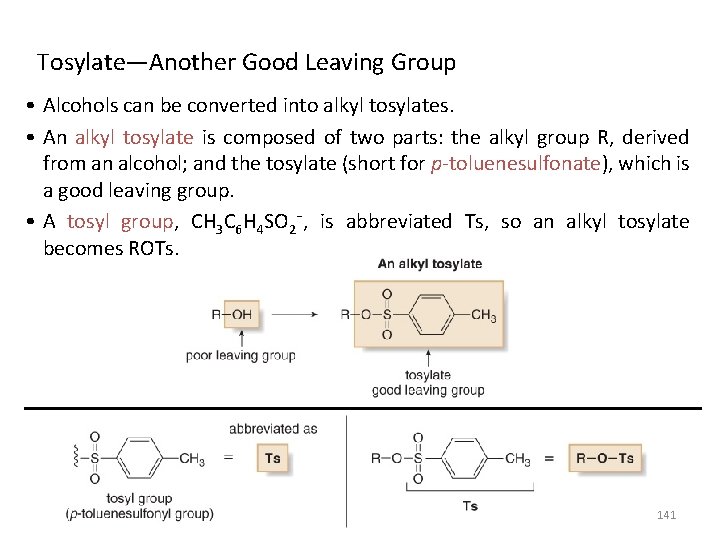
Tosylate—Another Good Leaving Group • Alcohols can be converted into alkyl tosylates. • An alkyl tosylate is composed of two parts: the alkyl group R, derived from an alcohol; and the tosylate (short for p-toluenesulfonate), which is a good leaving group. • A tosyl group, CH 3 C 6 H 4 SO 2¯, is abbreviated Ts, so an alkyl tosylate becomes ROTs. 141

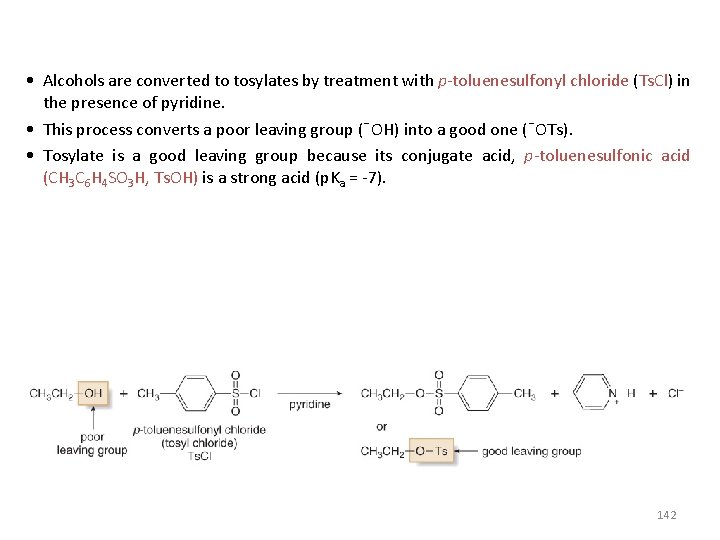
• Alcohols are converted to tosylates by treatment with p-toluenesulfonyl chloride (Ts. Cl) in the presence of pyridine. • This process converts a poor leaving group (¯OH) into a good one (¯OTs). • Tosylate is a good leaving group because its conjugate acid, p-toluenesulfonic acid (CH 3 C 6 H 4 SO 3 H, Ts. OH) is a strong acid (p. Ka = -7). 142

