ORGANIC VS INORGANIC COMPOUNDS Another way of classifying





- Slides: 12

ORGANIC VS. INORGANIC COMPOUNDS Another way of classifying chemical compounds…

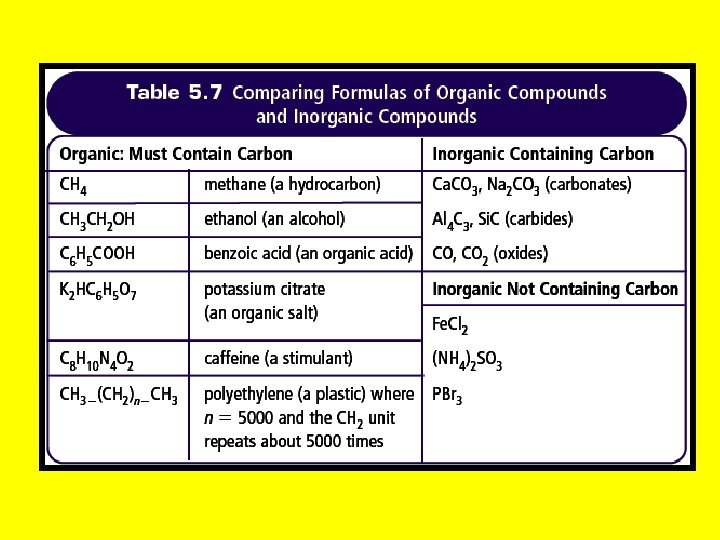
Organic Compounds • In science, organic compounds contain carbon (C), and usually hydrogen (H)

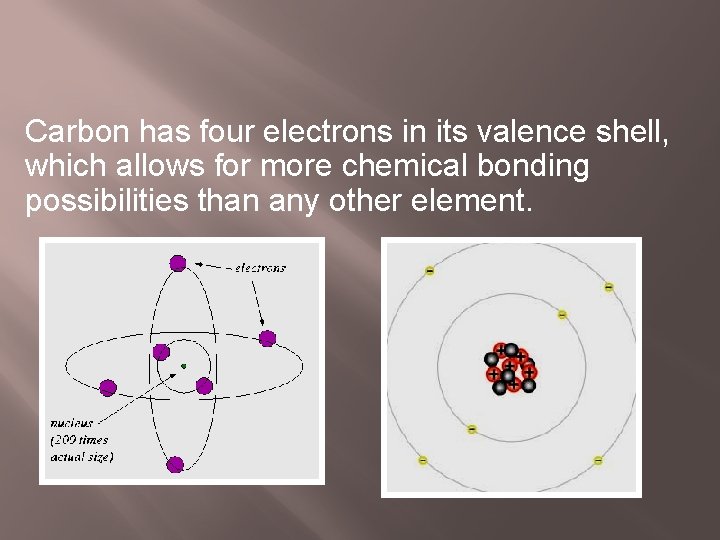
Carbon has four electrons in its valence shell, which allows for more chemical bonding possibilities than any other element.

Inorganic Compounds • Inorganic compounds do not contain carbon with 2 exceptions: • Carbon Monoxide and Carbon Dioxide • Compounds containing carbonate (CO 32 -) ions


