Mass Spectrometry Mass spectrometry MS is not true
- Slides: 25

Mass Spectrometry • Mass spectrometry (MS) is not true “spectroscopy” because it does not involve the absorption of electromagnetic radiation to form an excited state. • MS is very useful for • Determining a compound’s molecular weight • Detecting the presence of Br, Cl, and N atoms in a molecule • Structure determination • Two things happen in a mass spectrometer 1. A compound is vaporized in a vacuum and then ionized. 2. The masses of the ions are detected and graphed. Copyright 2012 John Wiley & Sons, Inc. 15 -1 Klein, Organic Chemistry 1 e

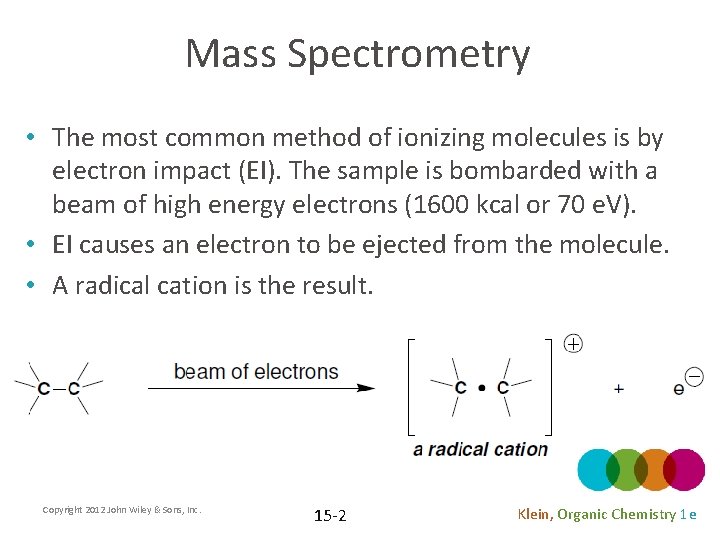
Mass Spectrometry • The most common method of ionizing molecules is by electron impact (EI). The sample is bombarded with a beam of high energy electrons (1600 kcal or 70 e. V). • EI causes an electron to be ejected from the molecule. • A radical cation is the result. Copyright 2012 John Wiley & Sons, Inc. 15 -2 Klein, Organic Chemistry 1 e

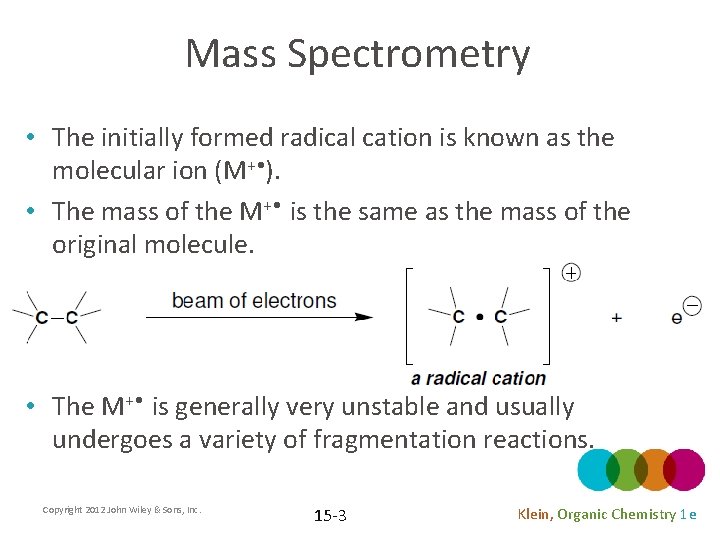
Mass Spectrometry • The initially formed radical cation is known as the molecular ion (M+ • ). • The mass of the M+ • is the same as the mass of the original molecule. • The M+ • is generally very unstable and usually undergoes a variety of fragmentation reactions. Copyright 2012 John Wiley & Sons, Inc. 15 -3 Klein, Organic Chemistry 1 e

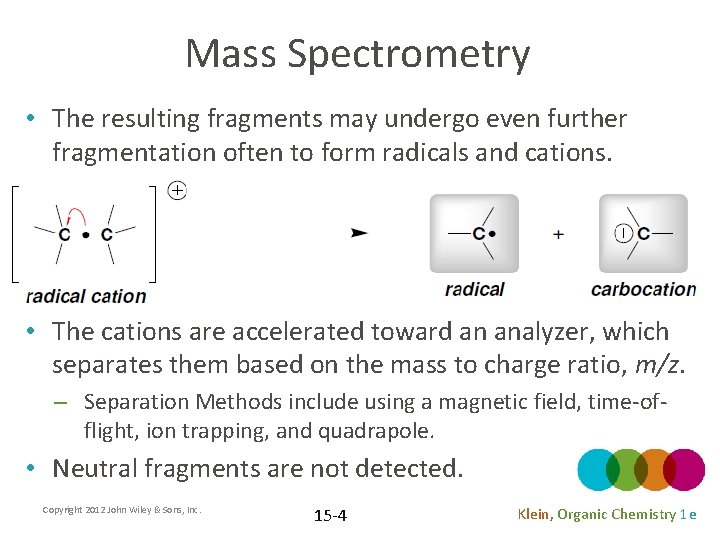
Mass Spectrometry • The resulting fragments may undergo even further fragmentation often to form radicals and cations. • The cations are accelerated toward an analyzer, which separates them based on the mass to charge ratio, m/z. – Separation Methods include using a magnetic field, time-offlight, ion trapping, and quadrapole. • Neutral fragments are not detected. Copyright 2012 John Wiley & Sons, Inc. 15 -4 Klein, Organic Chemistry 1 e

