Organic Chemistry Second Edition David Klein Chapter 22

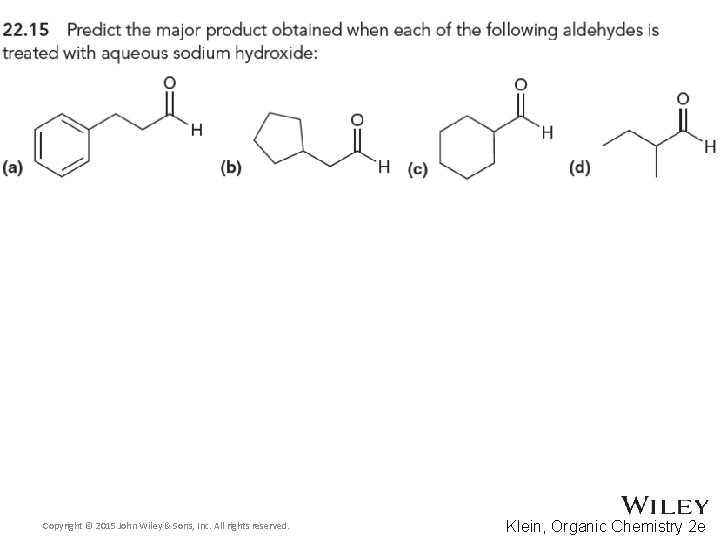

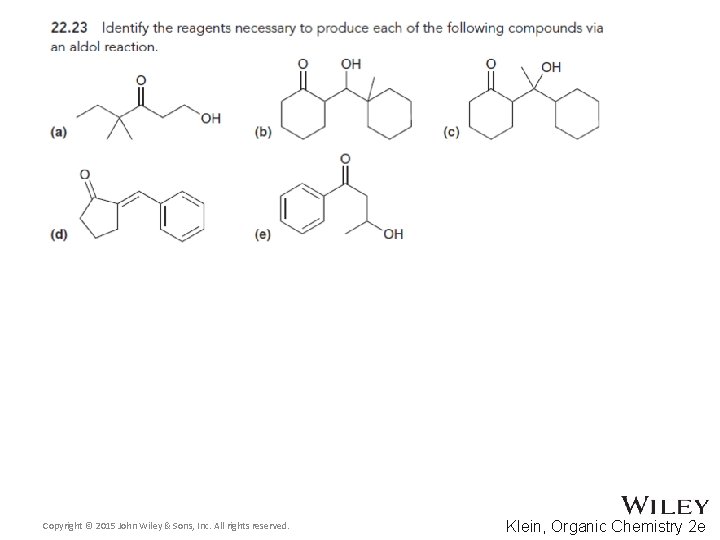
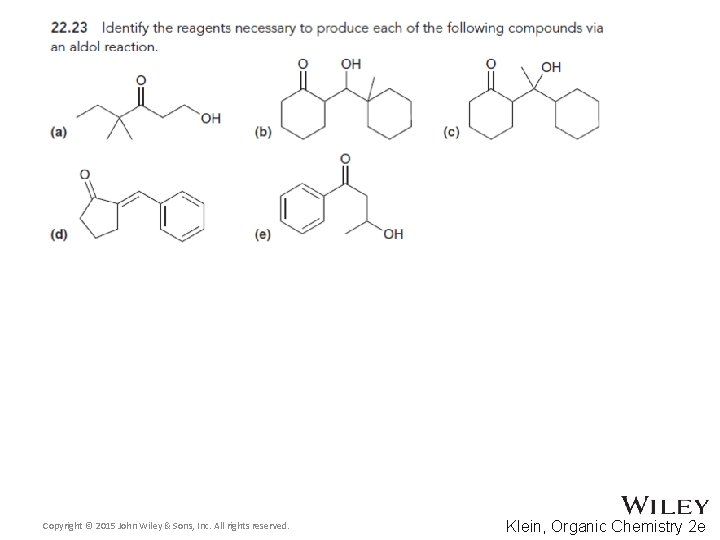





- Slides: 104

Organic Chemistry Second Edition David Klein Chapter 22 Alpha Carbon Chemistry: Enols and Enolates Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

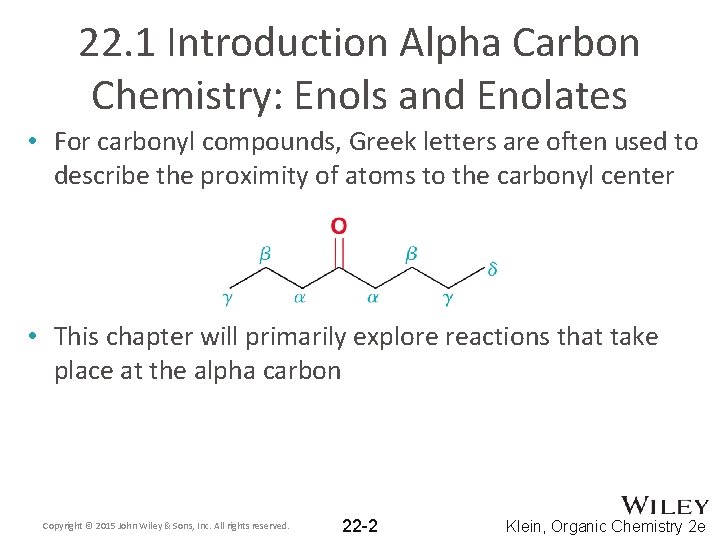
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • For carbonyl compounds, Greek letters are often used to describe the proximity of atoms to the carbonyl center • This chapter will primarily explore reactions that take place at the alpha carbon Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -2 Klein, Organic Chemistry 2 e

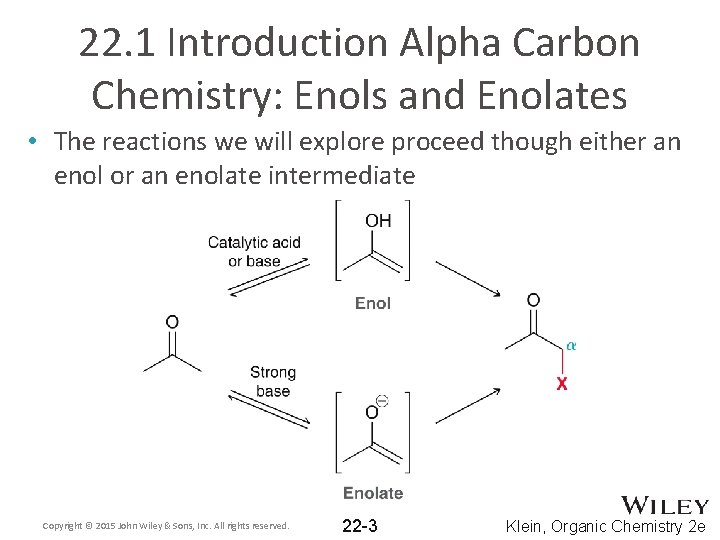
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • The reactions we will explore proceed though either an enol or an enolate intermediate Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -3 Klein, Organic Chemistry 2 e

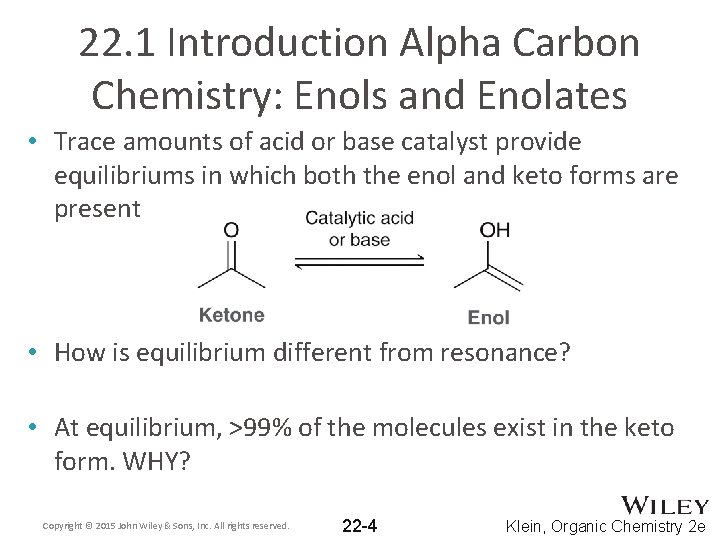
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • Trace amounts of acid or base catalyst provide equilibriums in which both the enol and keto forms are present • How is equilibrium different from resonance? • At equilibrium, >99% of the molecules exist in the keto form. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -4 Klein, Organic Chemistry 2 e

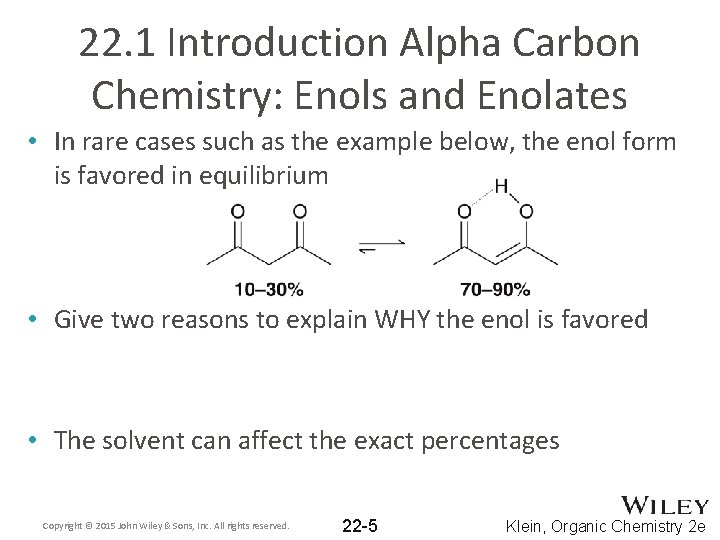
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • In rare cases such as the example below, the enol form is favored in equilibrium • Give two reasons to explain WHY the enol is favored • The solvent can affect the exact percentages Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -5 Klein, Organic Chemistry 2 e

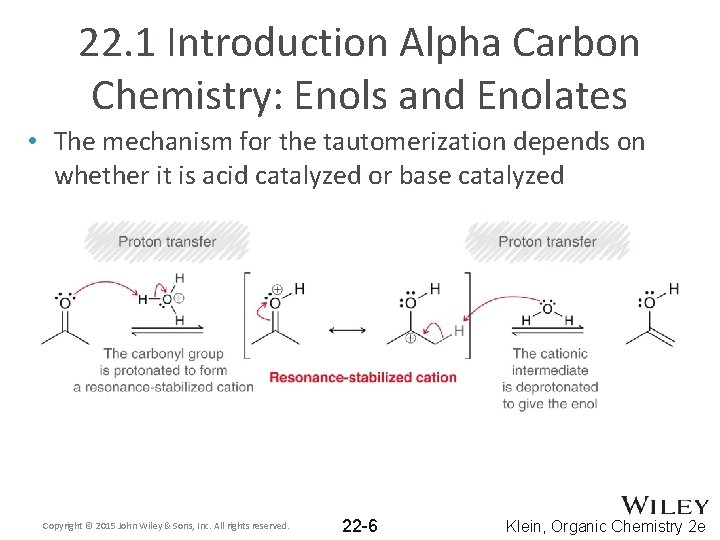
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • The mechanism for the tautomerization depends on whether it is acid catalyzed or base catalyzed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -6 Klein, Organic Chemistry 2 e

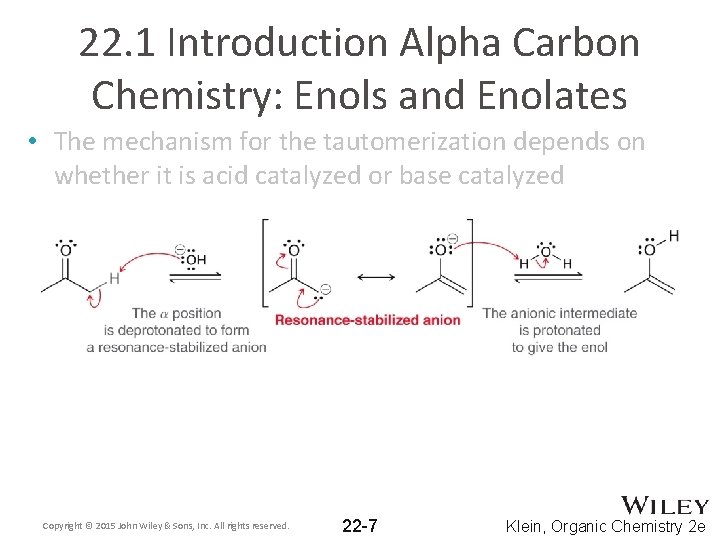
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • The mechanism for the tautomerization depends on whether it is acid catalyzed or base catalyzed Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -7 Klein, Organic Chemistry 2 e

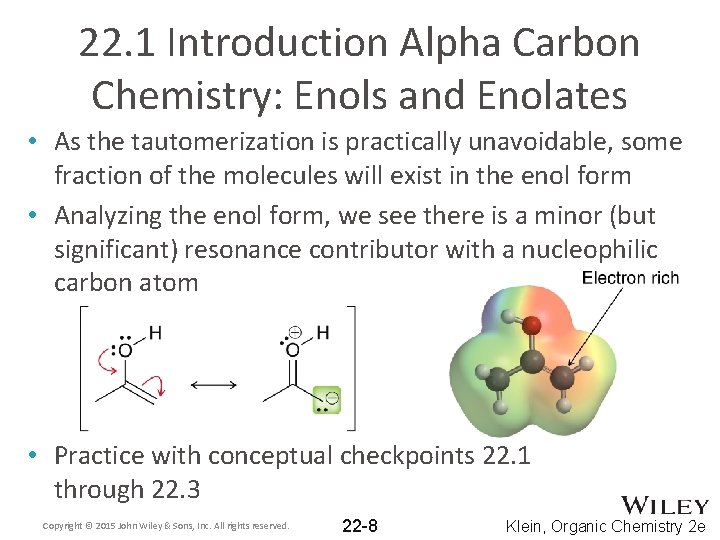
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • As the tautomerization is practically unavoidable, some fraction of the molecules will exist in the enol form • Analyzing the enol form, we see there is a minor (but significant) resonance contributor with a nucleophilic carbon atom • Practice with conceptual checkpoints 22. 1 through 22. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -8 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

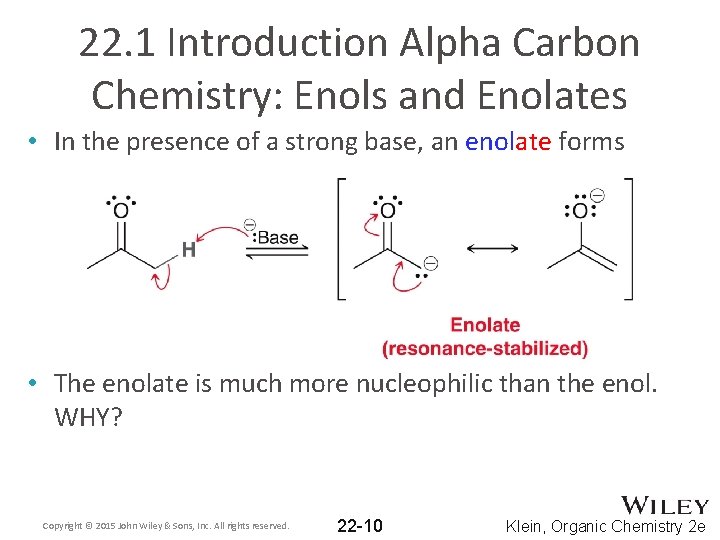
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • In the presence of a strong base, an enolate forms • The enolate is much more nucleophilic than the enol. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -10 Klein, Organic Chemistry 2 e