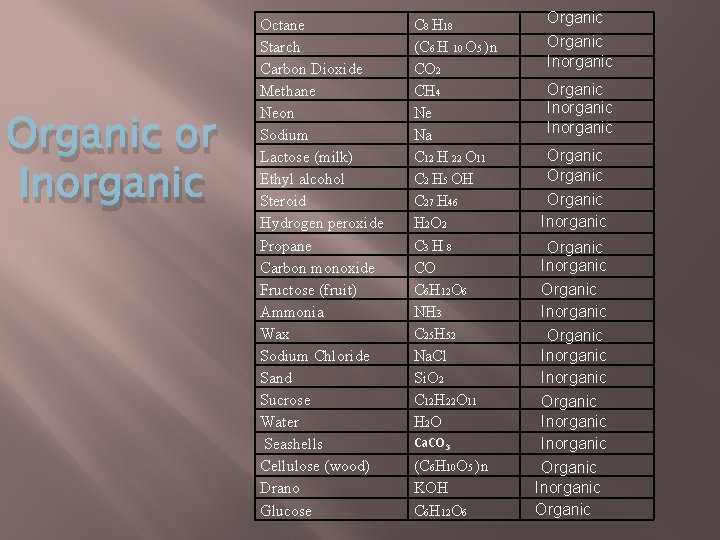
Organic or Inorganic Octane Starch Carbon Dioxide Methane Neon Sodium Lactose (milk) Ethyl alcohol Steroid Hydrogen peroxide Propane Carbon monoxide Fructose (fruit) Ammonia Wax Sodium Chloride Sand Sucrose Water Seashells Cellulose (wood) Drano Glucose C 8 H 18 (C 6 H 10 O 5 )n CO 2 CH 4 Ne Na C 12 H 22 O 11 C 2 H 5 OH C 27 H 46 H 2 O 2 C 3 H 8 CO C 6 H 12 O 6 NH 3 C 25 H 52 Na. Cl Si. O 2 C 12 H 22 O 11 H 2 O Ca. CO 3 (C 6 H 10 O 5 )n KOH C 6 H 12 O 6 Organic Inorganic Organic Inorganic Organic Inorganic Organic

� A hydrocarbon is an organic compound that contains only carbon and hydrogen. � Hydrocarbons are based on a carbon chain, with hydrogen atoms added on the sides. See pages 246 - 247

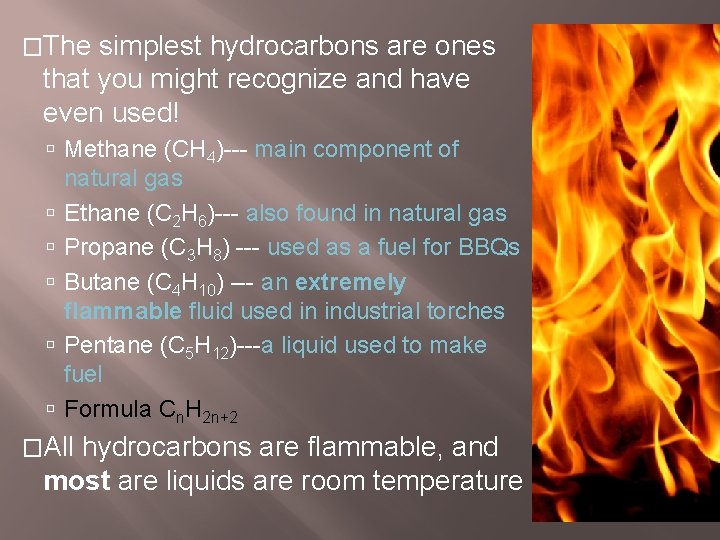
�The simplest hydrocarbons are ones that you might recognize and have even used! Methane (CH 4)--- main component of natural gas Ethane (C 2 H 6)--- also found in natural gas Propane (C 3 H 8) --- used as a fuel for BBQs Butane (C 4 H 10) --- an extremely flammable fluid used in industrial torches Pentane (C 5 H 12)---a liquid used to make fuel Formula Cn. H 2 n+2 �All hydrocarbons are flammable, and most are liquids are room temperature

ORGANIC COMPOUNDS � Alcohols are organic compounds with C, H, and O. � Alcohols are very good solvents (they dissolve other substances). � Alcohols are generally very flammable. See pages 246 - 247

Organic Compounds �The simplest alcohols are: Methanol (CH 4 O)--- used in labs as a solvent but causes blindness! Ethanol (C 2 H 6 O)---is a psychoactive drug (present in alcohol), but is now being considered as a fuel source Isopropyl alcohol (C 3 H 8 O)---rubbing alcohol used to sterile cuts �Alcohols are generally very flammable