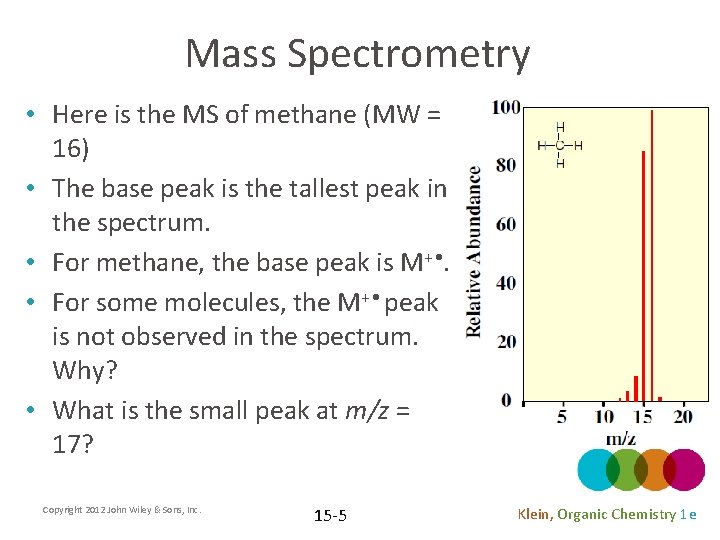
Mass Spectrometry • Here is the MS of methane (MW = 16) • The base peak is the tallest peak in the spectrum. • For methane, the base peak is M+ • . • For some molecules, the M+ • peak is not observed in the spectrum. Why? • What is the small peak at m/z = 17? Copyright 2012 John Wiley & Sons, Inc. 15 -5 Klein, Organic Chemistry 1 e

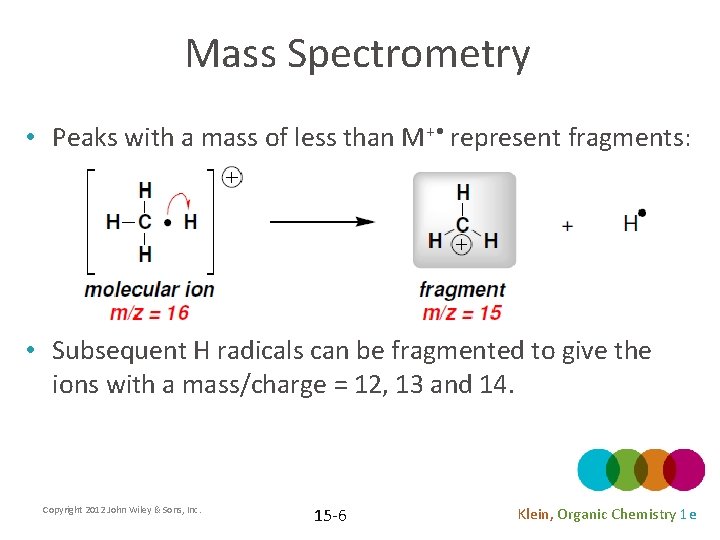
Mass Spectrometry • Peaks with a mass of less than M+ • represent fragments: • Subsequent H radicals can be fragmented to give the ions with a mass/charge = 12, 13 and 14. Copyright 2012 John Wiley & Sons, Inc. 15 -6 Klein, Organic Chemistry 1 e

Mass Spectrometry • MS is a very sensitive analytical method. • Many organic compounds can be identified: – – – – Pharmaceutical: drug discovery and drug metabolism, Organic Synthesis: reaction monitoring, product characterization Biotech: amino acid sequencing, analysis of macromolecules Clinical: neonatal screening, hemoglobin analysis Environmental: water quality, food contamination testing Geological: evaluating oil composition Forensic: explosives, illegal drugs Many More Copyright 2012 John Wiley & Sons, Inc. 15 -7 Klein, Organic Chemistry 1 e

