SOAPMAKING The chemistry of soap Organic chemistry review

- Slides: 10

SOAPMAKING The chemistry of soap

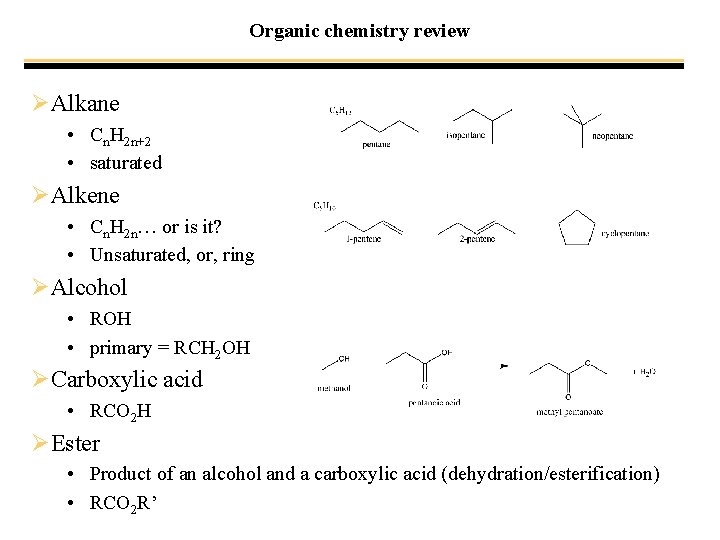
Organic chemistry review ØAlkane • Cn. H 2 n+2 • saturated ØAlkene • Cn. H 2 n… or is it? • Unsaturated, or, ring ØAlcohol • ROH • primary = RCH 2 OH ØCarboxylic acid • RCO 2 H ØEster • Product of an alcohol and a carboxylic acid (dehydration/esterification) • RCO 2 R’

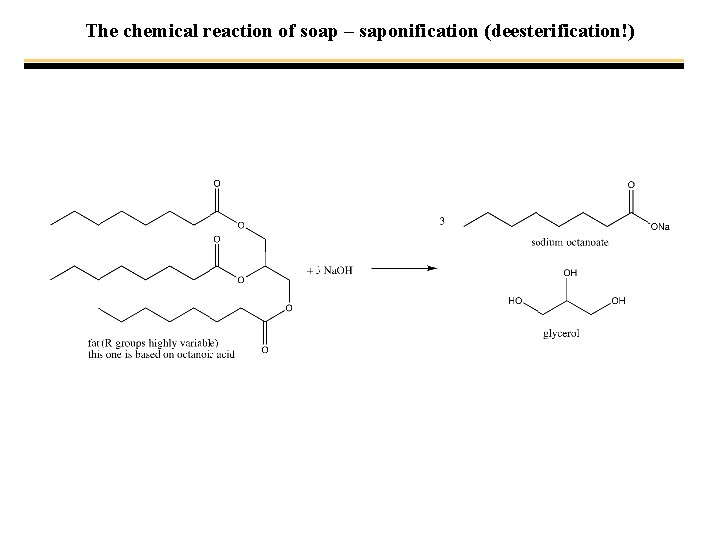
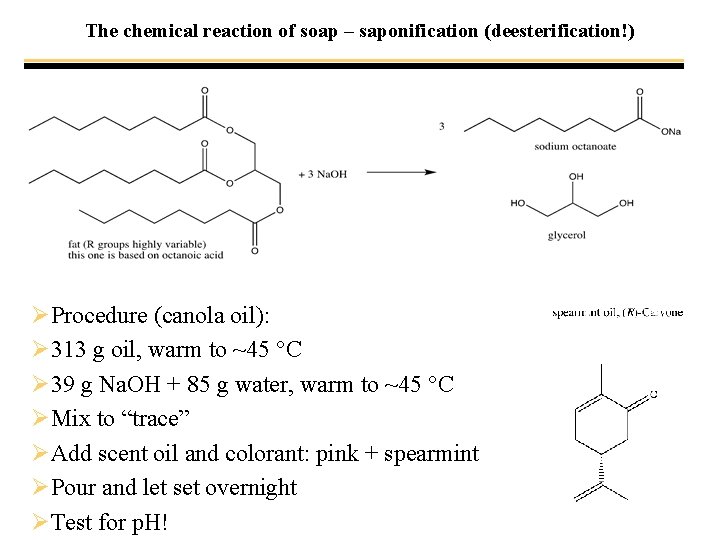
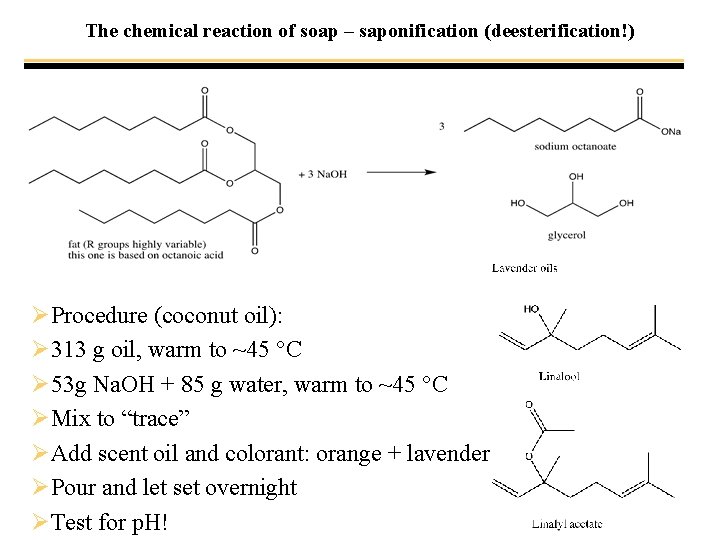
The chemical reaction of soap – saponification (deesterification!)