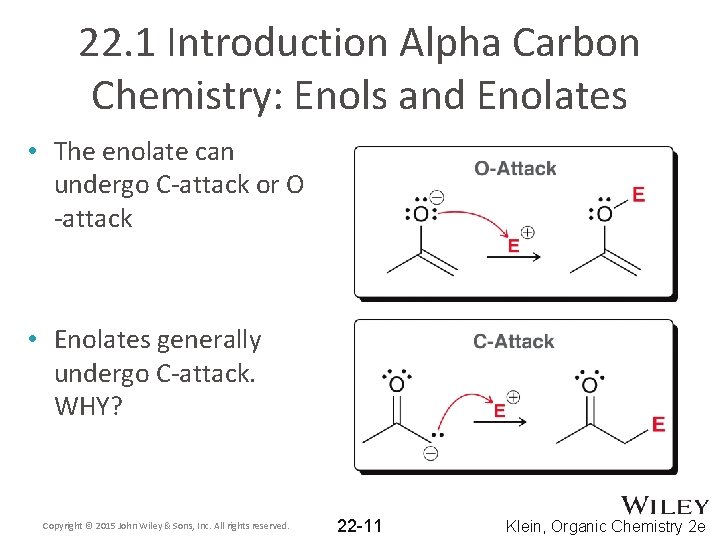
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • The enolate can undergo C-attack or O -attack • Enolates generally undergo C-attack. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -11 Klein, Organic Chemistry 2 e

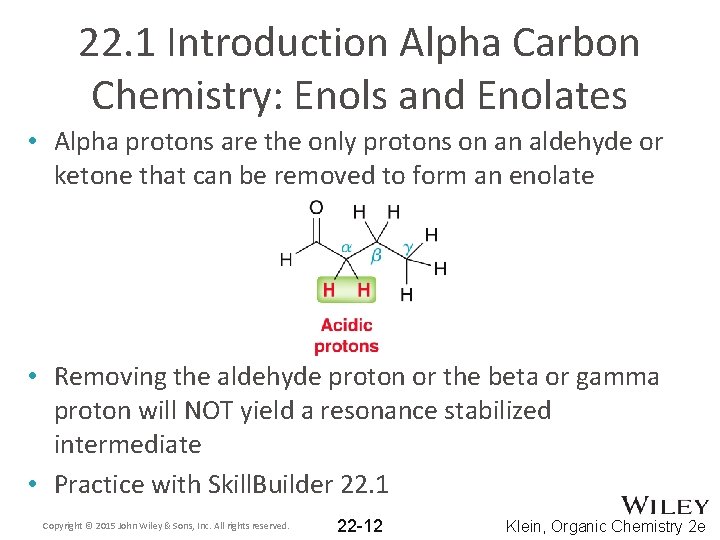
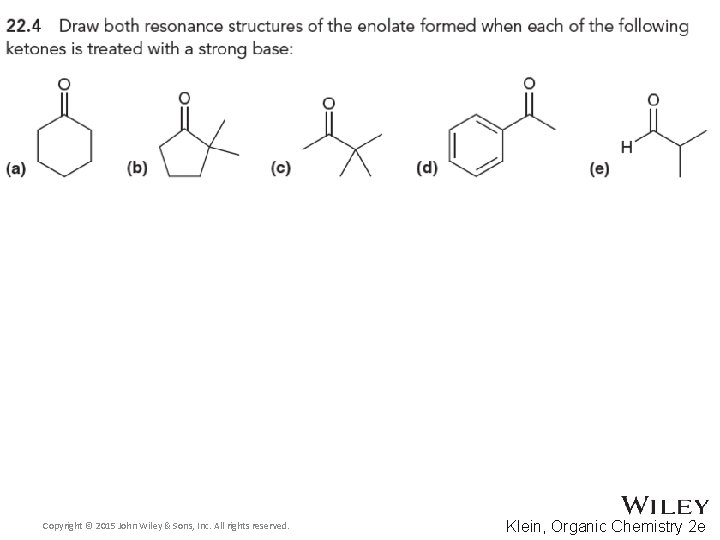
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • Alpha protons are the only protons on an aldehyde or ketone that can be removed to form an enolate • Removing the aldehyde proton or the beta or gamma proton will NOT yield a resonance stabilized intermediate • Practice with Skill. Builder 22. 1 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -12 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

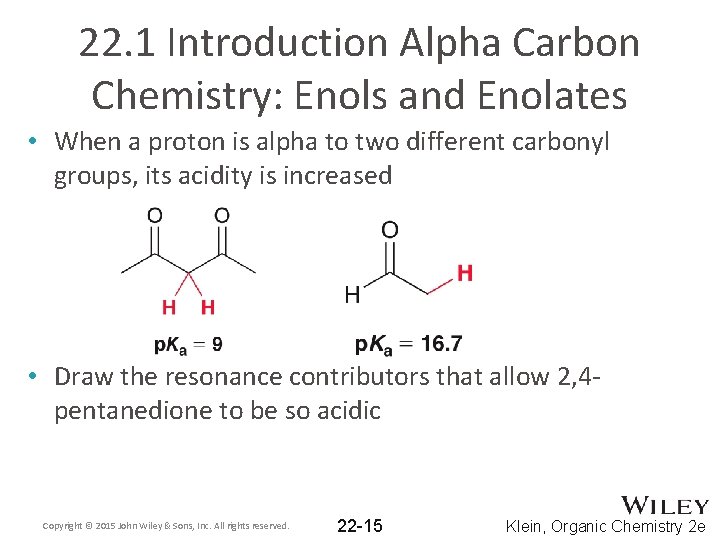
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • When a proton is alpha to two different carbonyl groups, its acidity is increased • Draw the resonance contributors that allow 2, 4 pentanedione to be so acidic Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -15 Klein, Organic Chemistry 2 e

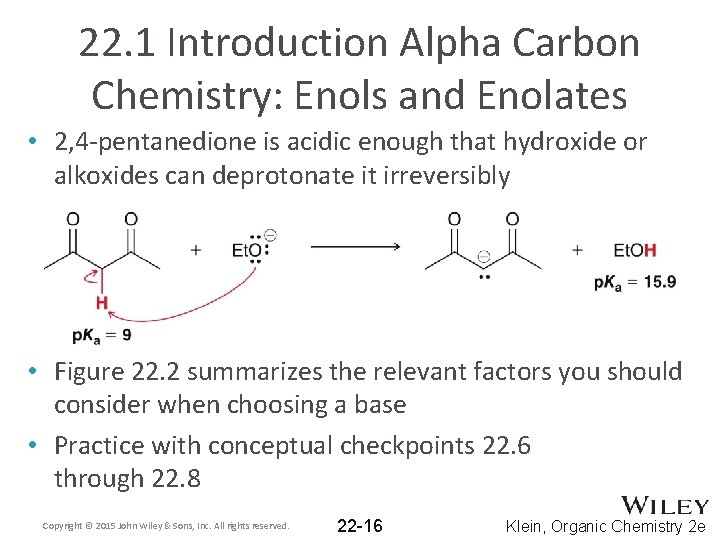
22. 1 Introduction Alpha Carbon Chemistry: Enols and Enolates • 2, 4 -pentanedione is acidic enough that hydroxide or alkoxides can deprotonate it irreversibly • Figure 22. 2 summarizes the relevant factors you should consider when choosing a base • Practice with conceptual checkpoints 22. 6 through 22. 8 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -16 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

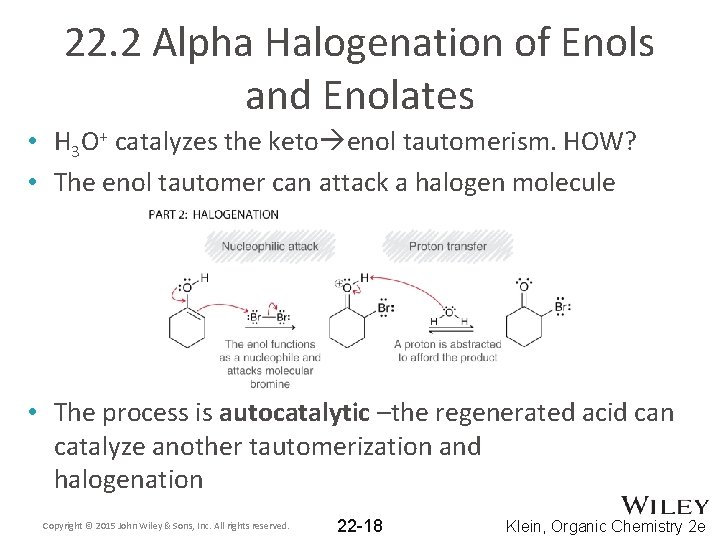
22. 2 Alpha Halogenation of Enols and Enolates • H 3 O+ catalyzes the keto enol tautomerism. HOW? • The enol tautomer can attack a halogen molecule • The process is autocatalytic –the regenerated acid can catalyze another tautomerization and halogenation Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -18 Klein, Organic Chemistry 2 e

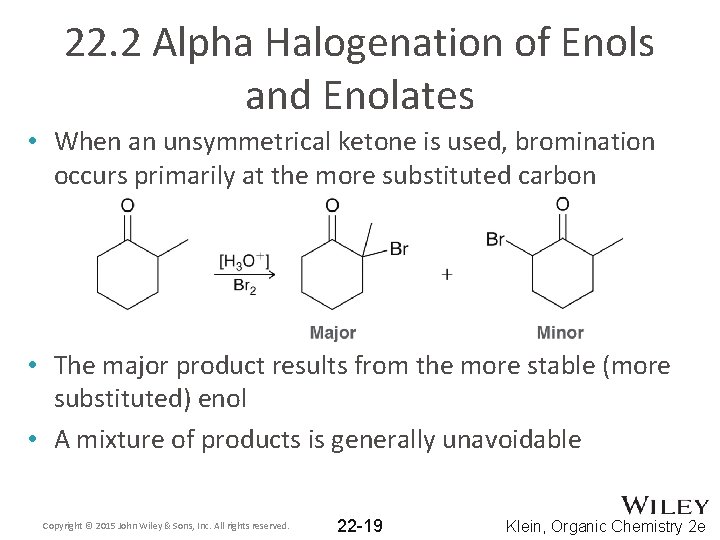
22. 2 Alpha Halogenation of Enols and Enolates • When an unsymmetrical ketone is used, bromination occurs primarily at the more substituted carbon • The major product results from the more stable (more substituted) enol • A mixture of products is generally unavoidable Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -19 Klein, Organic Chemistry 2 e

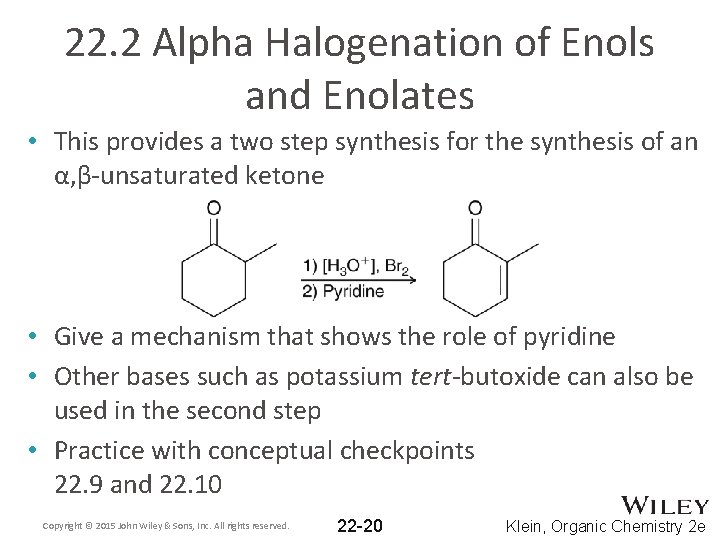
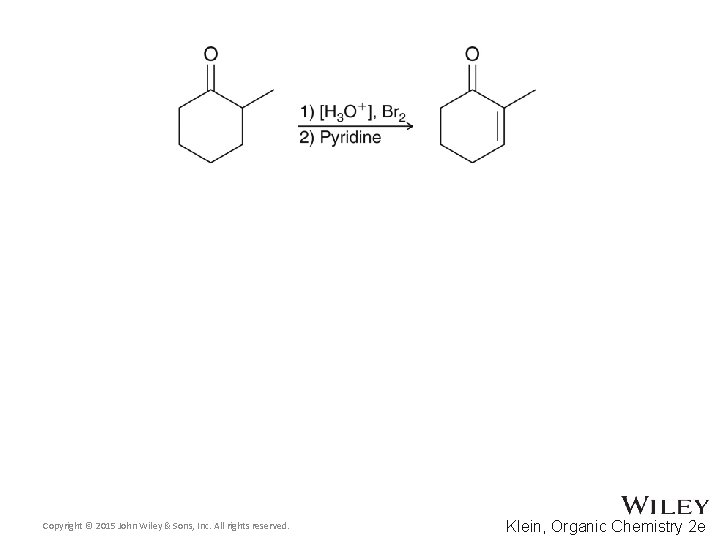
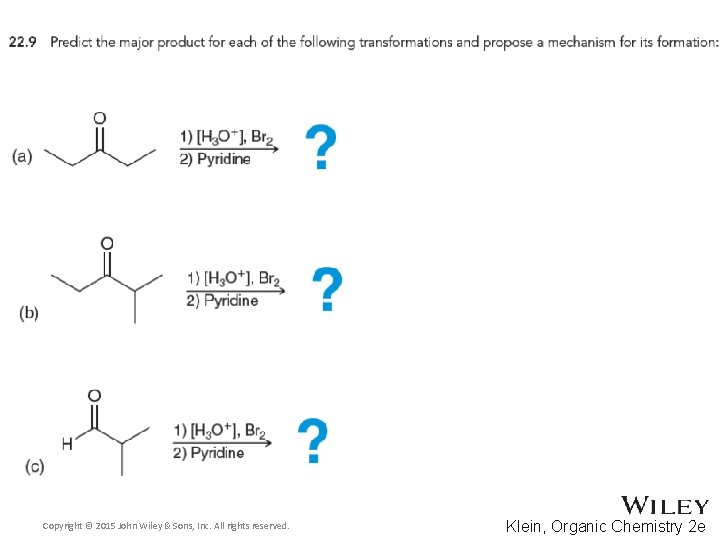
22. 2 Alpha Halogenation of Enols and Enolates • This provides a two step synthesis for the synthesis of an α, β-unsaturated ketone • Give a mechanism that shows the role of pyridine • Other bases such as potassium tert-butoxide can also be used in the second step • Practice with conceptual checkpoints 22. 9 and 22. 10 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -20 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