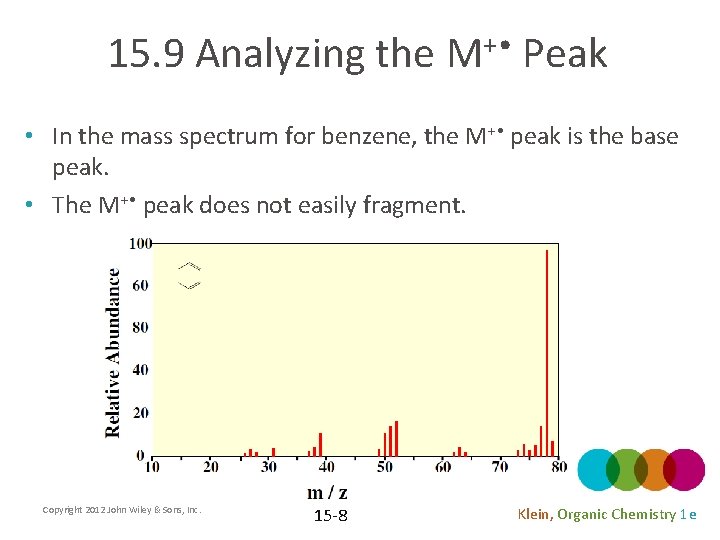
15. 9 Analyzing the M+ • Peak • In the mass spectrum for benzene, the M+ • peak is the base peak. • The M+ • peak does not easily fragment. Copyright 2012 John Wiley & Sons, Inc. 15 -8 Klein, Organic Chemistry 1 e

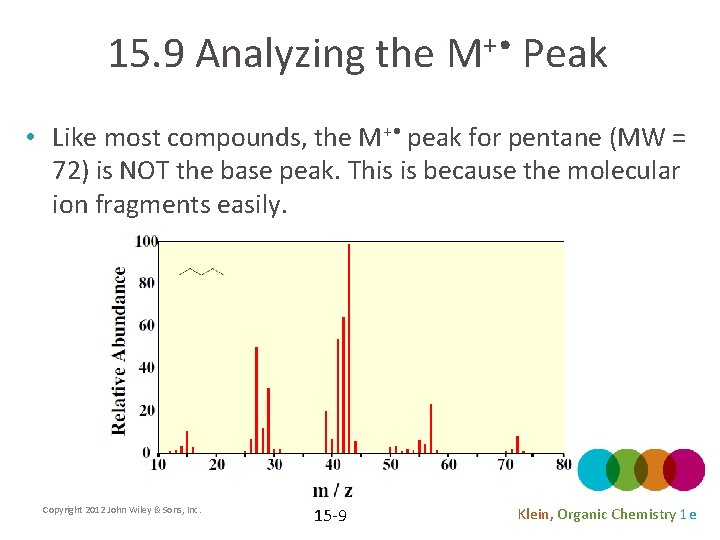
15. 9 Analyzing the M+ • Peak • Like most compounds, the M+ • peak for pentane (MW = 72) is NOT the base peak. This is because the molecular ion fragments easily. Copyright 2012 John Wiley & Sons, Inc. 15 -9 Klein, Organic Chemistry 1 e

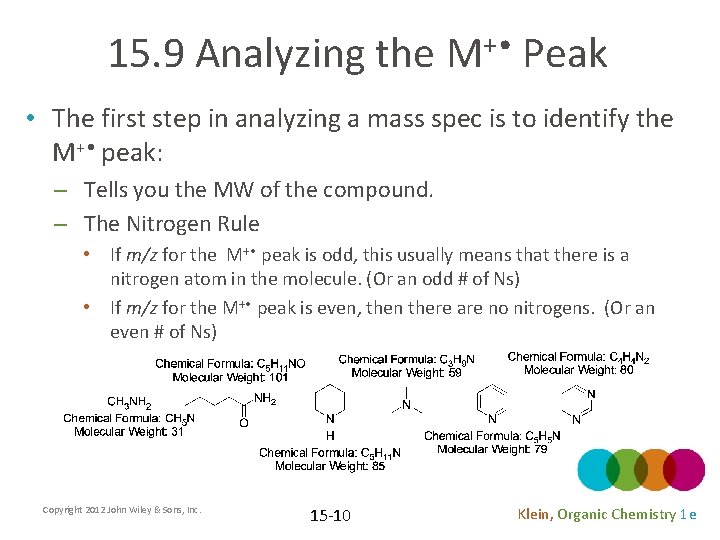
15. 9 Analyzing the M+ • Peak • The first step in analyzing a mass spec is to identify the M+ • peak: – Tells you the MW of the compound. – The Nitrogen Rule • If m/z for the M+ • peak is odd, this usually means that there is a nitrogen atom in the molecule. (Or an odd # of Ns) • If m/z for the M+ • peak is even, then there are no nitrogens. (Or an even # of Ns) Copyright 2012 John Wiley & Sons, Inc. 15 -10 Klein, Organic Chemistry 1 e