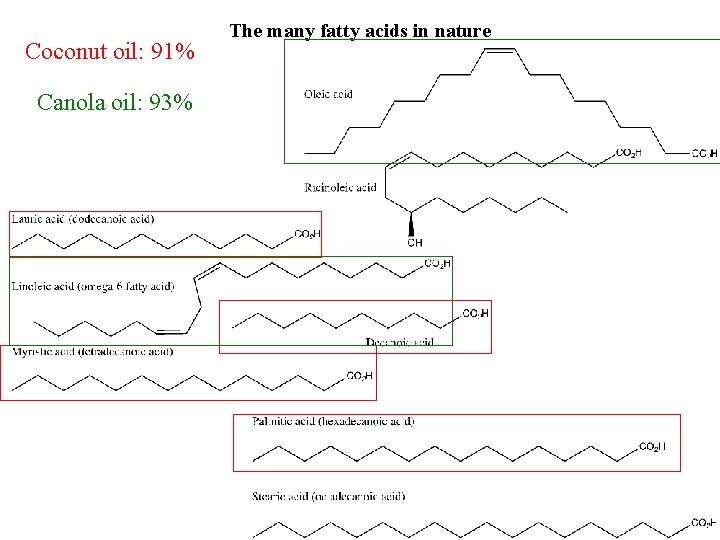
Coconut oil: 91% Canola oil: 93% The many fatty acids in nature

The chemical reaction of soap – saponification (deesterification!) ØProcedure (canola oil): Ø 313 g oil, warm to ~45 °C Ø 39 g Na. OH + 85 g water, warm to ~45 °C ØMix to “trace” ØAdd scent oil and colorant: pink + spearmint ØPour and let set overnight ØTest for p. H!

The chemical reaction of soap – saponification (deesterification!) ØProcedure (coconut oil): Ø 313 g oil, warm to ~45 °C Ø 53 g Na. OH + 85 g water, warm to ~45 °C ØMix to “trace” ØAdd scent oil and colorant: orange + lavender ØPour and let set overnight ØTest for p. H!

Calculating molecular weight ØMixture of substances ØNo single measure of molecular weight ØDifferent experimental techniques give different values ØNumber average, Mn (arithmetic mean of individual MWs) • Total weight of all the samples divided by total # of moles/sample • ∑Ni. Mi = number of moles·MW = mass; this is the mass of the sample • ∑Ni. Mi/∑Ni = mass/moles ØWeight average, Mw • • Find weight fraction of each molecule, WFi = Ni. Mi/∑Ni. Mi (unitless) This value is given as weight % Weight of that fraction, Wi = WFi·Mi (mass/moles) ∑Wi = sum of all molecular weights