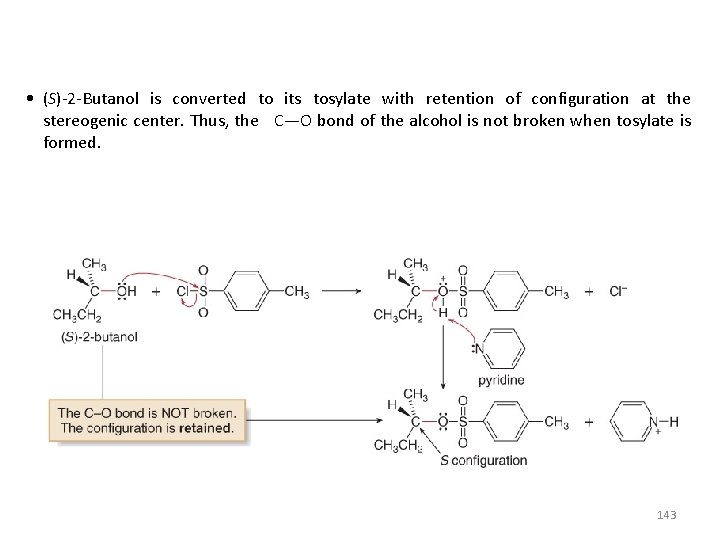
• (S)-2 -Butanol is converted to its tosylate with retention of configuration at the stereogenic center. Thus, the C—O bond of the alcohol is not broken when tosylate is formed. 143

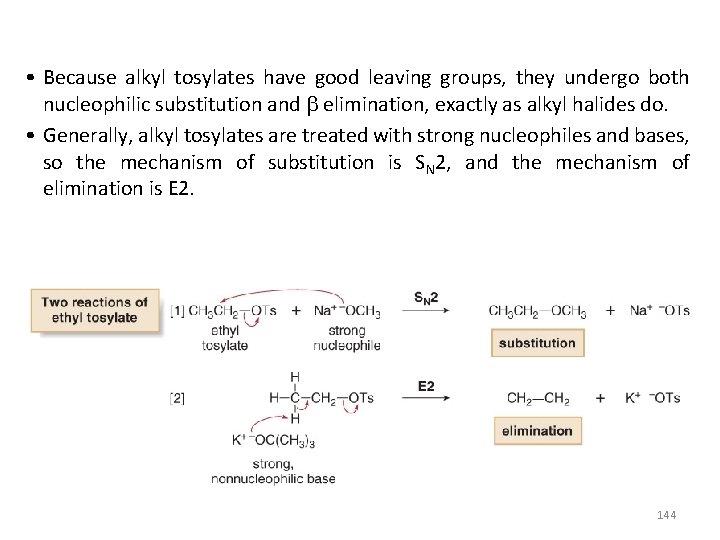
• Because alkyl tosylates have good leaving groups, they undergo both nucleophilic substitution and elimination, exactly as alkyl halides do. • Generally, alkyl tosylates are treated with strong nucleophiles and bases, so the mechanism of substitution is SN 2, and the mechanism of elimination is E 2. 144

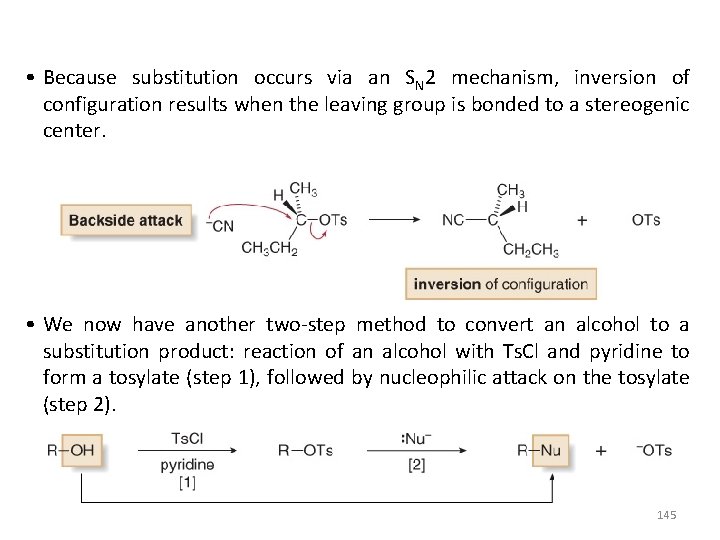
• Because substitution occurs via an SN 2 mechanism, inversion of configuration results when the leaving group is bonded to a stereogenic center. • We now have another two-step method to convert an alcohol to a substitution product: reaction of an alcohol with Ts. Cl and pyridine to form a tosylate (step 1), followed by nucleophilic attack on the tosylate (step 2). 145

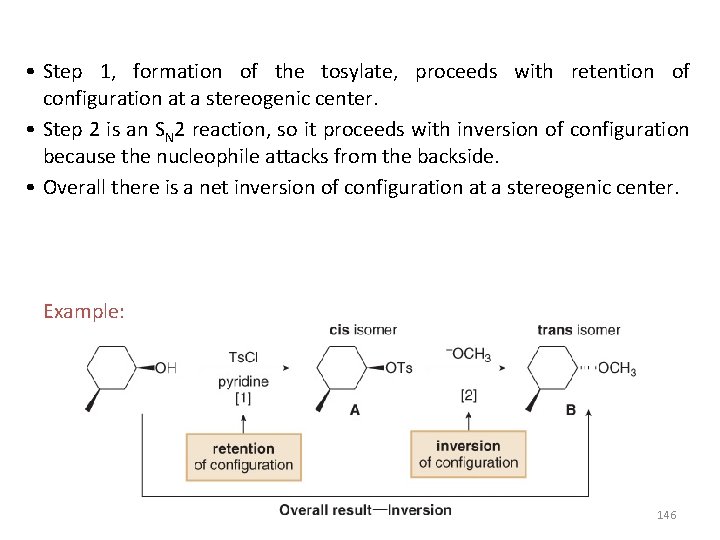
• Step 1, formation of the tosylate, proceeds with retention of configuration at a stereogenic center. • Step 2 is an SN 2 reaction, so it proceeds with inversion of configuration because the nucleophile attacks from the backside. • Overall there is a net inversion of configuration at a stereogenic center. Example: 146

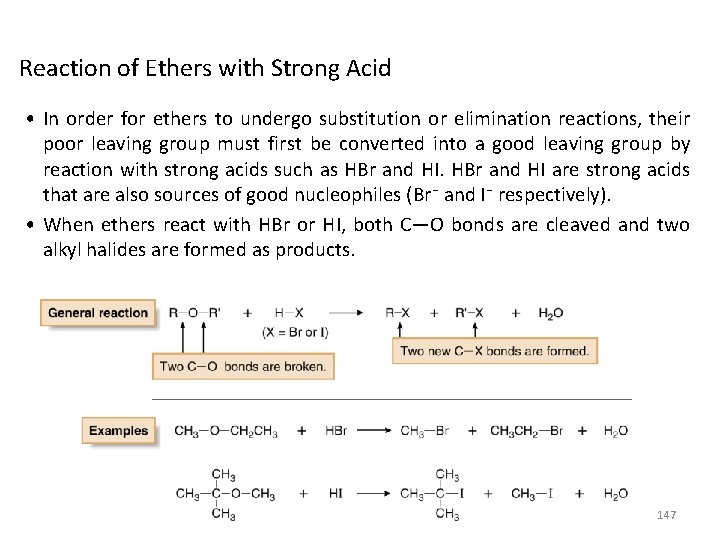
Reaction of Ethers with Strong Acid • In order for ethers to undergo substitution or elimination reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯ respectively). • When ethers react with HBr or HI, both C—O bonds are cleaved and two alkyl halides are formed as products. 147

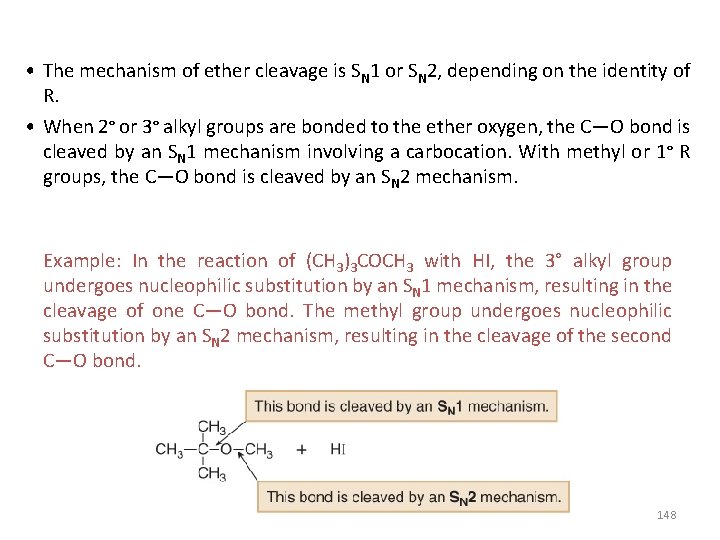
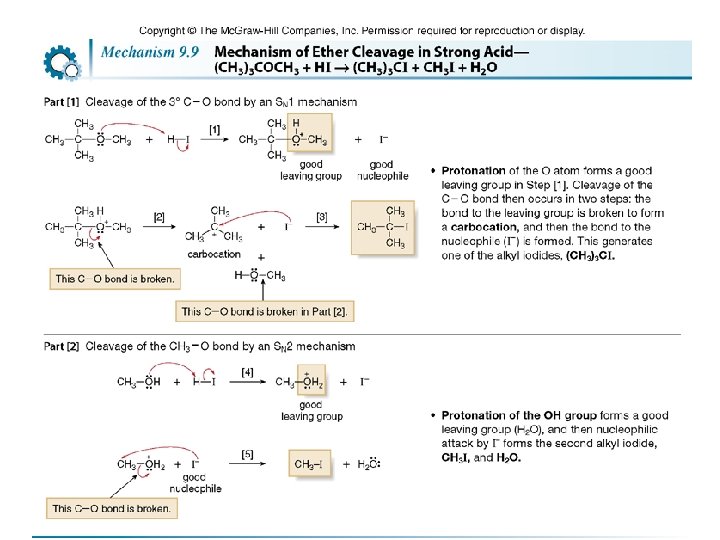
• The mechanism of ether cleavage is SN 1 or SN 2, depending on the identity of R. • When 2° or 3° alkyl groups are bonded to the ether oxygen, the C—O bond is cleaved by an SN 1 mechanism involving a carbocation. With methyl or 1° R groups, the C—O bond is cleaved by an SN 2 mechanism. Example: In the reaction of (CH 3)3 COCH 3 with HI, the 3° alkyl group undergoes nucleophilic substitution by an SN 1 mechanism, resulting in the cleavage of one C—O bond. The methyl group undergoes nucleophilic substitution by an SN 2 mechanism, resulting in the cleavage of the second C—O bond. 148

149

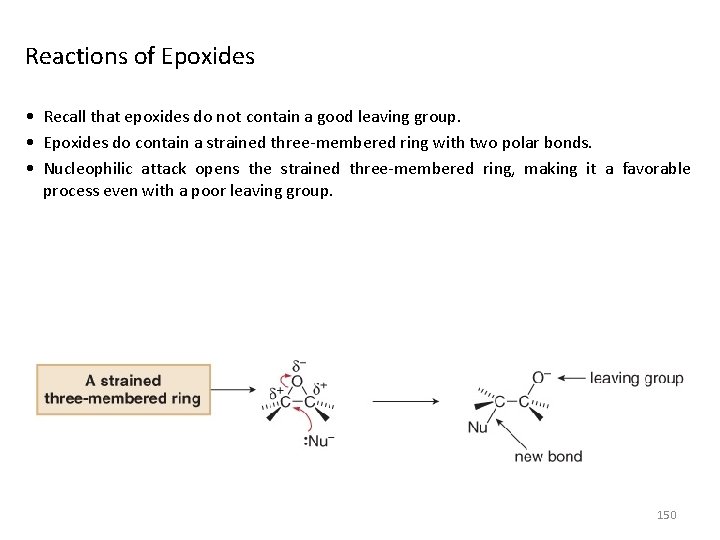
Reactions of Epoxides • Recall that epoxides do not contain a good leaving group. • Epoxides do contain a strained three-membered ring with two polar bonds. • Nucleophilic attack opens the strained three-membered ring, making it a favorable process even with a poor leaving group. 150

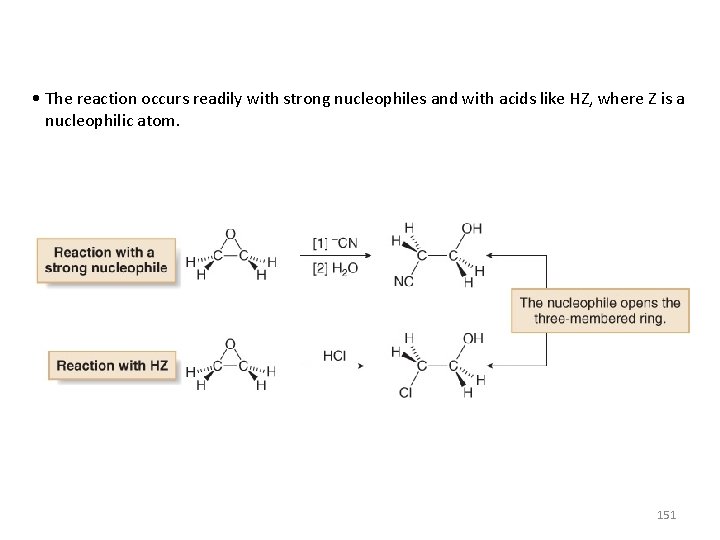
• The reaction occurs readily with strong nucleophiles and with acids like HZ, where Z is a nucleophilic atom. 151

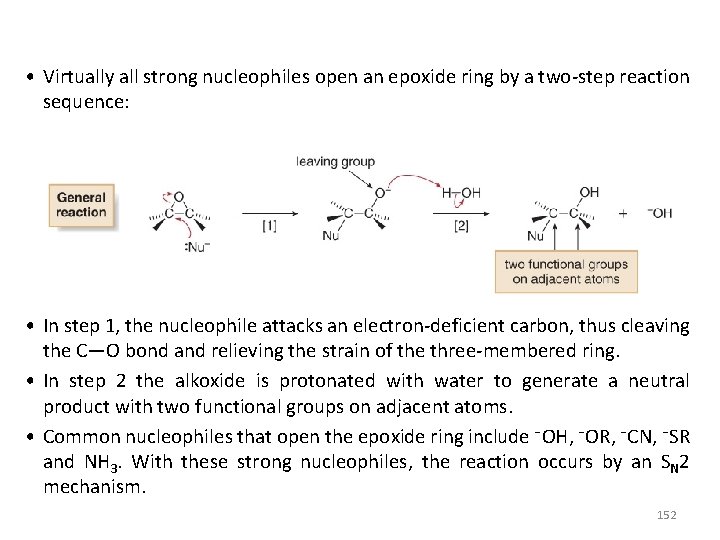
• Virtually all strong nucleophiles open an epoxide ring by a two-step reaction sequence: • In step 1, the nucleophile attacks an electron-deficient carbon, thus cleaving the C—O bond and relieving the strain of the three-membered ring. • In step 2 the alkoxide is protonated with water to generate a neutral product with two functional groups on adjacent atoms. • Common nucleophiles that open the epoxide ring include ¯OH, ¯OR, ¯CN, ¯SR and NH 3. With these strong nucleophiles, the reaction occurs by an SN 2 mechanism. 152