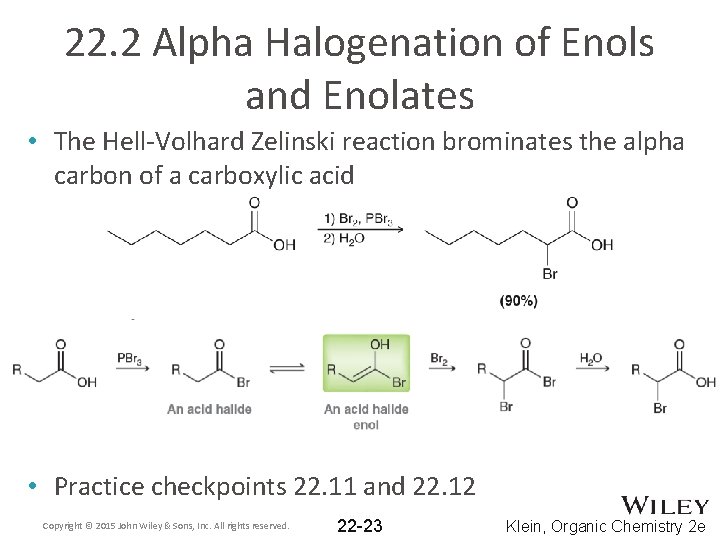
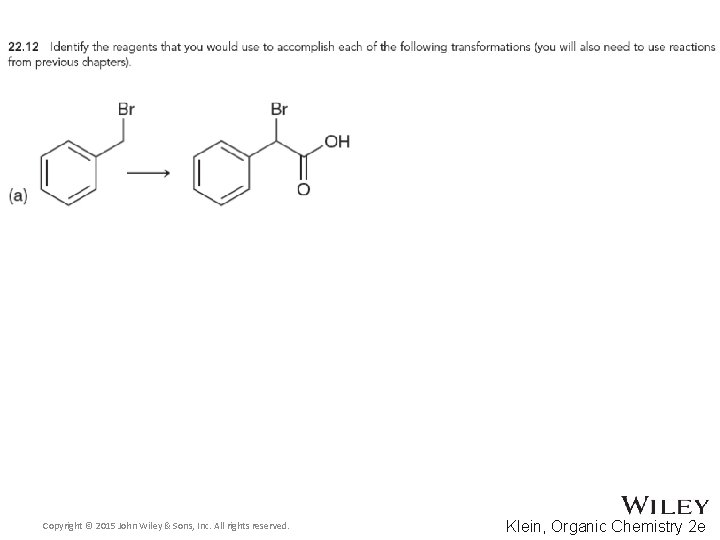
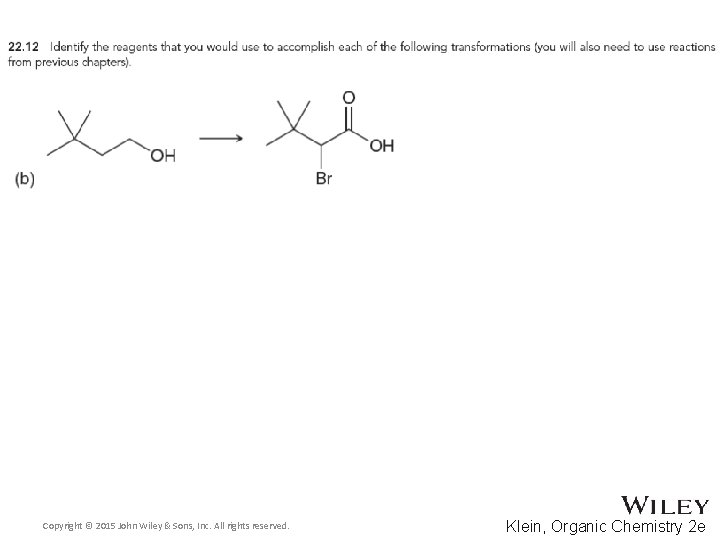
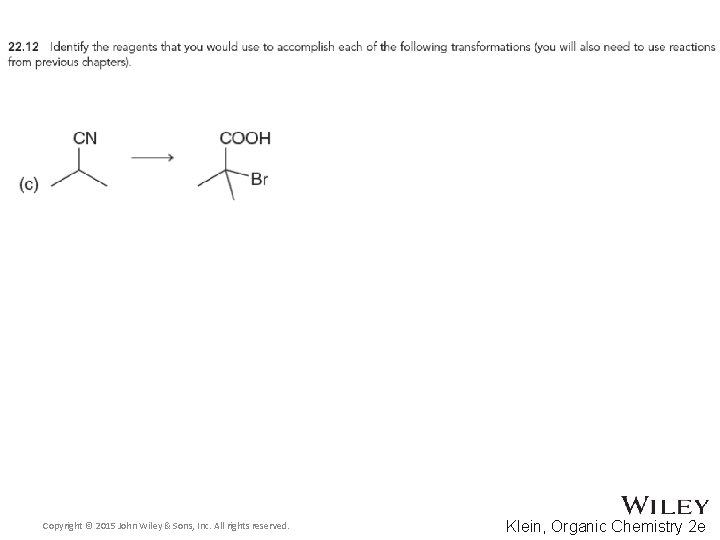
22. 2 Alpha Halogenation of Enols and Enolates • The Hell-Volhard Zelinski reaction brominates the alpha carbon of a carboxylic acid • Practice checkpoints 22. 11 and 22. 12 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -23 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

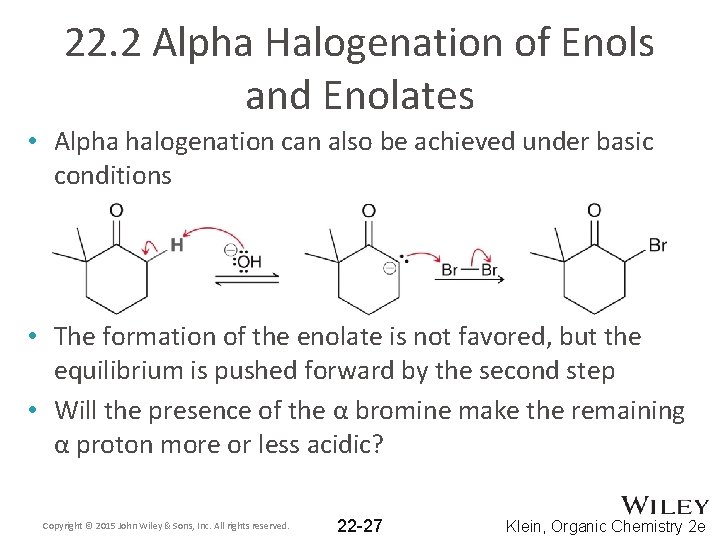
22. 2 Alpha Halogenation of Enols and Enolates • Alpha halogenation can also be achieved under basic conditions • The formation of the enolate is not favored, but the equilibrium is pushed forward by the second step • Will the presence of the α bromine make the remaining α proton more or less acidic? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -27 Klein, Organic Chemistry 2 e

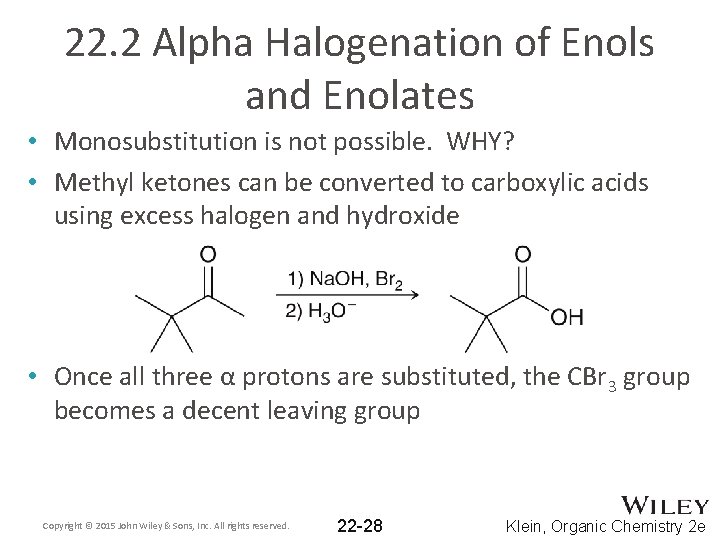
22. 2 Alpha Halogenation of Enols and Enolates • Monosubstitution is not possible. WHY? • Methyl ketones can be converted to carboxylic acids using excess halogen and hydroxide • Once all three α protons are substituted, the CBr 3 group becomes a decent leaving group Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -28 Klein, Organic Chemistry 2 e

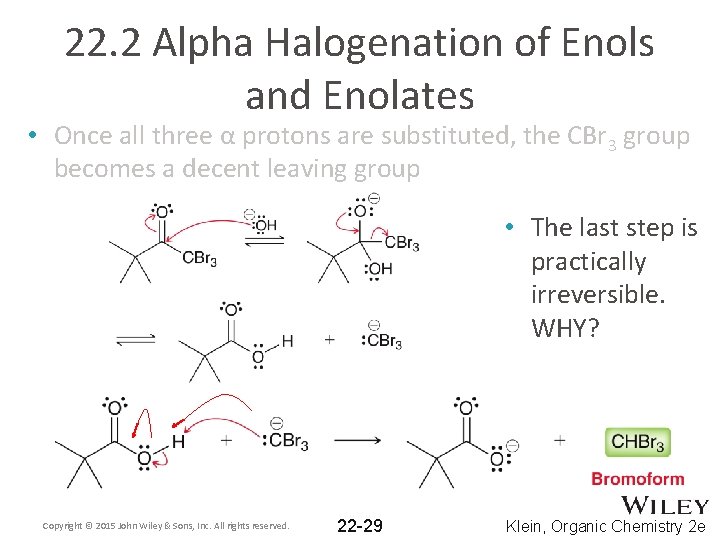
22. 2 Alpha Halogenation of Enols and Enolates • Once all three α protons are substituted, the CBr 3 group becomes a decent leaving group • The last step is practically irreversible. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -29 Klein, Organic Chemistry 2 e

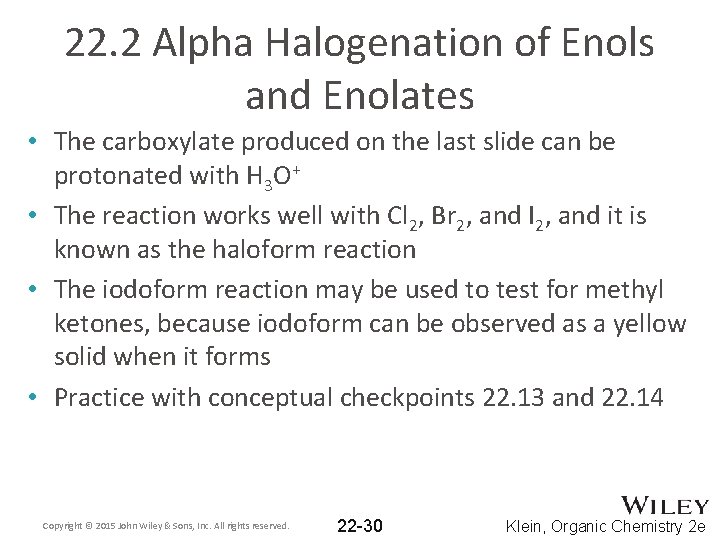
22. 2 Alpha Halogenation of Enols and Enolates • The carboxylate produced on the last slide can be protonated with H 3 O+ • The reaction works well with Cl 2, Br 2, and I 2, and it is known as the haloform reaction • The iodoform reaction may be used to test for methyl ketones, because iodoform can be observed as a yellow solid when it forms • Practice with conceptual checkpoints 22. 13 and 22. 14 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -30 Klein, Organic Chemistry 2 e

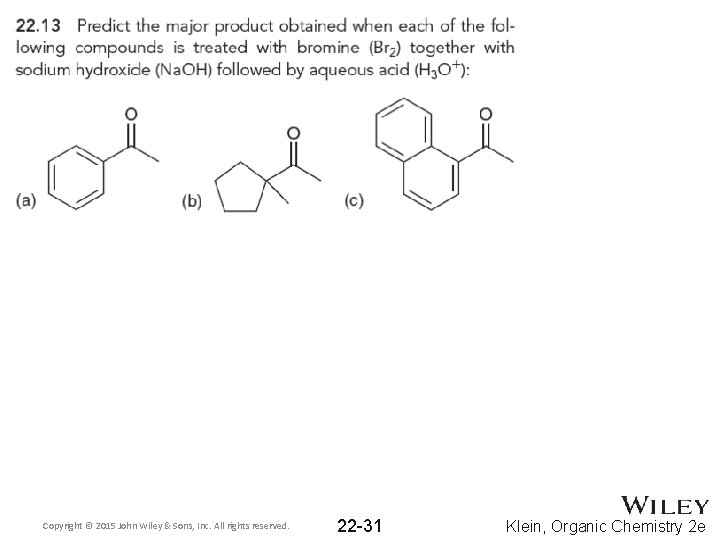
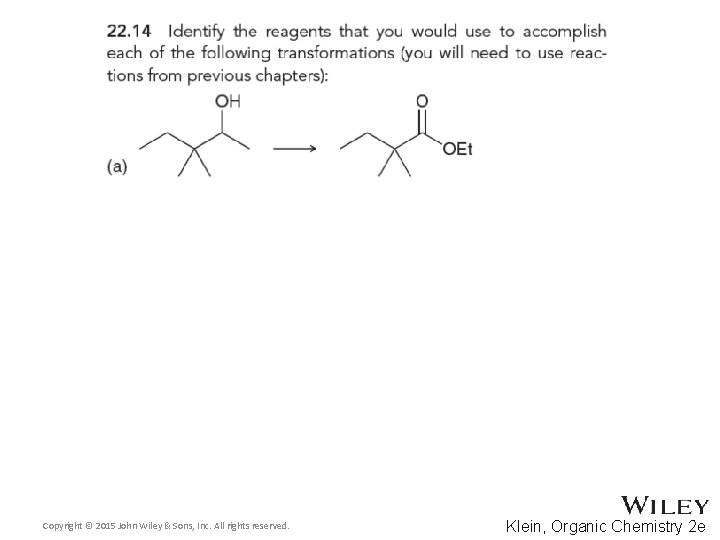
Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -31 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

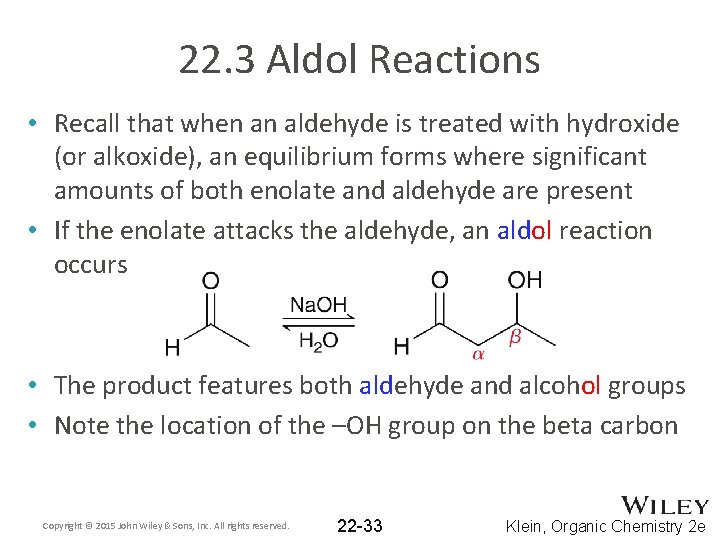
22. 3 Aldol Reactions • Recall that when an aldehyde is treated with hydroxide (or alkoxide), an equilibrium forms where significant amounts of both enolate and aldehyde are present • If the enolate attacks the aldehyde, an aldol reaction occurs • The product features both aldehyde and alcohol groups • Note the location of the –OH group on the beta carbon Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -33 Klein, Organic Chemistry 2 e

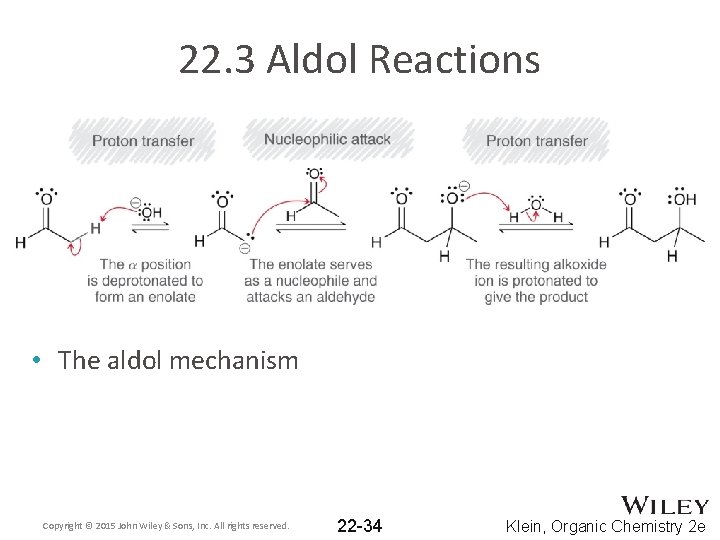
22. 3 Aldol Reactions • The aldol mechanism Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -34 Klein, Organic Chemistry 2 e

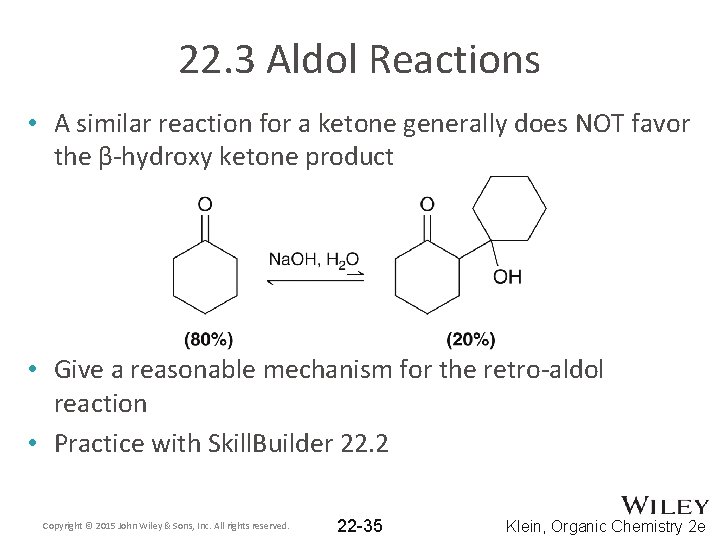
22. 3 Aldol Reactions • A similar reaction for a ketone generally does NOT favor the β-hydroxy ketone product • Give a reasonable mechanism for the retro-aldol reaction • Practice with Skill. Builder 22. 2 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -35 Klein, Organic Chemistry 2 e