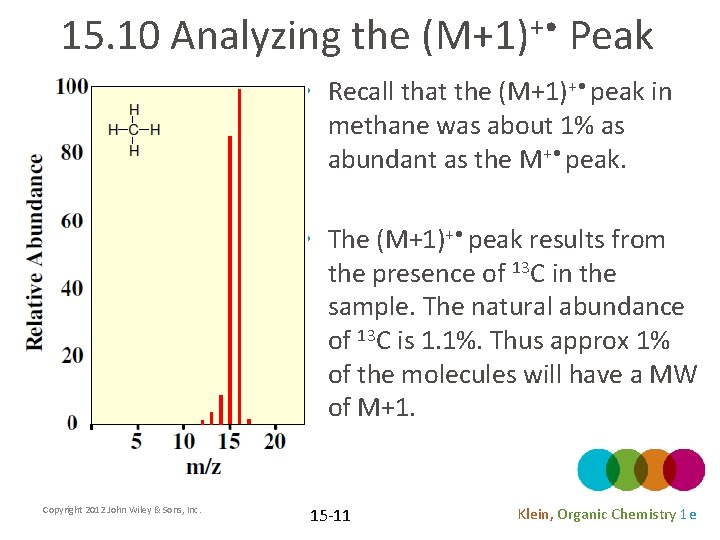
15. 10 Analyzing the (M+1)+ • Peak • Recall that the (M+1)+ • peak in methane was about 1% as abundant as the M+ • peak. • The (M+1)+ • peak results from the presence of 13 C in the sample. The natural abundance of 13 C is 1. 1%. Thus approx 1% of the molecules will have a MW of M+1. Copyright 2012 John Wiley & Sons, Inc. 15 -11 Klein, Organic Chemistry 1 e

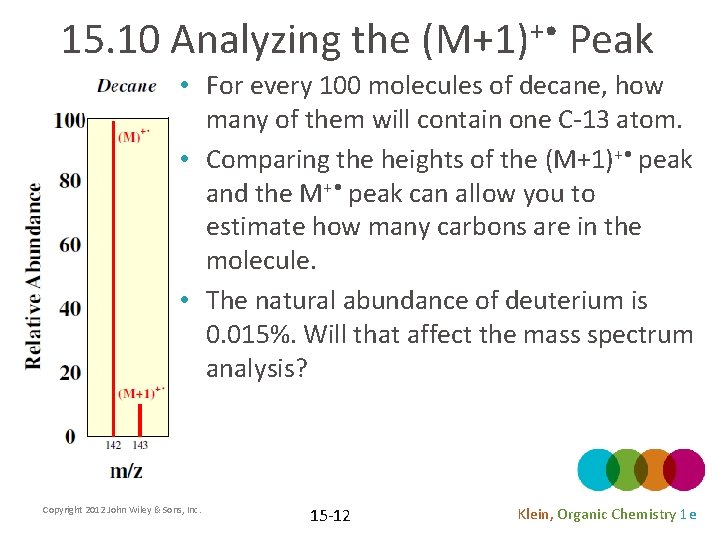
15. 10 Analyzing the (M+1)+ • Peak • For every 100 molecules of decane, how many of them will contain one C-13 atom. • Comparing the heights of the (M+1)+ • peak and the M+ • peak can allow you to estimate how many carbons are in the molecule. • The natural abundance of deuterium is 0. 015%. Will that affect the mass spectrum analysis? Copyright 2012 John Wiley & Sons, Inc. 15 -12 Klein, Organic Chemistry 1 e

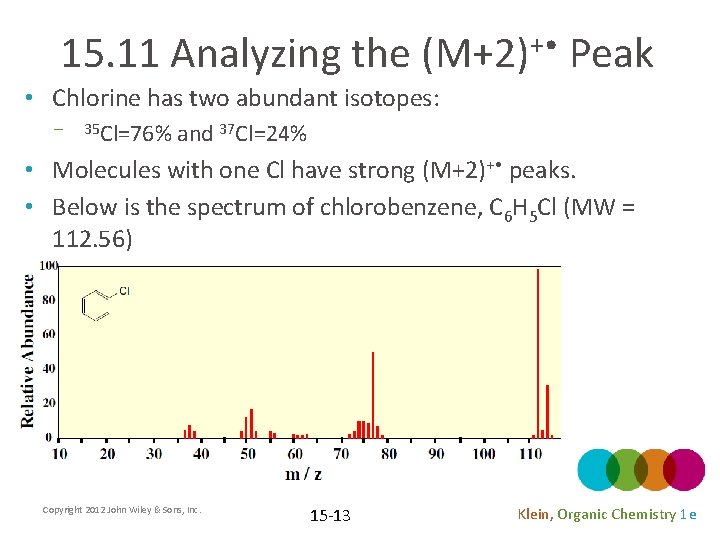
15. 11 Analyzing the (M+2)+ • Peak • Chlorine has two abundant isotopes: – 35 Cl=76% and 37 Cl=24% • Molecules with one Cl have strong (M+2)+ • peaks. • Below is the spectrum of chlorobenzene, C 6 H 5 Cl (MW = 112. 56) Copyright 2012 John Wiley & Sons, Inc. 15 -13 Klein, Organic Chemistry 1 e

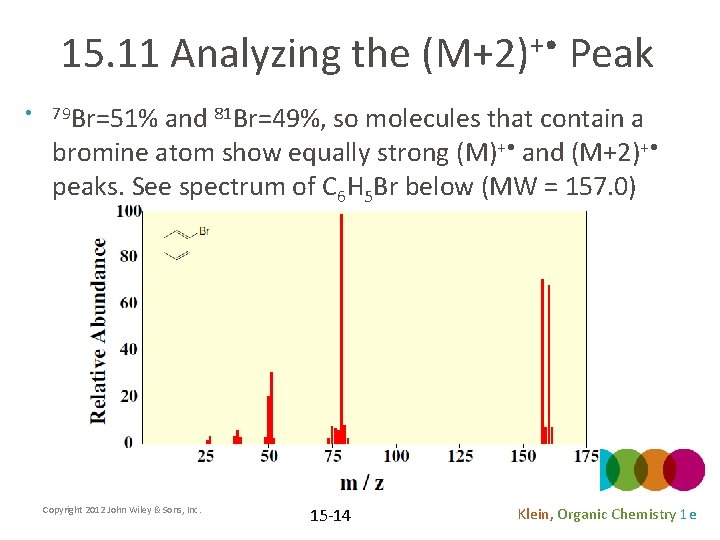
15. 11 Analyzing the (M+2)+ • Peak • 79 Br=51% and 81 Br=49%, so molecules that contain a bromine atom show equally strong (M)+ • and (M+2)+ • peaks. See spectrum of C 6 H 5 Br below (MW = 157. 0) Copyright 2012 John Wiley & Sons, Inc. 15 -14 Klein, Organic Chemistry 1 e

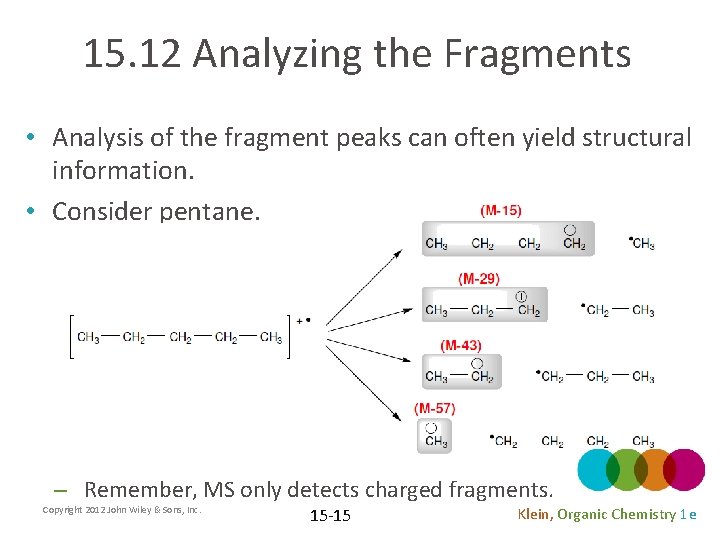
15. 12 Analyzing the Fragments • Analysis of the fragment peaks can often yield structural information. • Consider pentane. – Remember, MS only detects charged fragments. Copyright 2012 John Wiley & Sons, Inc. 15 -15 Klein, Organic Chemistry 1 e

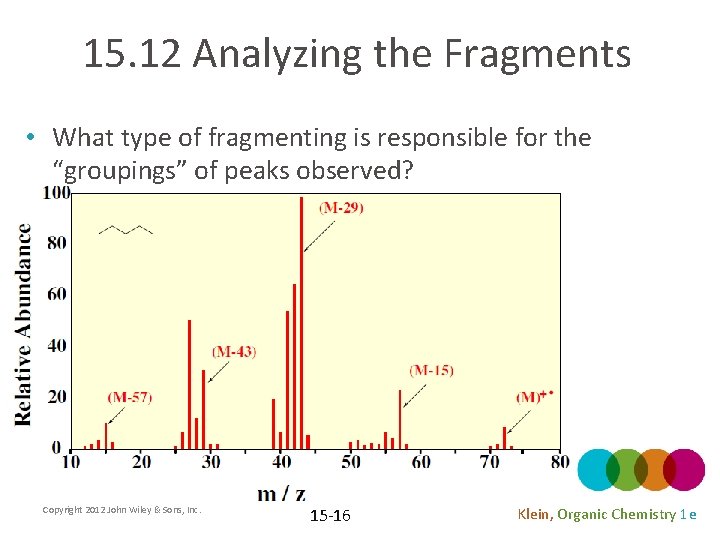
15. 12 Analyzing the Fragments • What type of fragmenting is responsible for the “groupings” of peaks observed? Copyright 2012 John Wiley & Sons, Inc. 15 -16 Klein, Organic Chemistry 1 e

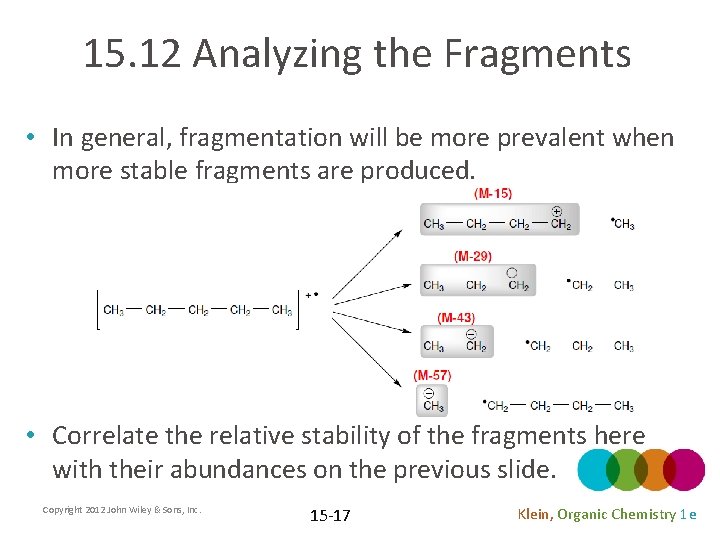
15. 12 Analyzing the Fragments • In general, fragmentation will be more prevalent when more stable fragments are produced. • Correlate the relative stability of the fragments here with their abundances on the previous slide. Copyright 2012 John Wiley & Sons, Inc. 15 -17 Klein, Organic Chemistry 1 e

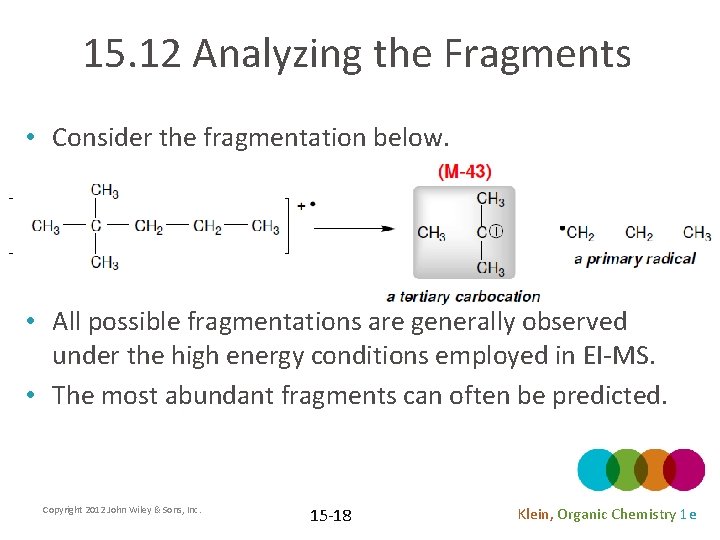
15. 12 Analyzing the Fragments • Consider the fragmentation below. • All possible fragmentations are generally observed under the high energy conditions employed in EI-MS. • The most abundant fragments can often be predicted. Copyright 2012 John Wiley & Sons, Inc. 15 -18 Klein, Organic Chemistry 1 e

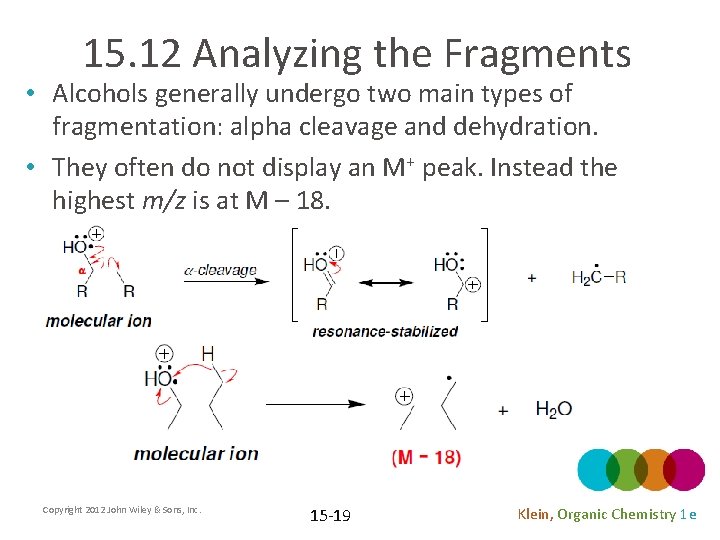
15. 12 Analyzing the Fragments • Alcohols generally undergo two main types of fragmentation: alpha cleavage and dehydration. • They often do not display an M+ peak. Instead the highest m/z is at M – 18. Copyright 2012 John Wiley & Sons, Inc. 15 -19 Klein, Organic Chemistry 1 e

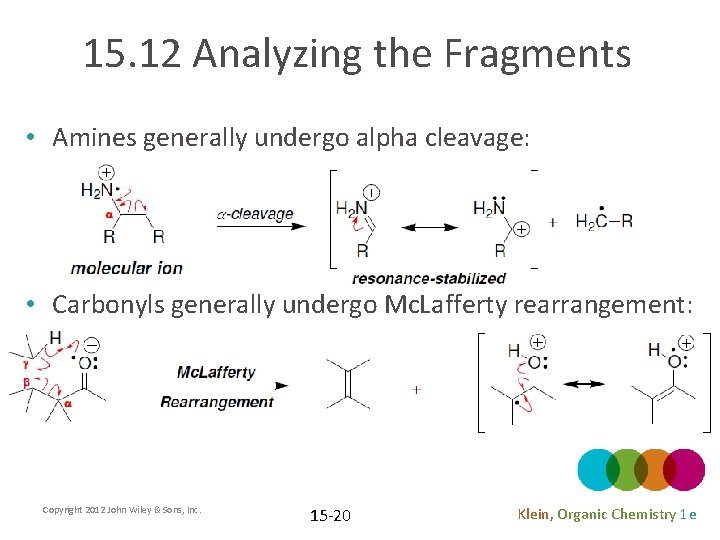
15. 12 Analyzing the Fragments • Amines generally undergo alpha cleavage: • Carbonyls generally undergo Mc. Lafferty rearrangement: Copyright 2012 John Wiley & Sons, Inc. 15 -20 Klein, Organic Chemistry 1 e

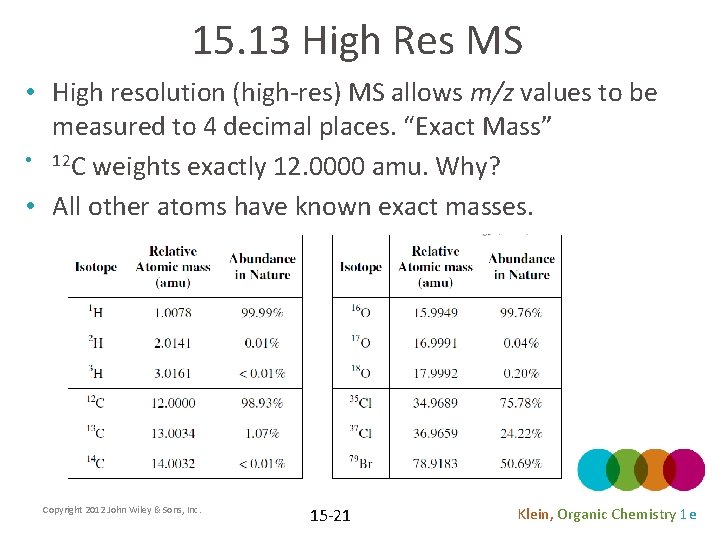
15. 13 High Res MS • High resolution (high-res) MS allows m/z values to be measured to 4 decimal places. “Exact Mass” • 12 C weights exactly 12. 0000 amu. Why? • All other atoms have known exact masses. Copyright 2012 John Wiley & Sons, Inc. 15 -21 Klein, Organic Chemistry 1 e

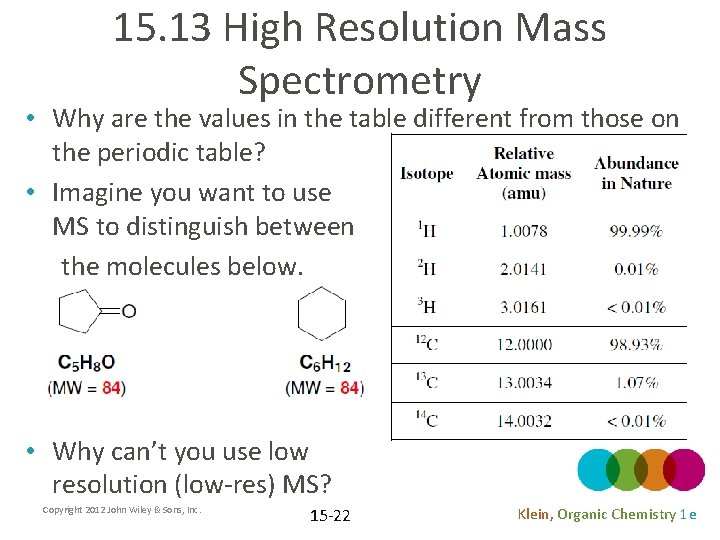
15. 13 High Resolution Mass Spectrometry • Why are the values in the table different from those on the periodic table? • Imagine you want to use MS to distinguish between the molecules below. • Why can’t you use low resolution (low-res) MS? Copyright 2012 John Wiley & Sons, Inc. 15 -22 Klein, Organic Chemistry 1 e

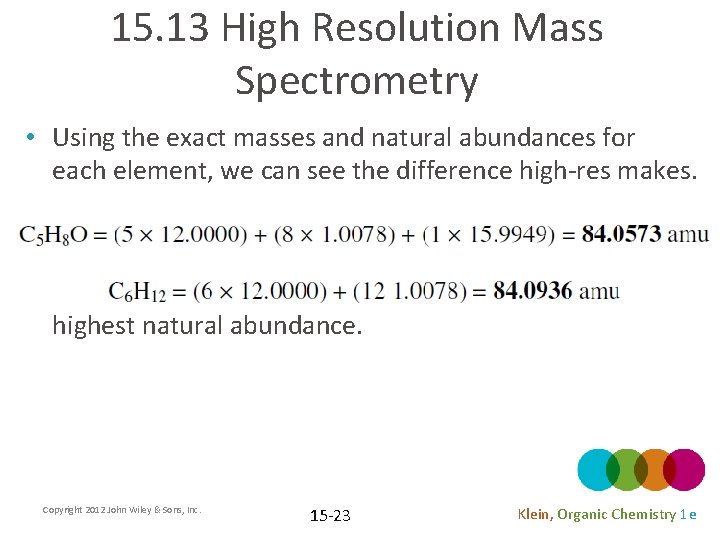
15. 13 High Resolution Mass Spectrometry • Using the exact masses and natural abundances for each element, we can see the difference high-res makes. • The molecular ion results from the molecule with the highest natural abundance. Copyright 2012 John Wiley & Sons, Inc. 15 -23 Klein, Organic Chemistry 1 e

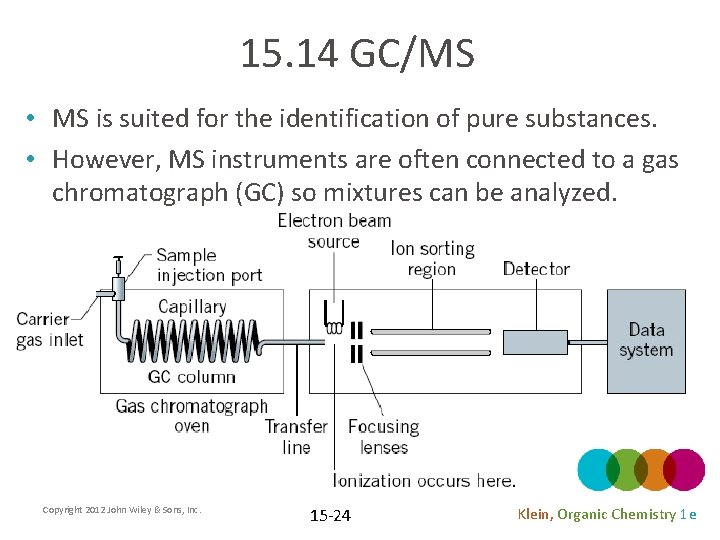
15. 14 GC/MS • MS is suited for the identification of pure substances. • However, MS instruments are often connected to a gas chromatograph (GC) so mixtures can be analyzed. Copyright 2012 John Wiley & Sons, Inc. 15 -24 Klein, Organic Chemistry 1 e

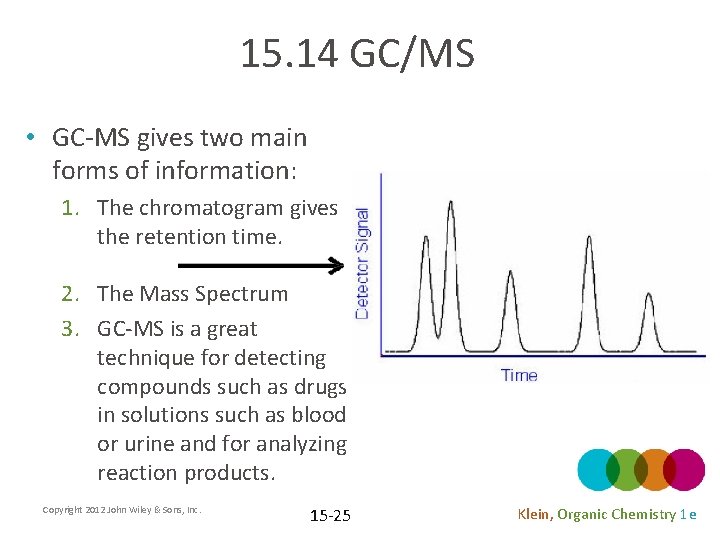
15. 14 GC/MS • GC-MS gives two main forms of information: 1. The chromatogram gives the retention time. 2. The Mass Spectrum 3. GC-MS is a great technique for detecting compounds such as drugs in solutions such as blood or urine and for analyzing reaction products. Copyright 2012 John Wiley & Sons, Inc. 15 -25 Klein, Organic Chemistry 1 e