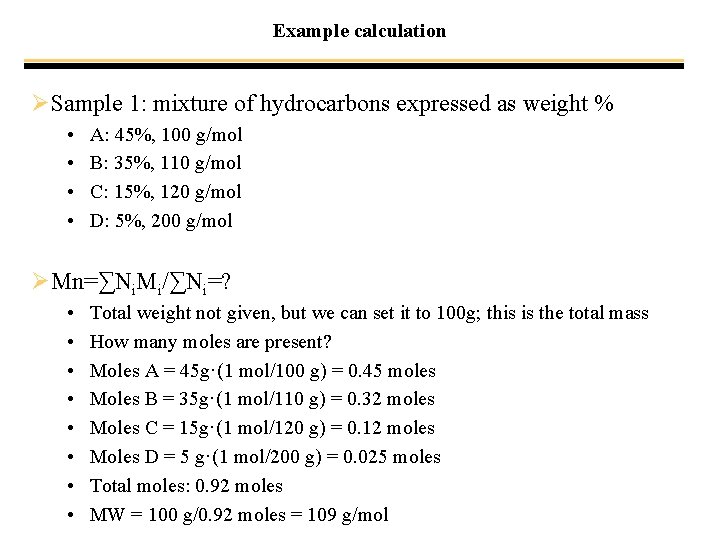
Example calculation ØSample 1: mixture of hydrocarbons expressed as weight % • • A: 45%, 100 g/mol B: 35%, 110 g/mol C: 15%, 120 g/mol D: 5%, 200 g/mol ØMn=∑Ni. Mi/∑Ni=? • • Total weight not given, but we can set it to 100 g; this is the total mass How many moles are present? Moles A = 45 g·(1 mol/100 g) = 0. 45 moles Moles B = 35 g·(1 mol/110 g) = 0. 32 moles Moles C = 15 g·(1 mol/120 g) = 0. 12 moles Moles D = 5 g·(1 mol/200 g) = 0. 025 moles Total moles: 0. 92 moles MW = 100 g/0. 92 moles = 109 g/mol

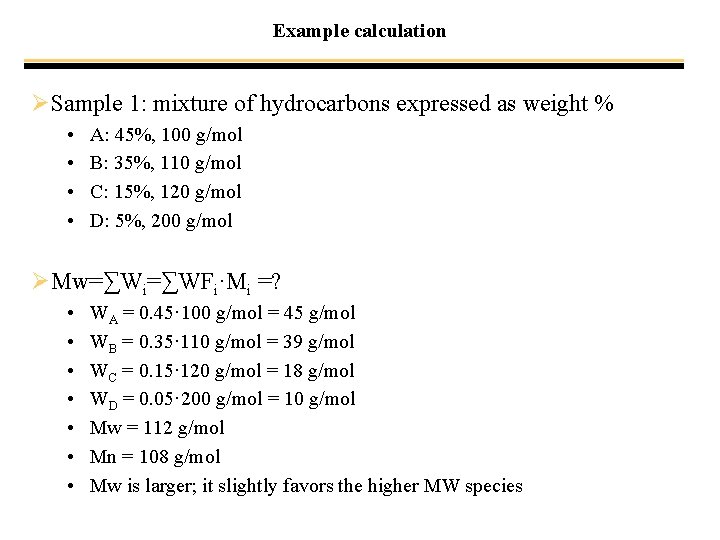
Example calculation ØSample 1: mixture of hydrocarbons expressed as weight % • • A: 45%, 100 g/mol B: 35%, 110 g/mol C: 15%, 120 g/mol D: 5%, 200 g/mol ØMw=∑Wi=∑WFi·Mi =? • • WA = 0. 45· 100 g/mol = 45 g/mol WB = 0. 35· 110 g/mol = 39 g/mol WC = 0. 15· 120 g/mol = 18 g/mol WD = 0. 05· 200 g/mol = 10 g/mol Mw = 112 g/mol Mn = 108 g/mol Mw is larger; it slightly favors the higher MW species

Reminder of Wednesday exercises and reading Øa) Verify that the two soaps in class are overfatted. Turn in your work, clearly demonstrating how you arrived at your answer, at the beginning of the next class. The molecular weight to use for coconut oil and canola oils are 698 g/mol and 932 g/mol respectively. I used 313 g of each oil, and 39. 0 g (canola) or 53. 0 g (coconut) of Na. OH. Øb) draw a picture or cartoon that explains how soap works at the molecular level. Include several sentences of description. ØI calculated these molecular weights using one of the two techniques; the other technique gives a different value!
 Canola oil
Canola oil Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Cycloalkanes
Cycloalkanes Ester organic chemistry
Ester organic chemistry Chemistry organic
Chemistry organic Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s



