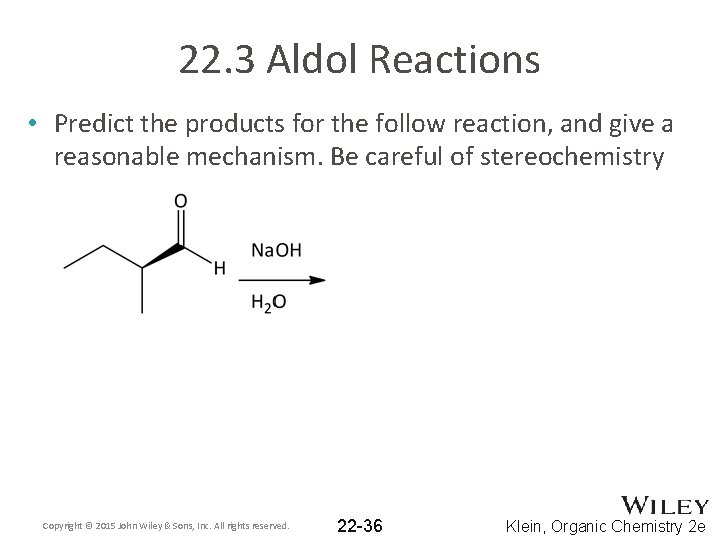
22. 3 Aldol Reactions • Predict the products for the follow reaction, and give a reasonable mechanism. Be careful of stereochemistry Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -36 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

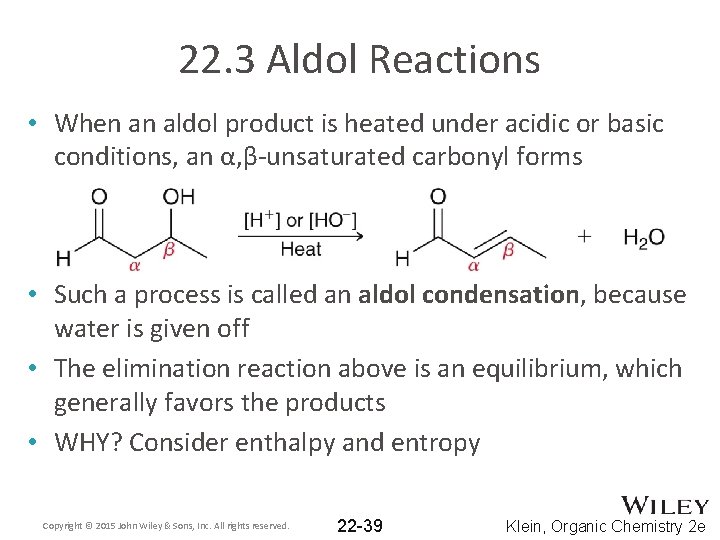
22. 3 Aldol Reactions • When an aldol product is heated under acidic or basic conditions, an α, β-unsaturated carbonyl forms • Such a process is called an aldol condensation, because water is given off • The elimination reaction above is an equilibrium, which generally favors the products • WHY? Consider enthalpy and entropy Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -39 Klein, Organic Chemistry 2 e

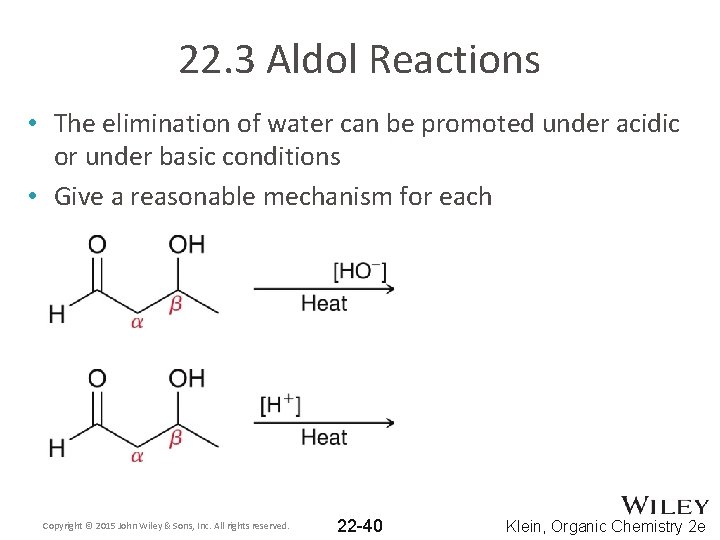
22. 3 Aldol Reactions • The elimination of water can be promoted under acidic or under basic conditions • Give a reasonable mechanism for each Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -40 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

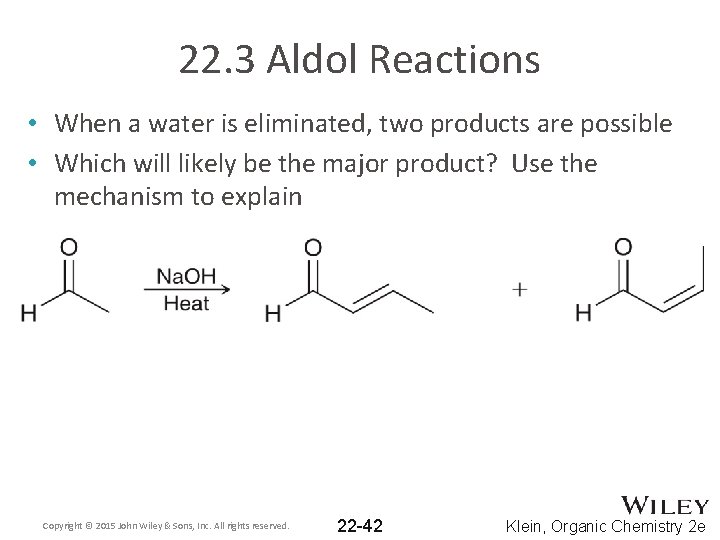
22. 3 Aldol Reactions • When a water is eliminated, two products are possible • Which will likely be the major product? Use the mechanism to explain Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -42 Klein, Organic Chemistry 2 e

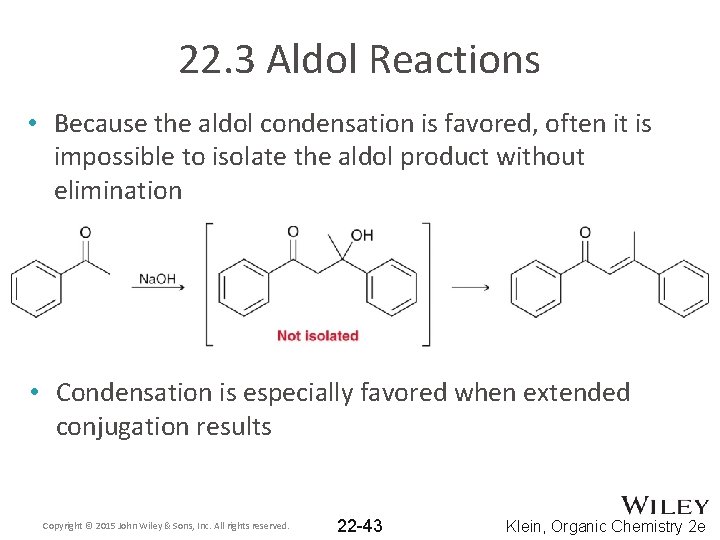
22. 3 Aldol Reactions • Because the aldol condensation is favored, often it is impossible to isolate the aldol product without elimination • Condensation is especially favored when extended conjugation results Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -43 Klein, Organic Chemistry 2 e

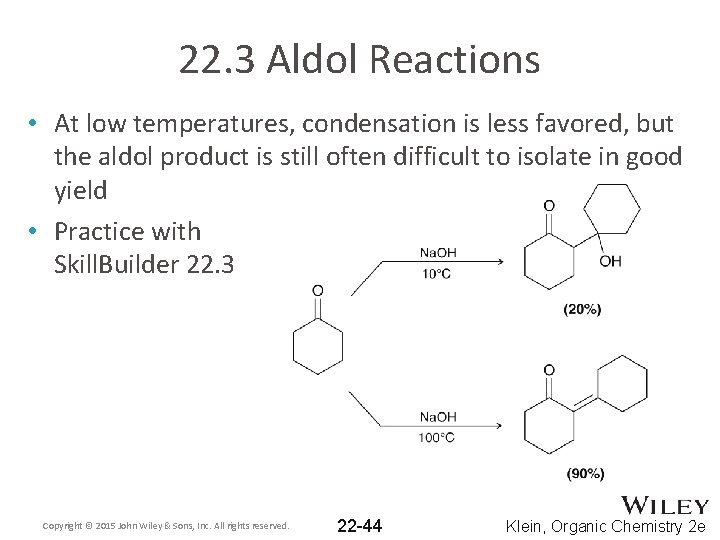
22. 3 Aldol Reactions • At low temperatures, condensation is less favored, but the aldol product is still often difficult to isolate in good yield • Practice with Skill. Builder 22. 3 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -44 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

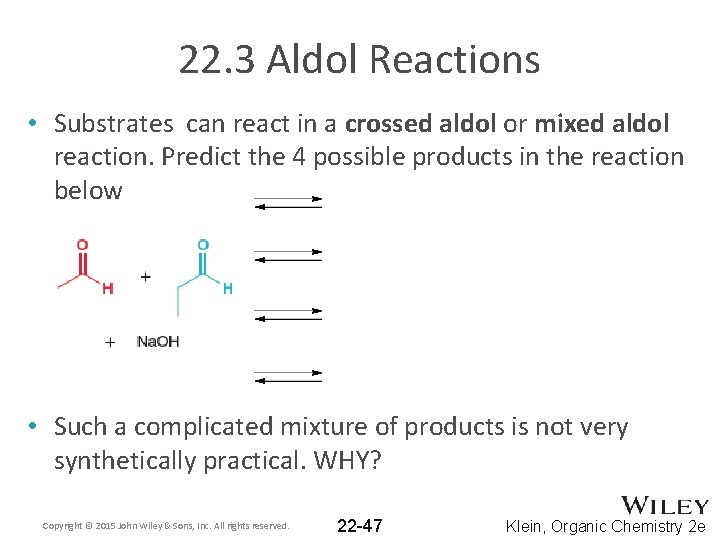
22. 3 Aldol Reactions • Substrates can react in a crossed aldol or mixed aldol reaction. Predict the 4 possible products in the reaction below • Such a complicated mixture of products is not very synthetically practical. WHY? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -47 Klein, Organic Chemistry 2 e

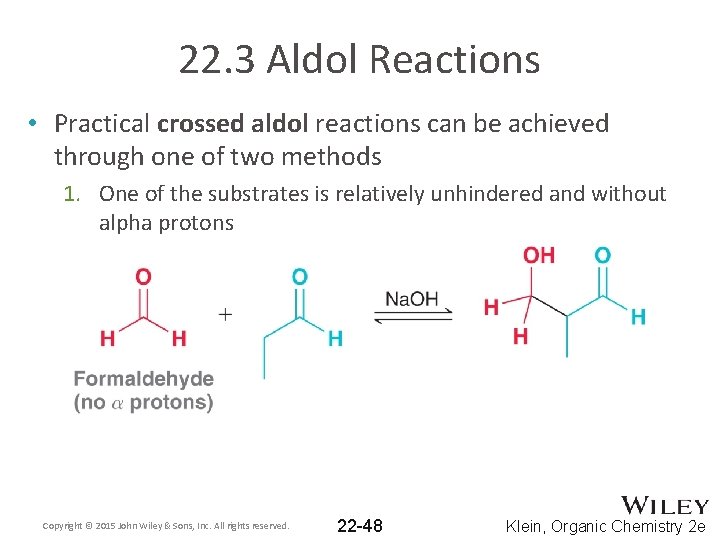
22. 3 Aldol Reactions • Practical crossed aldol reactions can be achieved through one of two methods 1. One of the substrates is relatively unhindered and without alpha protons Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -48 Klein, Organic Chemistry 2 e

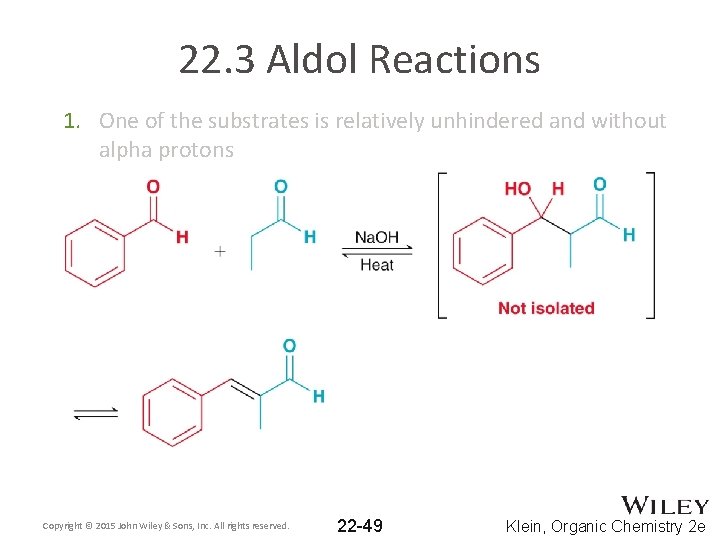
22. 3 Aldol Reactions 1. One of the substrates is relatively unhindered and without alpha protons Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -49 Klein, Organic Chemistry 2 e

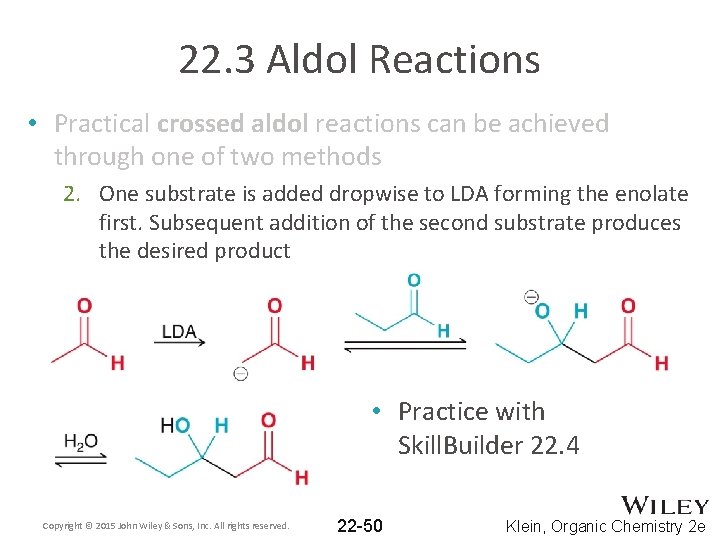
22. 3 Aldol Reactions • Practical crossed aldol reactions can be achieved through one of two methods 2. One substrate is added dropwise to LDA forming the enolate first. Subsequent addition of the second substrate produces the desired product • Practice with Skill. Builder 22. 4 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -50 Klein, Organic Chemistry 2 e

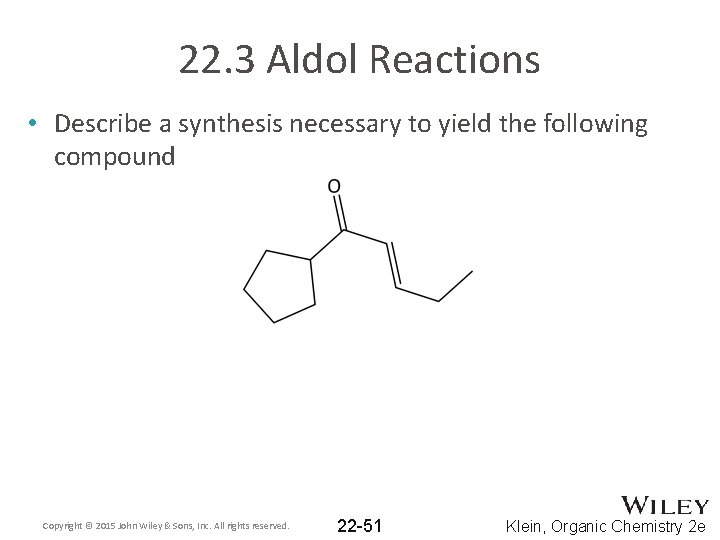
22. 3 Aldol Reactions • Describe a synthesis necessary to yield the following compound Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -51 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

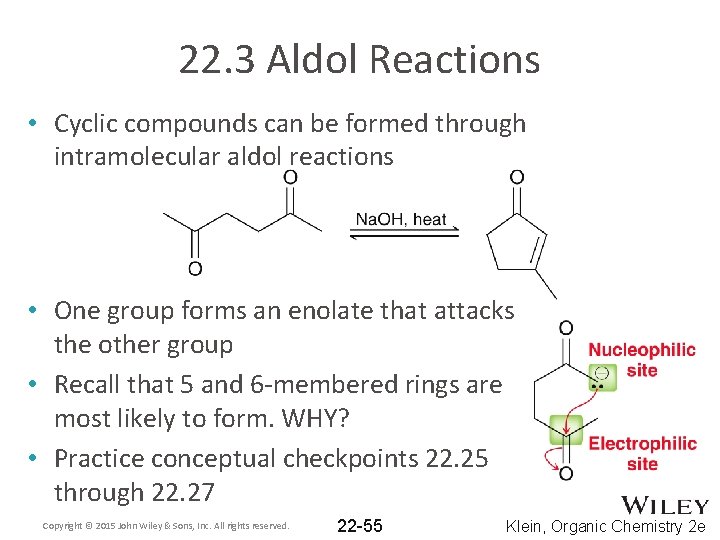
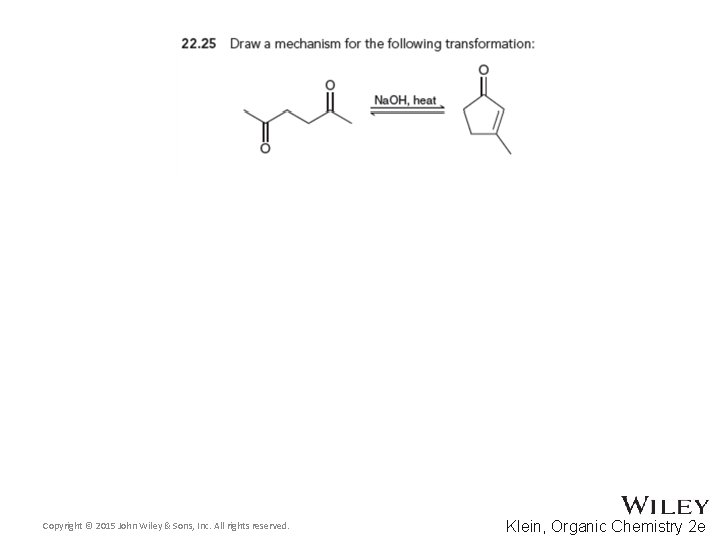
22. 3 Aldol Reactions • Cyclic compounds can be formed through intramolecular aldol reactions • One group forms an enolate that attacks the other group • Recall that 5 and 6 -membered rings are most likely to form. WHY? • Practice conceptual checkpoints 22. 25 through 22. 27 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -55 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

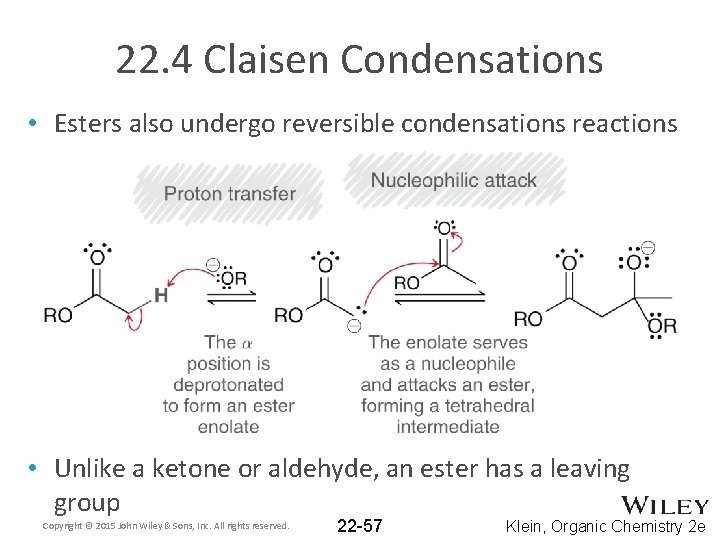
22. 4 Claisen Condensations • Esters also undergo reversible condensations reactions • Unlike a ketone or aldehyde, an ester has a leaving group Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -57 Klein, Organic Chemistry 2 e

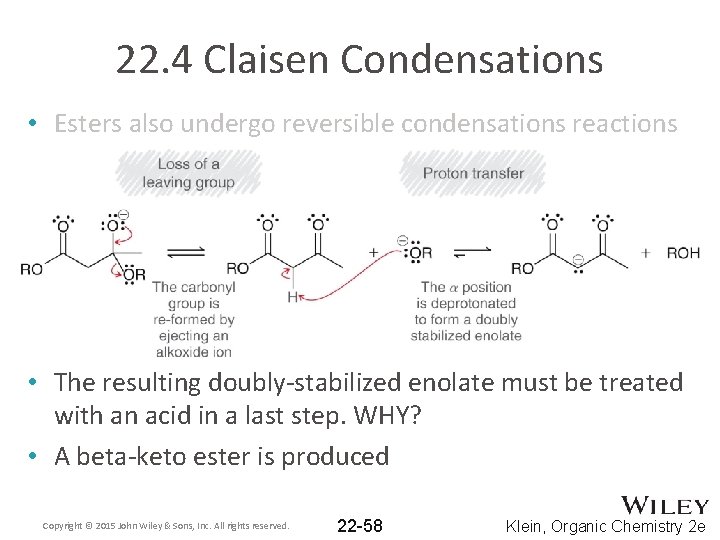
22. 4 Claisen Condensations • Esters also undergo reversible condensations reactions • The resulting doubly-stabilized enolate must be treated with an acid in a last step. WHY? • A beta-keto ester is produced Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -58 Klein, Organic Chemistry 2 e

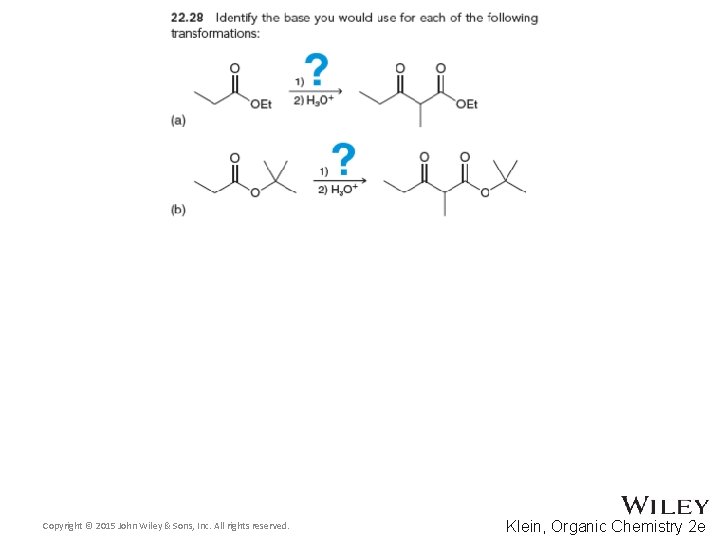
22. 4 Claisen Condensations • There are some limitations to the Claisen condensation 1. The starting ester must have 2 alpha protons, because removal of the second proton by the alkoxide ion is what drives the equilibrium forward 2. Hydroxide cannot be used as the base to promote Claisen condensations, because a hydrolysis reaction occurs between hydroxide and the ester 3. An alkoxide equivalent to the –OR group of the ester is a good base, because transesterification is avoided • Practice conceptual checkpoints 22. 28 and 22. 29 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -59 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

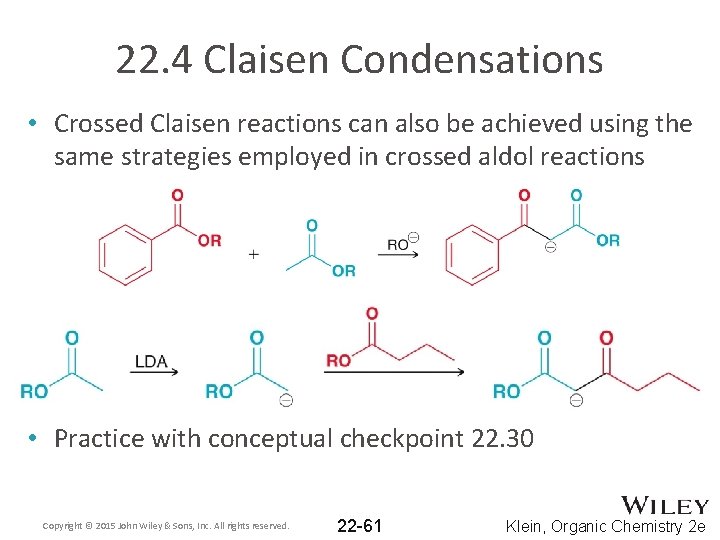
22. 4 Claisen Condensations • Crossed Claisen reactions can also be achieved using the same strategies employed in crossed aldol reactions • Practice with conceptual checkpoint 22. 30 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -61 Klein, Organic Chemistry 2 e

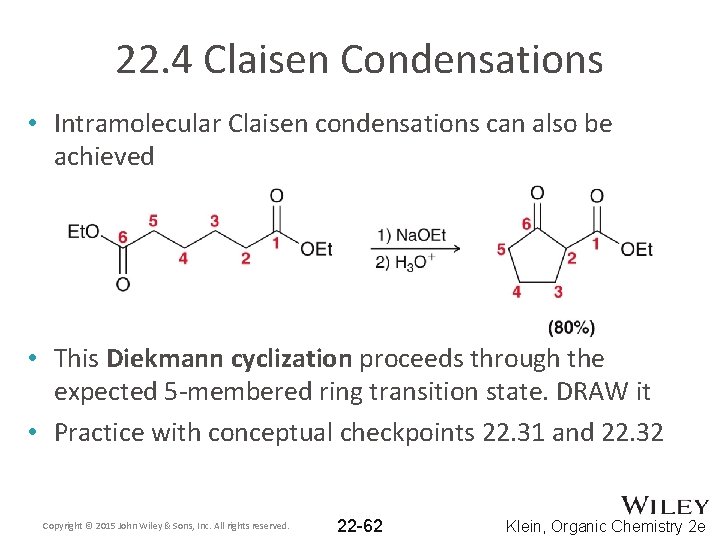
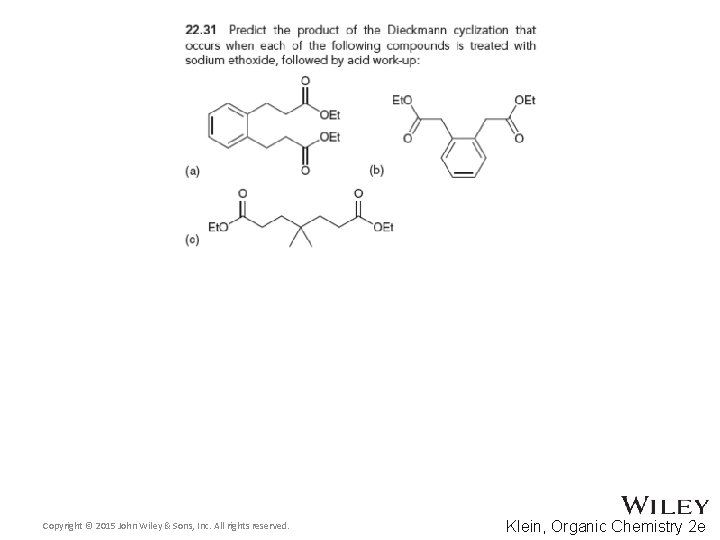
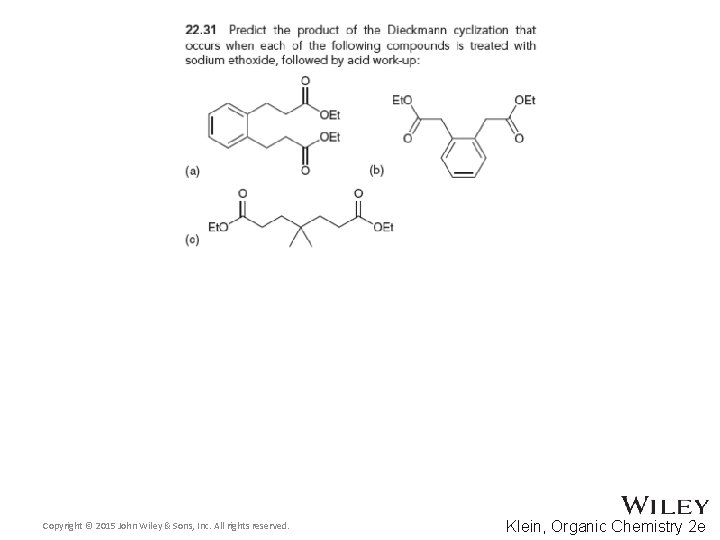
22. 4 Claisen Condensations • Intramolecular Claisen condensations can also be achieved • This Diekmann cyclization proceeds through the expected 5 -membered ring transition state. DRAW it • Practice with conceptual checkpoints 22. 31 and 22. 32 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -62 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

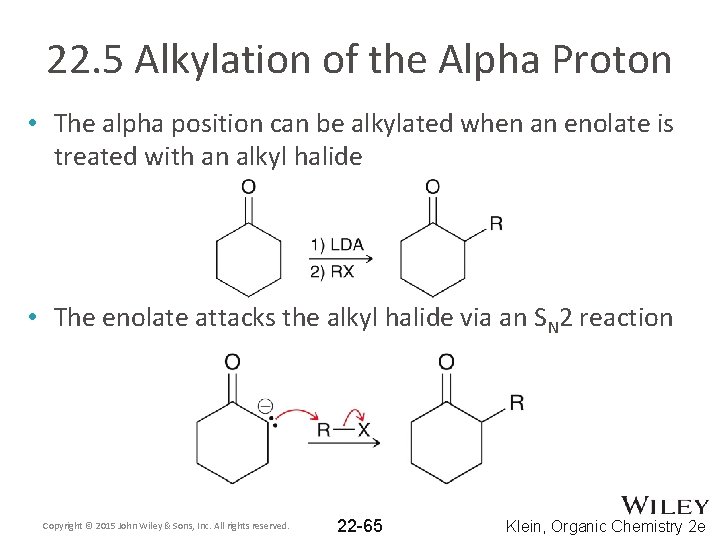
22. 5 Alkylation of the Alpha Proton • The alpha position can be alkylated when an enolate is treated with an alkyl halide • The enolate attacks the alkyl halide via an SN 2 reaction Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -65 Klein, Organic Chemistry 2 e

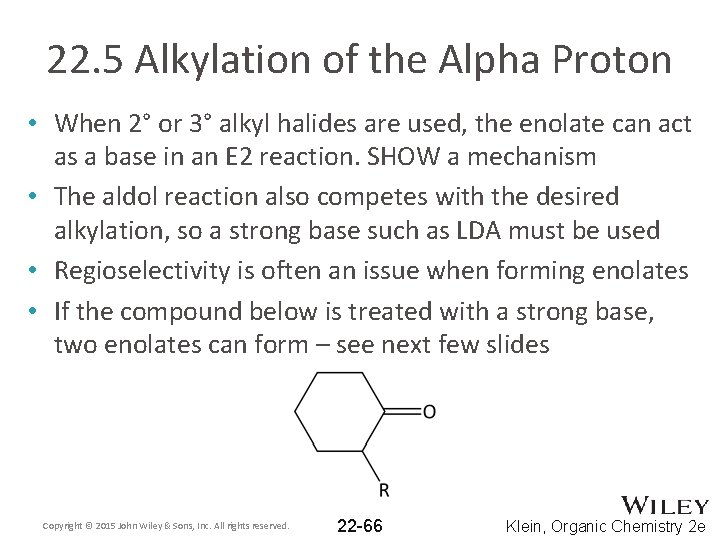
22. 5 Alkylation of the Alpha Proton • When 2° or 3° alkyl halides are used, the enolate can act as a base in an E 2 reaction. SHOW a mechanism • The aldol reaction also competes with the desired alkylation, so a strong base such as LDA must be used • Regioselectivity is often an issue when forming enolates • If the compound below is treated with a strong base, two enolates can form – see next few slides Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -66 Klein, Organic Chemistry 2 e

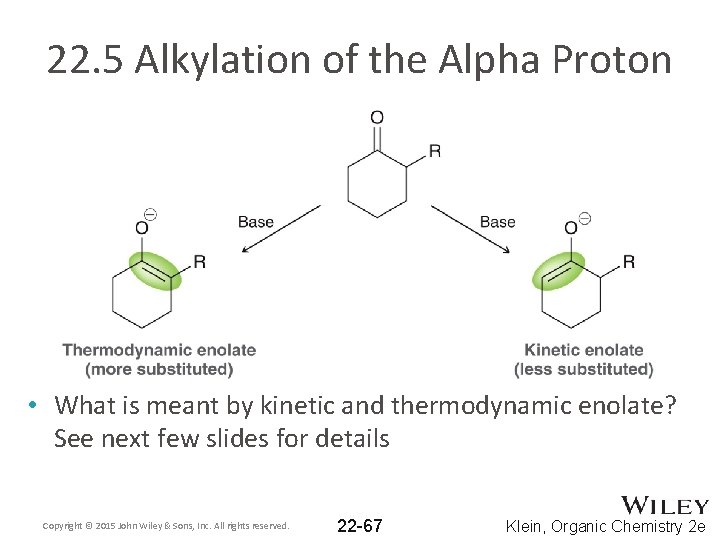
22. 5 Alkylation of the Alpha Proton • What is meant by kinetic and thermodynamic enolate? See next few slides for details Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -67 Klein, Organic Chemistry 2 e

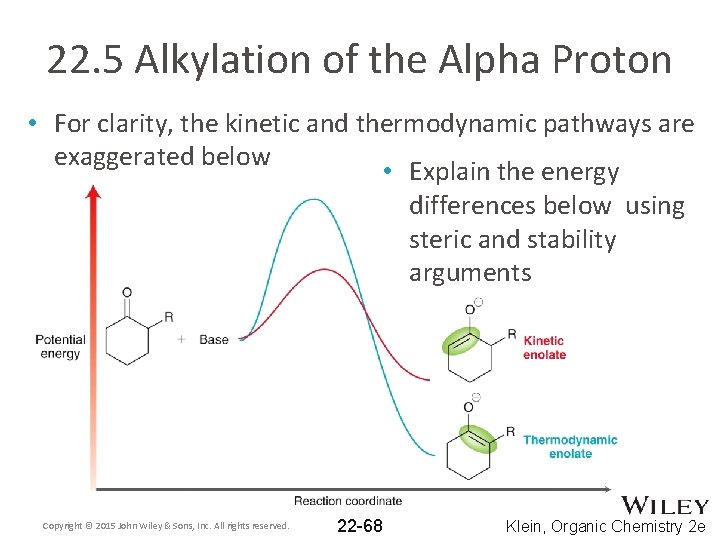
22. 5 Alkylation of the Alpha Proton • For clarity, the kinetic and thermodynamic pathways are exaggerated below • Explain the energy differences below using steric and stability arguments Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -68 Klein, Organic Chemistry 2 e

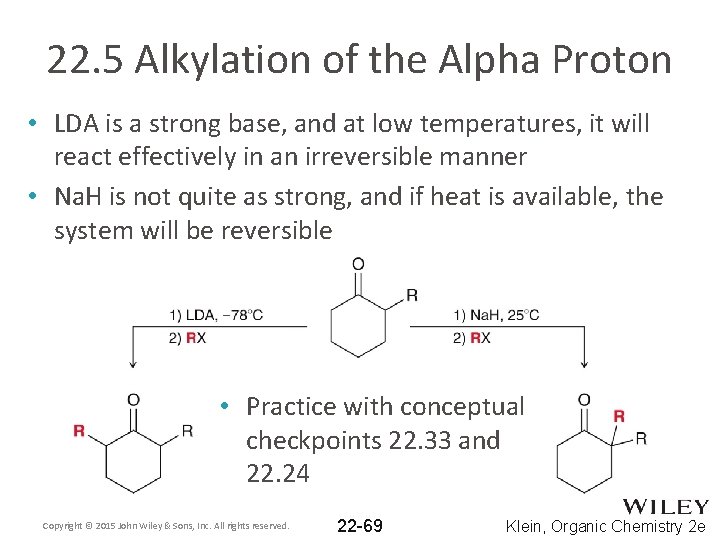
22. 5 Alkylation of the Alpha Proton • LDA is a strong base, and at low temperatures, it will react effectively in an irreversible manner • Na. H is not quite as strong, and if heat is available, the system will be reversible • Practice with conceptual checkpoints 22. 33 and 22. 24 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -69 Klein, Organic Chemistry 2 e

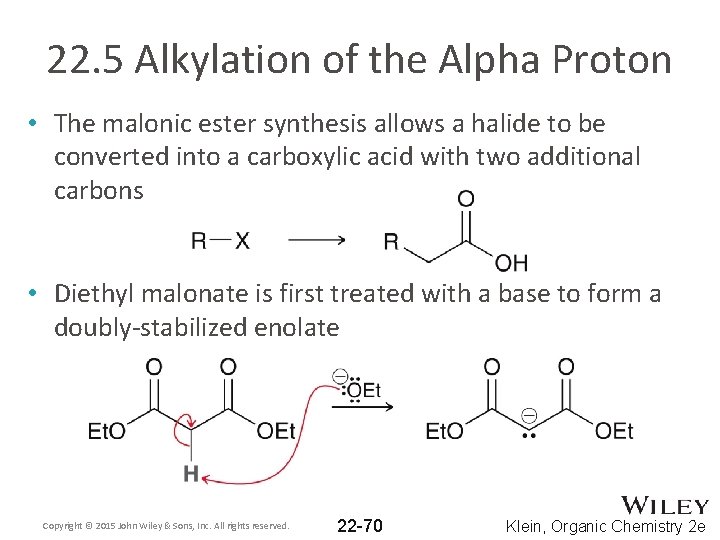
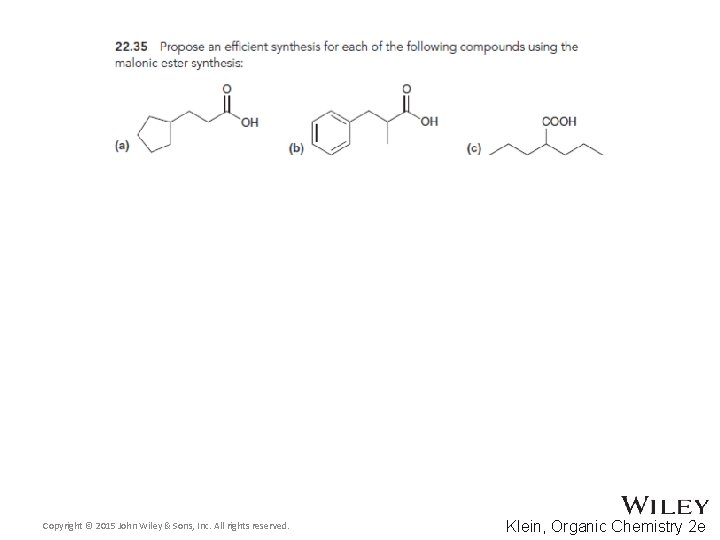
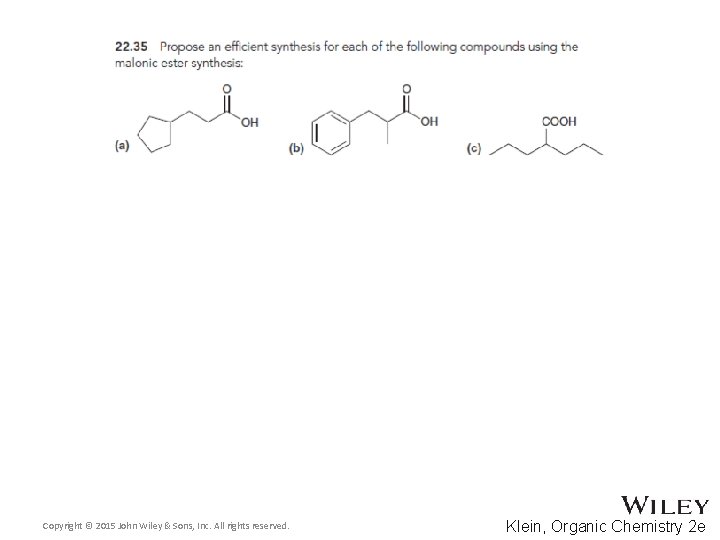
22. 5 Alkylation of the Alpha Proton • The malonic ester synthesis allows a halide to be converted into a carboxylic acid with two additional carbons • Diethyl malonate is first treated with a base to form a doubly-stabilized enolate Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -70 Klein, Organic Chemistry 2 e

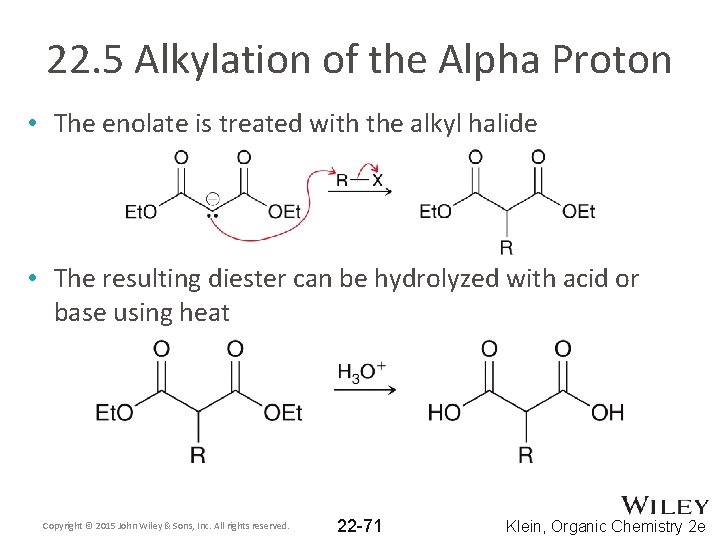
22. 5 Alkylation of the Alpha Proton • The enolate is treated with the alkyl halide • The resulting diester can be hydrolyzed with acid or base using heat Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -71 Klein, Organic Chemistry 2 e

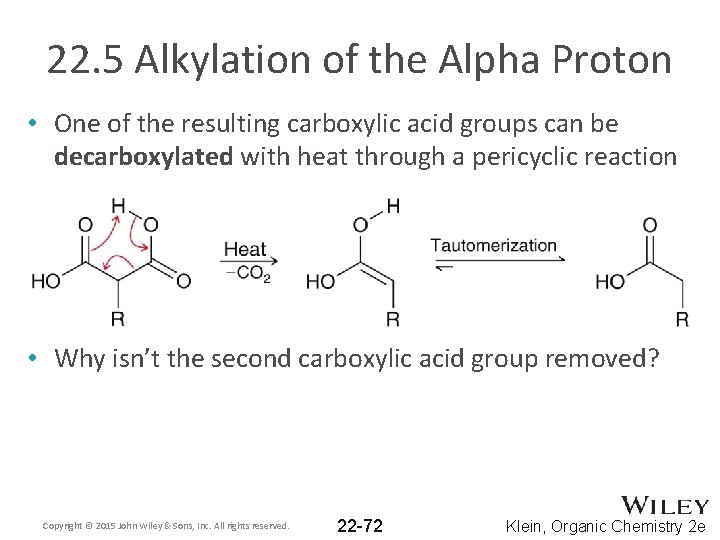
22. 5 Alkylation of the Alpha Proton • One of the resulting carboxylic acid groups can be decarboxylated with heat through a pericyclic reaction • Why isn’t the second carboxylic acid group removed? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -72 Klein, Organic Chemistry 2 e

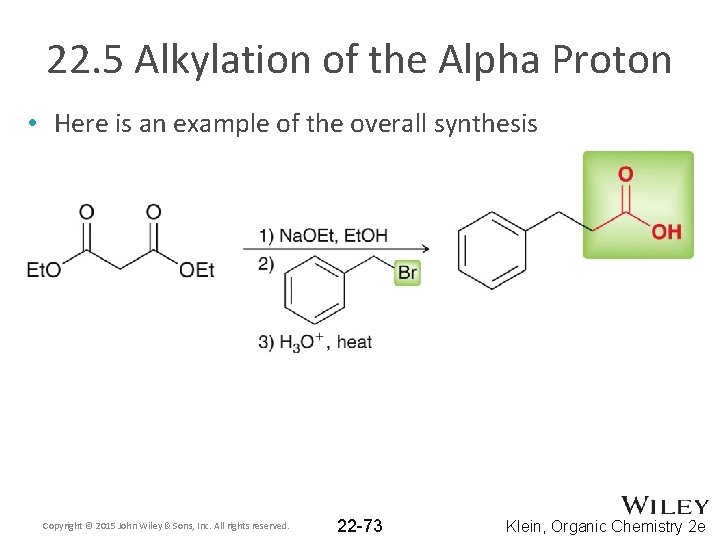
22. 5 Alkylation of the Alpha Proton • Here is an example of the overall synthesis Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -73 Klein, Organic Chemistry 2 e

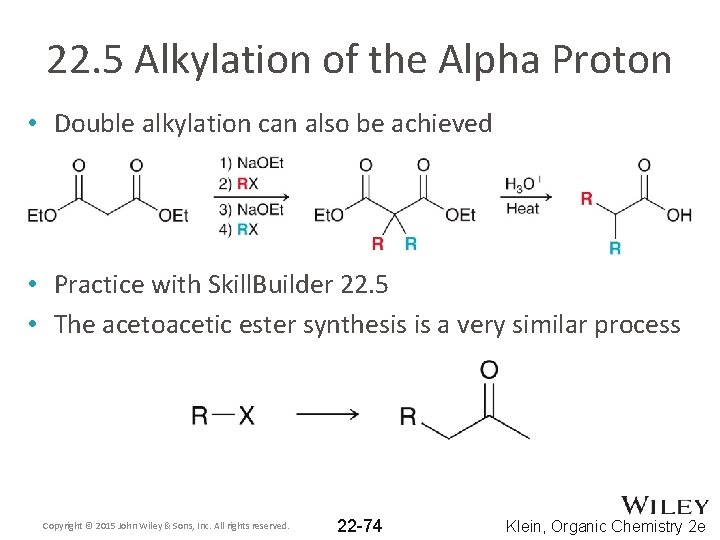
22. 5 Alkylation of the Alpha Proton • Double alkylation can also be achieved • Practice with Skill. Builder 22. 5 • The acetoacetic ester synthesis is a very similar process Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -74 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

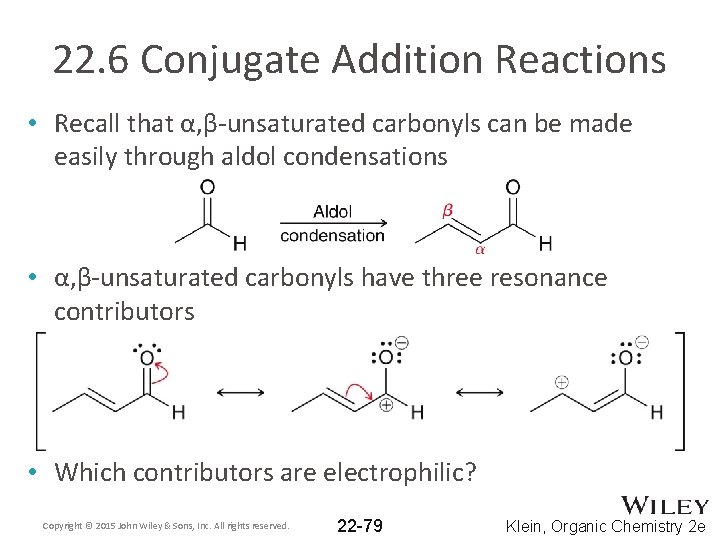
22. 6 Conjugate Addition Reactions • Recall that α, β-unsaturated carbonyls can be made easily through aldol condensations • α, β-unsaturated carbonyls have three resonance contributors • Which contributors are electrophilic? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -79 Klein, Organic Chemistry 2 e

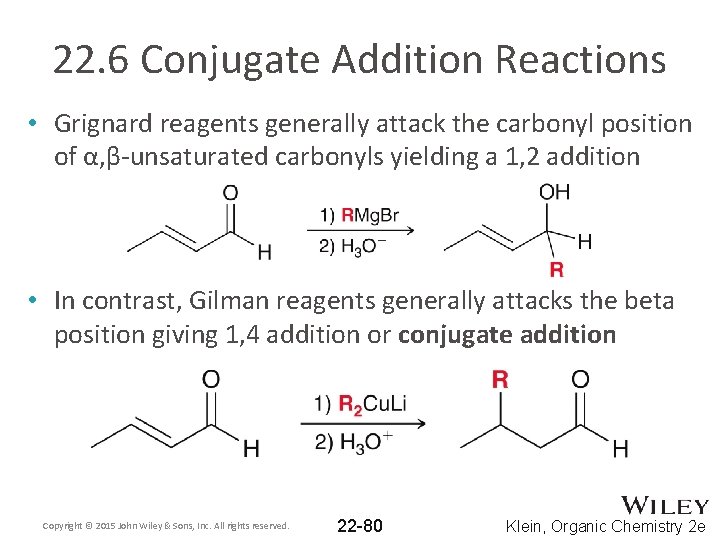
22. 6 Conjugate Addition Reactions • Grignard reagents generally attack the carbonyl position of α, β-unsaturated carbonyls yielding a 1, 2 addition • In contrast, Gilman reagents generally attacks the beta position giving 1, 4 addition or conjugate addition Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -80 Klein, Organic Chemistry 2 e

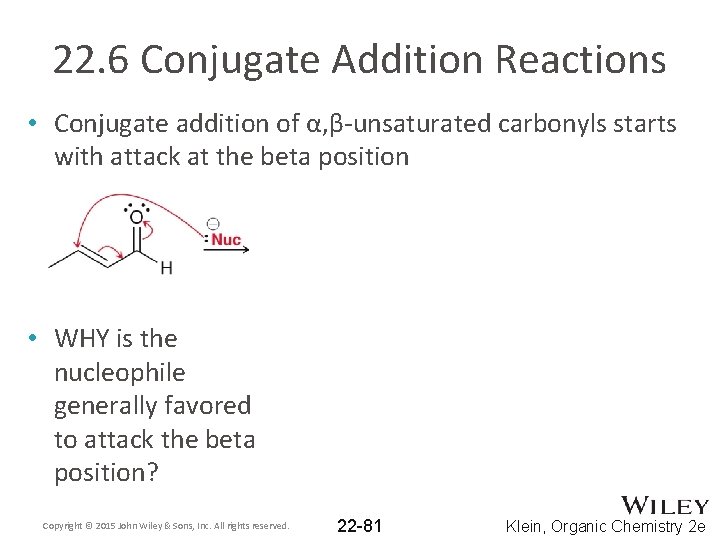
22. 6 Conjugate Addition Reactions • Conjugate addition of α, β-unsaturated carbonyls starts with attack at the beta position • WHY is the nucleophile generally favored to attack the beta position? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -81 Klein, Organic Chemistry 2 e

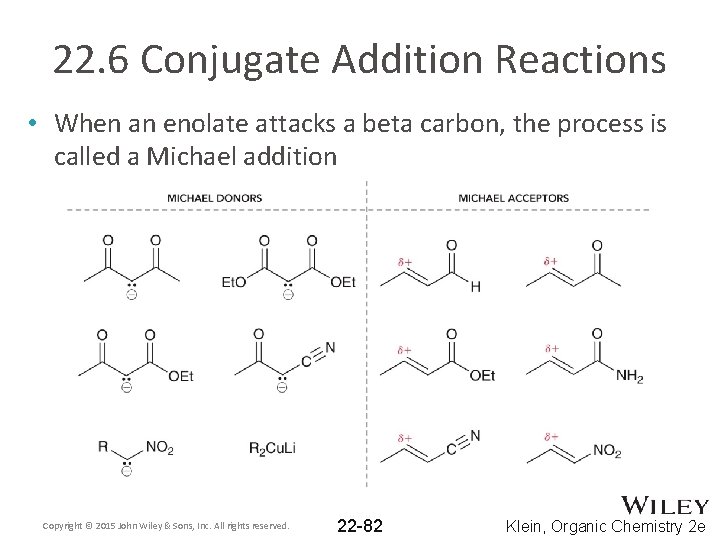
22. 6 Conjugate Addition Reactions • When an enolate attacks a beta carbon, the process is called a Michael addition Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -82 Klein, Organic Chemistry 2 e

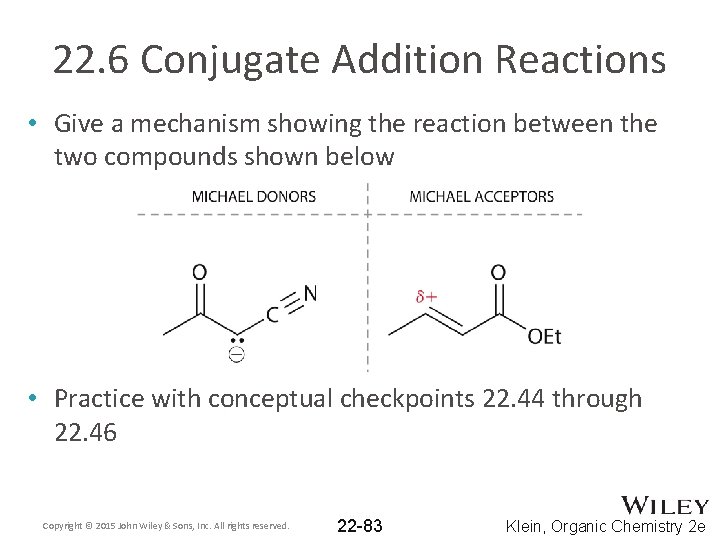
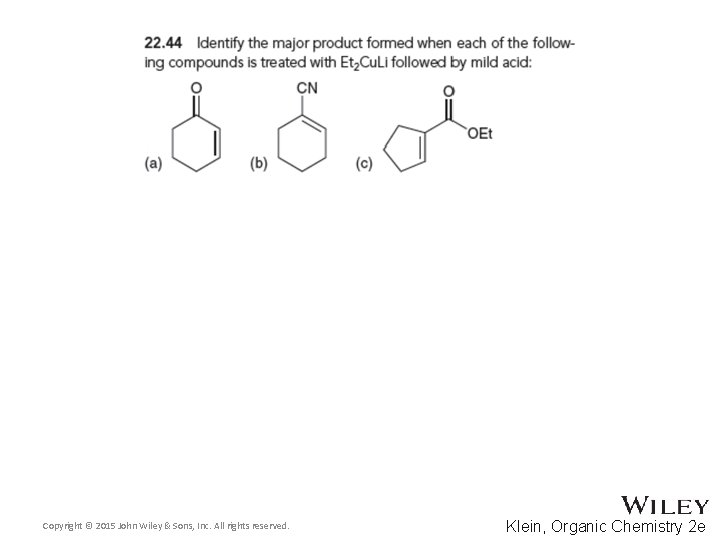
22. 6 Conjugate Addition Reactions • Give a mechanism showing the reaction between the two compounds shown below • Practice with conceptual checkpoints 22. 44 through 22. 46 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -83 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

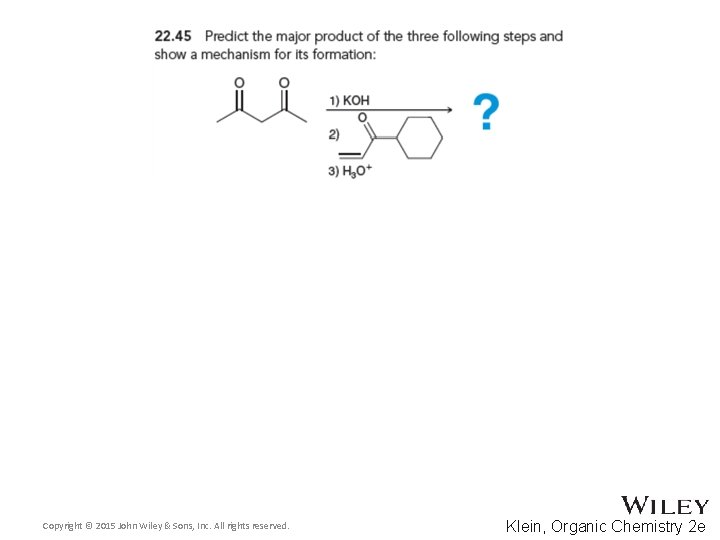
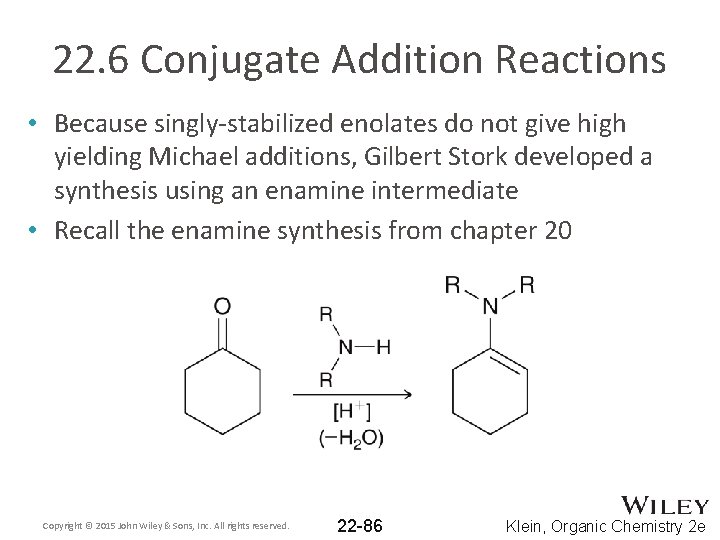
22. 6 Conjugate Addition Reactions • Because singly-stabilized enolates do not give high yielding Michael additions, Gilbert Stork developed a synthesis using an enamine intermediate • Recall the enamine synthesis from chapter 20 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -86 Klein, Organic Chemistry 2 e

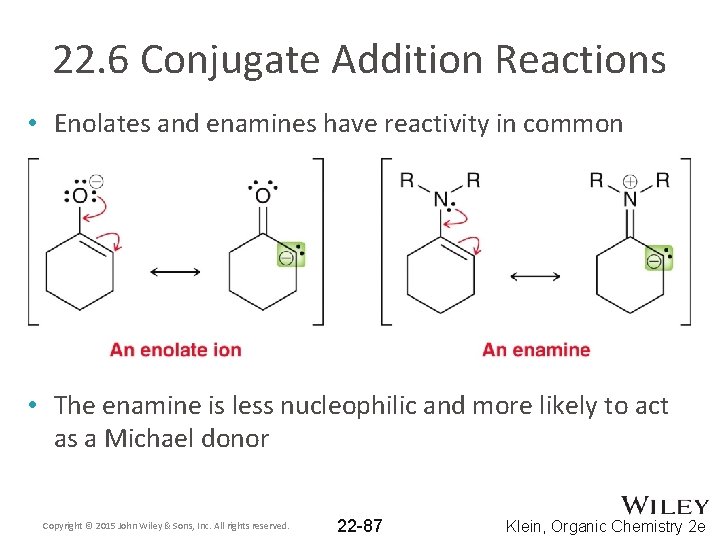
22. 6 Conjugate Addition Reactions • Enolates and enamines have reactivity in common • The enamine is less nucleophilic and more likely to act as a Michael donor Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -87 Klein, Organic Chemistry 2 e

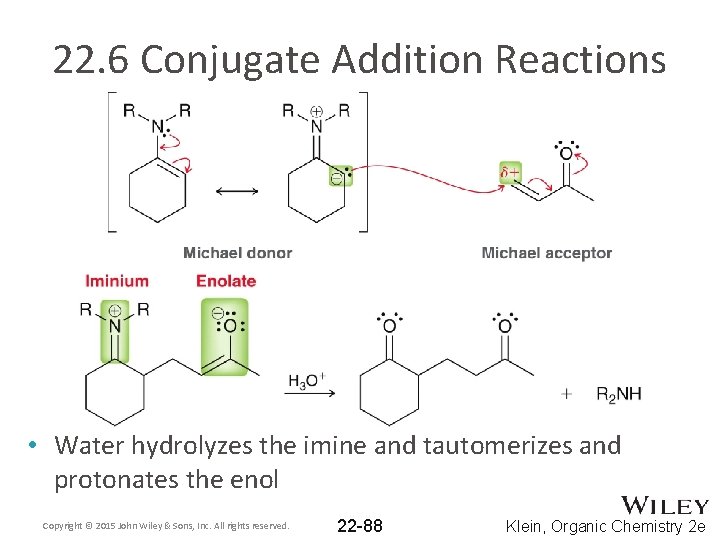
22. 6 Conjugate Addition Reactions • Water hydrolyzes the imine and tautomerizes and protonates the enol Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -88 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

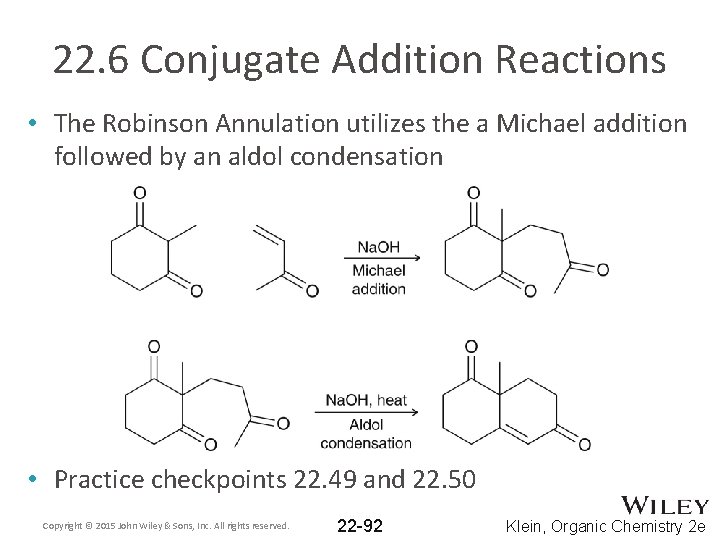
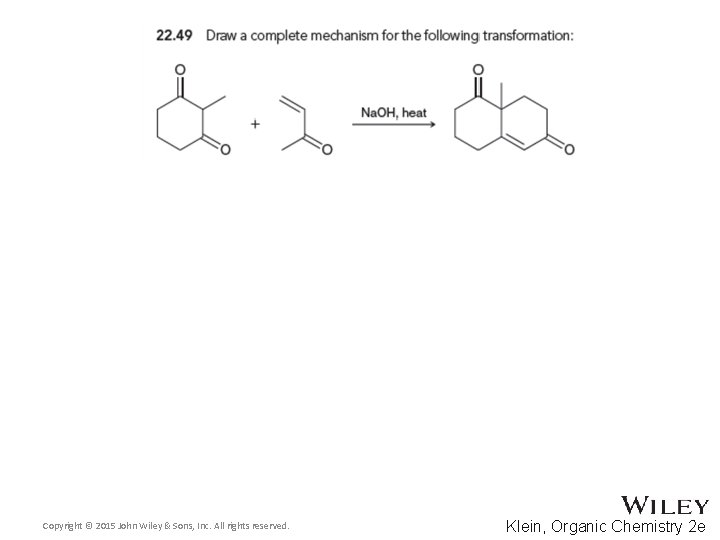
22. 6 Conjugate Addition Reactions • The Robinson Annulation utilizes the a Michael addition followed by an aldol condensation • Practice checkpoints 22. 49 and 22. 50 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -92 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