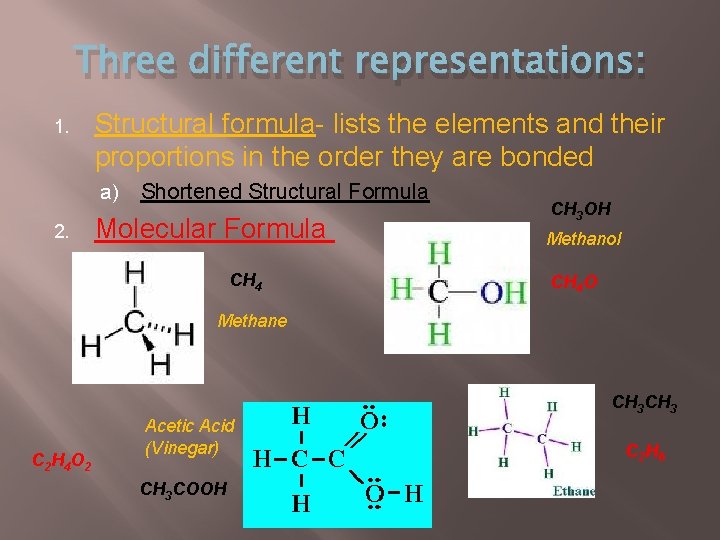
Three different representations: 1. Structural formula- lists the elements and their proportions in the order they are bonded a) 2. Shortened Structural Formula Molecular Formula CH 4 CH 3 OH Methanol CH 4 O Methane CH 3 C 2 H 4 O 2 Acetic Acid (Vinegar) CH 3 COOH C 2 H 6

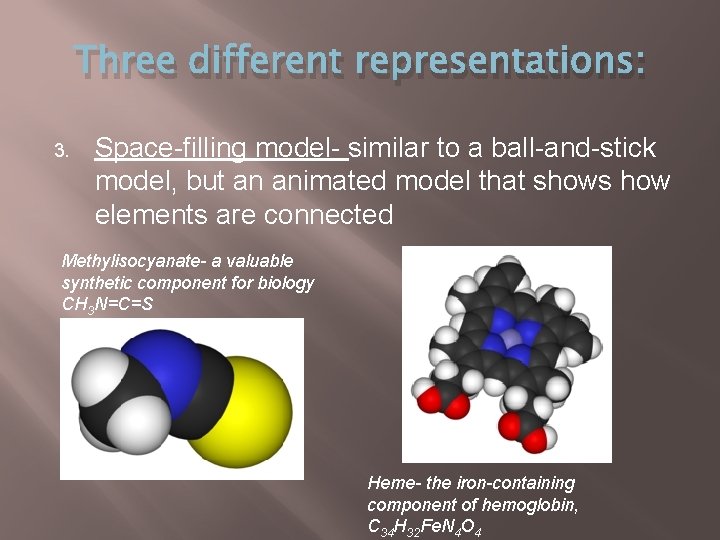
Three different representations: 3. Space-filling model- similar to a ball-and-stick model, but an animated model that shows how elements are connected Methylisocyanate- a valuable synthetic component for biology CH 3 N=C=S Heme- the iron-containing component of hemoglobin, C 34 H 32 Fe. N 4 O 4
 Organic versus inorganic compounds
Organic versus inorganic compounds Organic chemistry chapter 1
Organic chemistry chapter 1 Organic vs inorganic compounds
Organic vs inorganic compounds Organic vs inorganic compounds
Organic vs inorganic compounds Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Organic vs inorganic
Organic vs inorganic Inorganic vs organic
Inorganic vs organic C10h22 organic or inorganic
C10h22 organic or inorganic Smear layer in endodontics ppt
Smear layer in endodontics ppt Organic vs inorganic
Organic vs inorganic Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Organic vs inorganic growth
Organic vs inorganic growth Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry