Organic Chemistry 1 Crude oil and cracking Cracking















- Slides: 39

Organic Chemistry 1 Crude oil and cracking

Cracking video Cracking class practical

Crude oil and cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe the process of fractional distillation of crude oil • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why alkanes are reformed • Explain why the boiling points of different alkanes are different

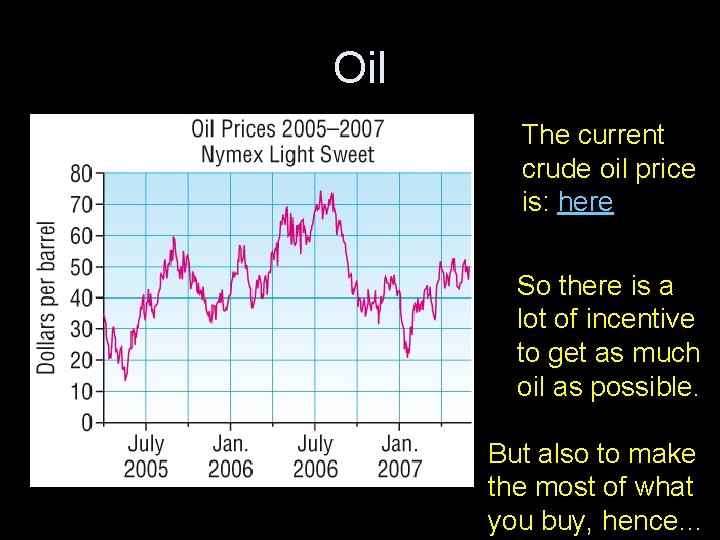
Oil The current crude oil price is: here So there is a lot of incentive to get as much oil as possible. But also to make the most of what you buy, hence…

Crude oil and alkanes Crude oil is a mixture composed mainly of straight and branched chain alkanes. It also includes lesser amounts of cycloalkanes and arenes, both of which are hydrocarbons containing a ring of carbon atoms, as well as impurities such as sulfur compounds. The exact composition of crude oil depends on the conditions under which it formed, so crude oil extracted at different locations has different compositions. 5 of 37 © Boardworks Ltd 2009

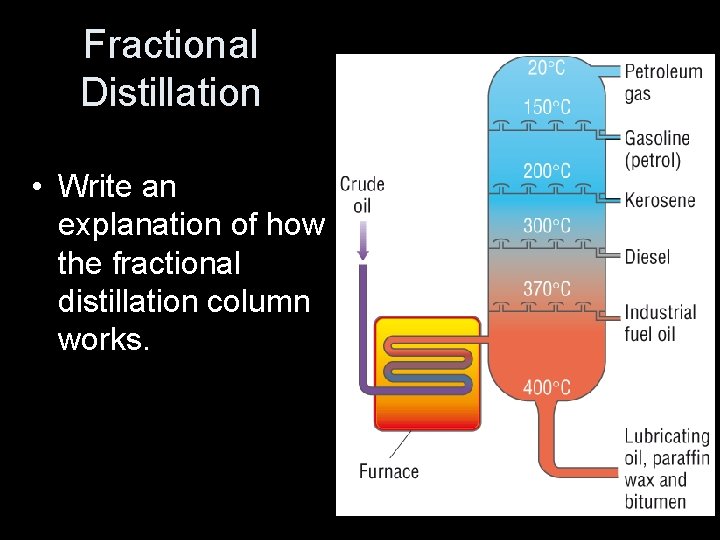
Fractional Distillation • Write an explanation of how the fractional distillation column works.

How does fractional distillation work?

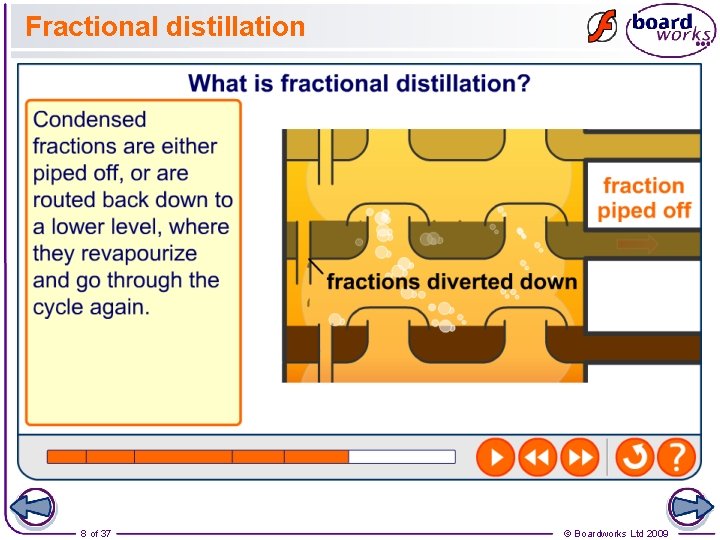
Fractional distillation 8 of 37 © Boardworks Ltd 2009