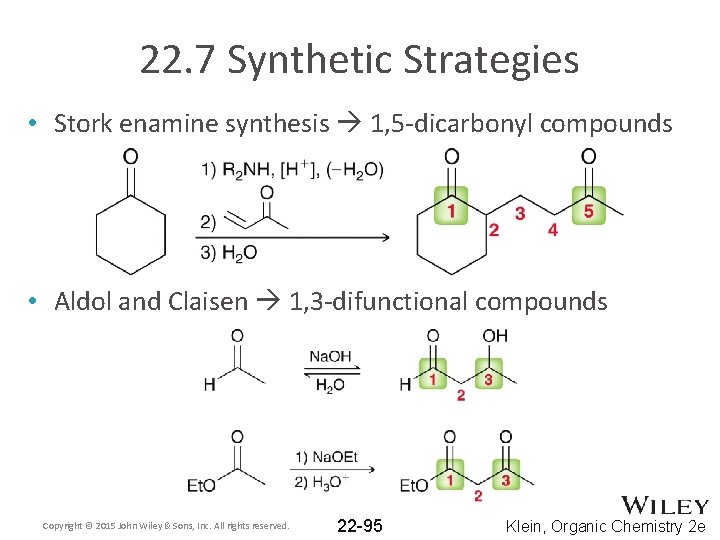
22. 7 Synthetic Strategies • Most of the reactions in this chapter are C-C bond forming • Three of the reactions yield a product with two functional groups • The positions of the functional groups in the product can be used to design necessary reagents in the synthesis – see next few slides • Practice with Skill. Builder 22. 8 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -94 Klein, Organic Chemistry 2 e

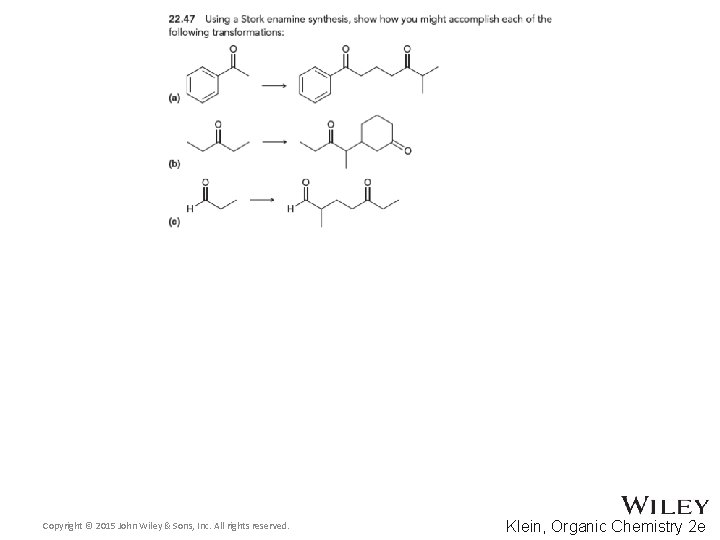
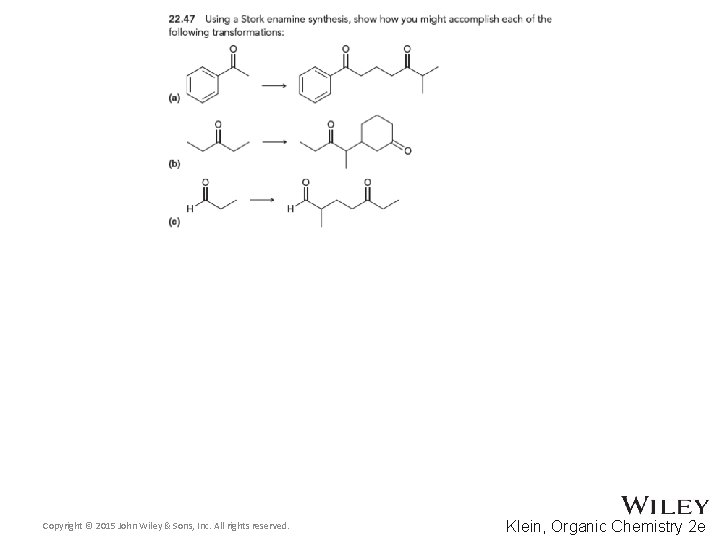
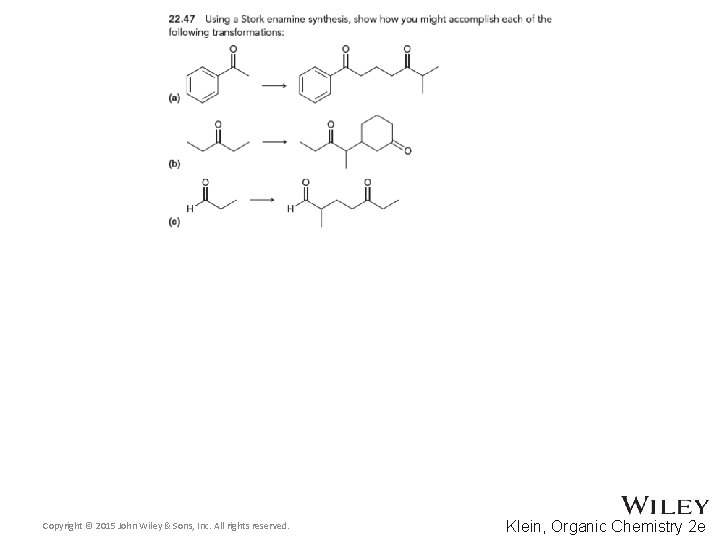
22. 7 Synthetic Strategies • Stork enamine synthesis 1, 5 -dicarbonyl compounds • Aldol and Claisen 1, 3 -difunctional compounds Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -95 Klein, Organic Chemistry 2 e

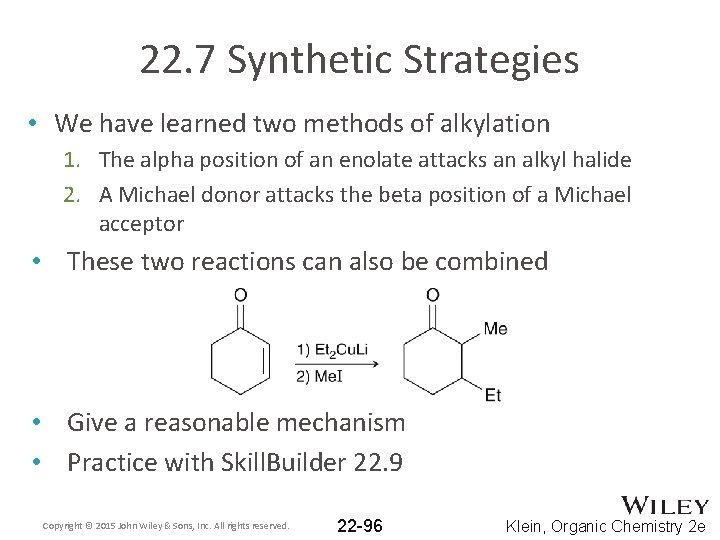
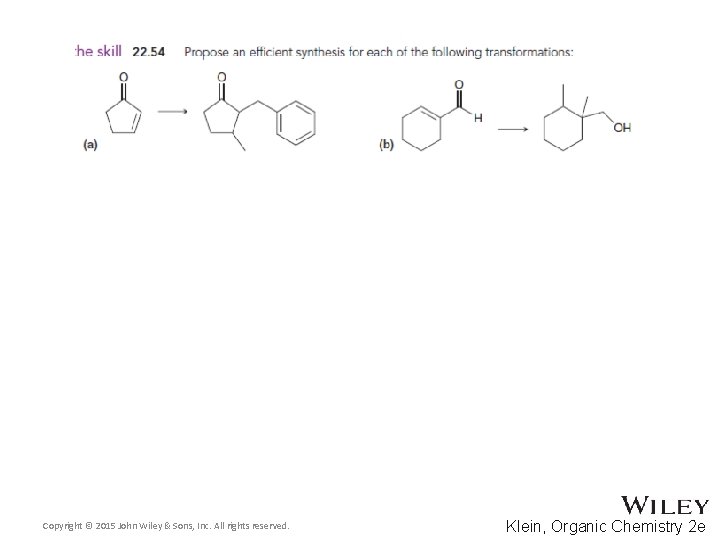
22. 7 Synthetic Strategies • We have learned two methods of alkylation 1. The alpha position of an enolate attacks an alkyl halide 2. A Michael donor attacks the beta position of a Michael acceptor • These two reactions can also be combined • Give a reasonable mechanism • Practice with Skill. Builder 22. 9 Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -96 Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2 e

Additional Practice Problems • Explain why an enolate is more likely to produce products resulting from attack by the alpha carbon than direct attack by the oxygen. Is the argument kinetic or thermodynamic or both? Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -99 Klein, Organic Chemistry 2 e

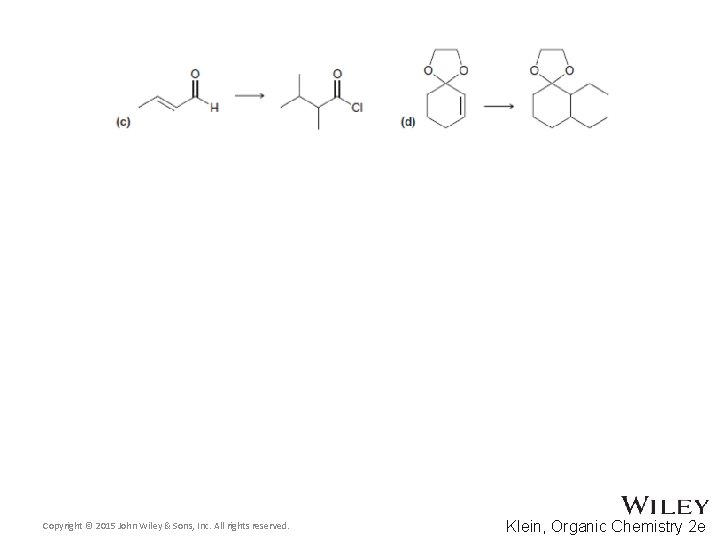
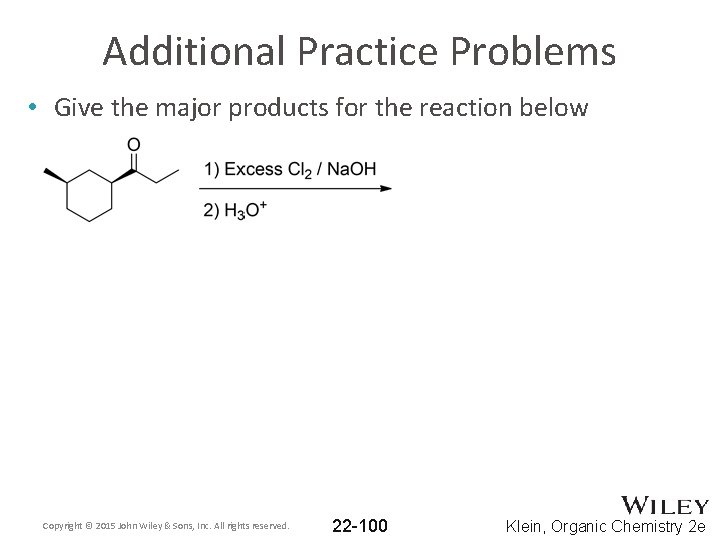
Additional Practice Problems • Give the major products for the reaction below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -100 Klein, Organic Chemistry 2 e

Additional Practice Problems • Using both kinetic and thermodynamic arguments, explain why aldol reactions involving a ketone are less product favored than those involving aldehydes Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -101 Klein, Organic Chemistry 2 e

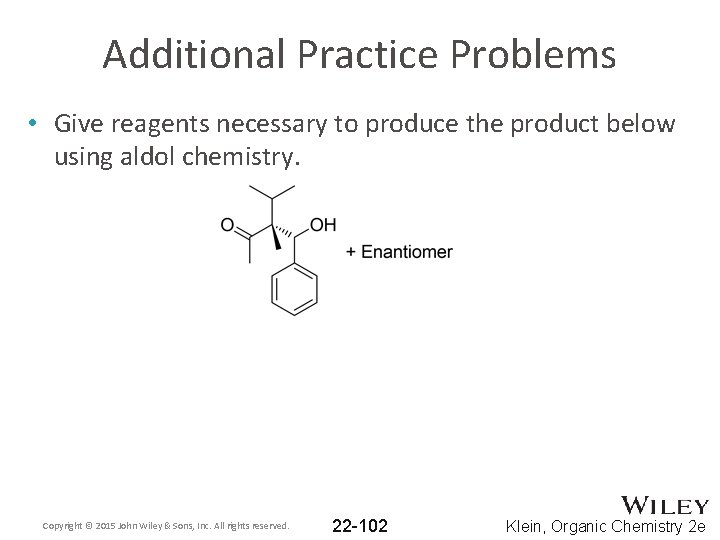
Additional Practice Problems • Give reagents necessary to produce the product below using aldol chemistry. Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -102 Klein, Organic Chemistry 2 e

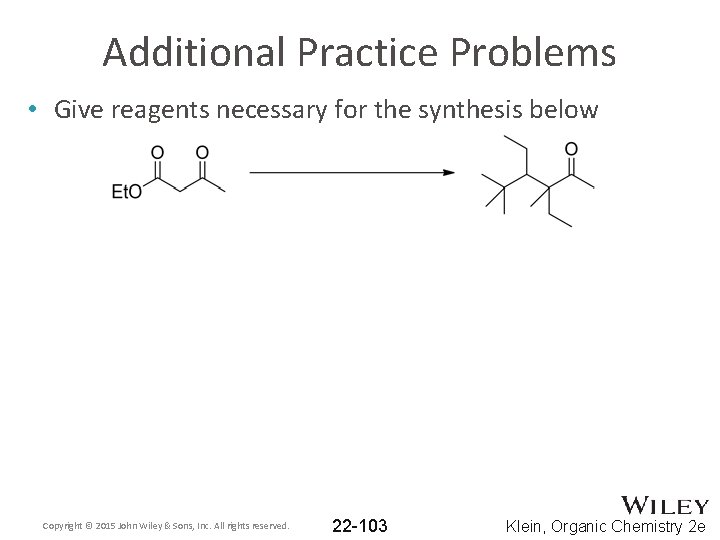
Additional Practice Problems • Give reagents necessary for the synthesis below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -103 Klein, Organic Chemistry 2 e

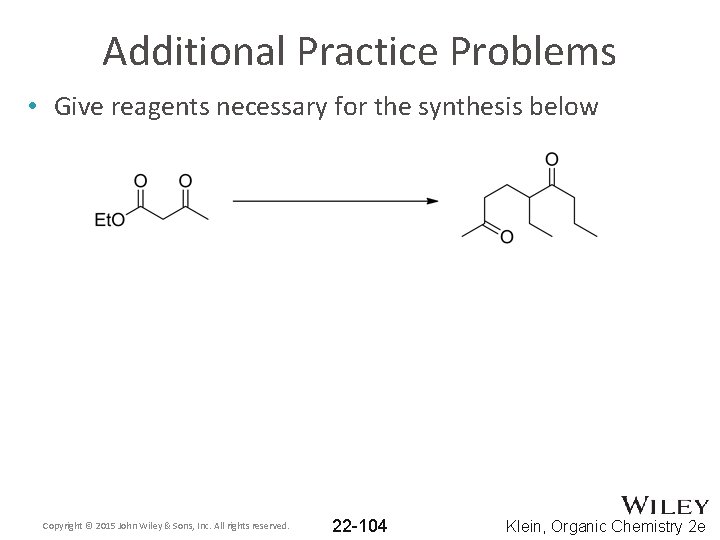
Additional Practice Problems • Give reagents necessary for the synthesis below Copyright © 2015 John Wiley & Sons, Inc. All rights reserved. 22 -104 Klein, Organic Chemistry 2 e