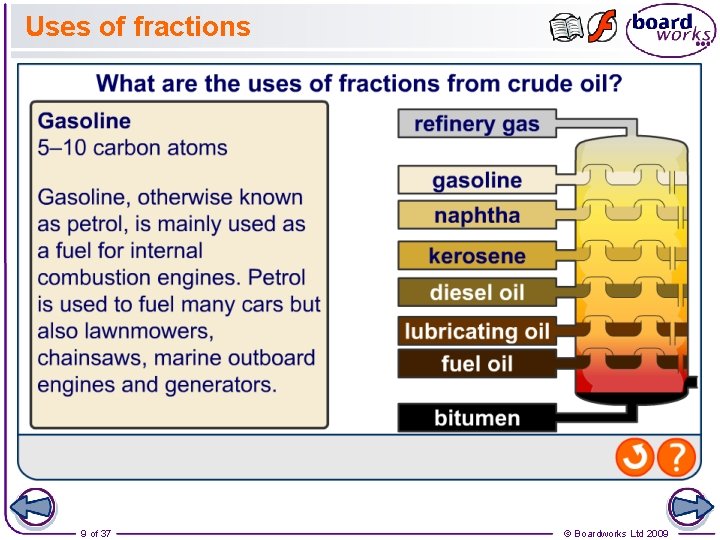
Uses of fractions 9 of 37 © Boardworks Ltd 2009

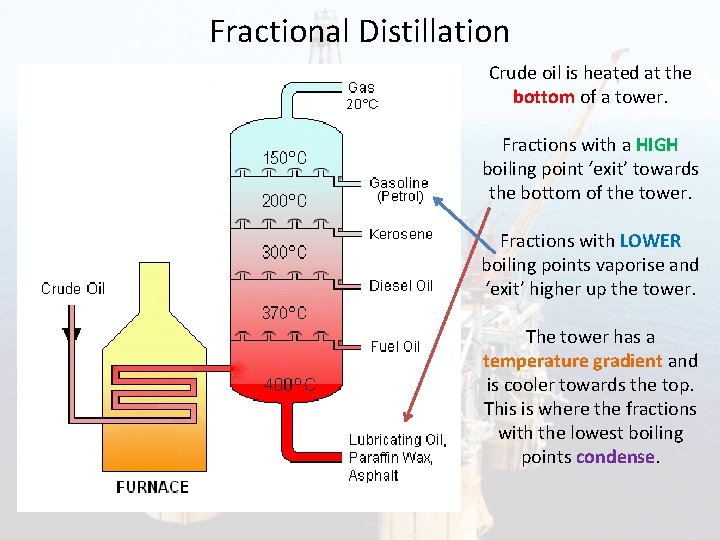
Fractional distillation of crude oil 1. Oil is heated to about 370 °C and pumped into the bottom of a tall tower called a fractionating column, where it vaporizes. 2. The column is very hot at the bottom but much cooler at the top. As the vaporized oil rises, it cools and condenses. 3. Large molecules have high boiling points and condense near the bottom of the column. 4. Small molecules have lower boiling points and condense further up the column.

Fractional Distillation Crude oil is heated at the bottom of a tower. Fractions with a HIGH boiling point ‘exit’ towards the bottom of the tower. Fractions with LOWER boiling points vaporise and ‘exit’ higher up the tower. The tower has a temperature gradient and is cooler towards the top. This is where the fractions with the lowest boiling points condense.

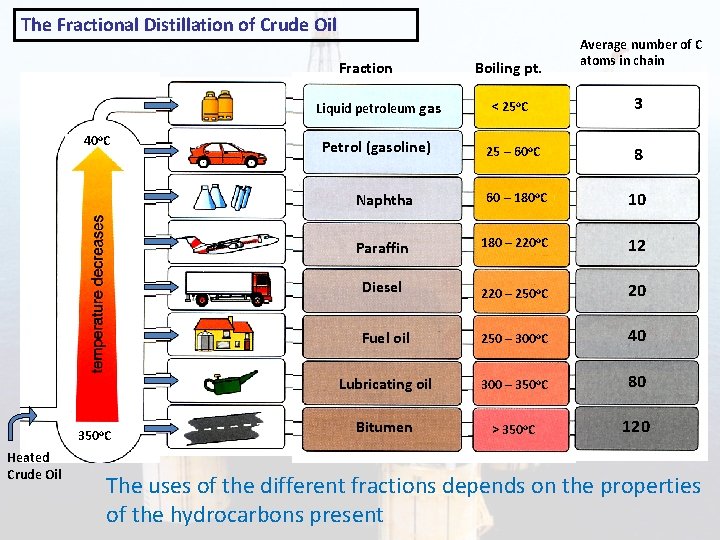
The Fractional Distillation of Crude Oil Fraction 40 o. C 350 o. C Heated Crude Oil Boiling pt. Average number of C atoms in chain Liquid petroleum gas < 25 o. C 3 Petrol (gasoline) 25 – 60 o. C 8 Naphtha 60 – 180 o. C 10 Paraffin 180 – 220 o. C 12 Diesel 220 – 250 o. C 20 Fuel oil 250 – 300 o. C 40 Lubricating oil 300 – 350 o. C 80 Bitumen > 350 o. C 120 The uses of the different fractions depends on the properties of the hydrocarbons present

Boiling Points of Alkanes • Fractional distillation works because the bigger the hydrocarbon, the higher the boiling point. • But why is that? • What kind of forces hold alkane molecules together? • Alkanes are non-polar… • …so only Van-der Waals’

Crude oil and cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe the process of fractional distillation of crude oil • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why alkanes are reformed • Explain why the boiling points of different alkanes are different

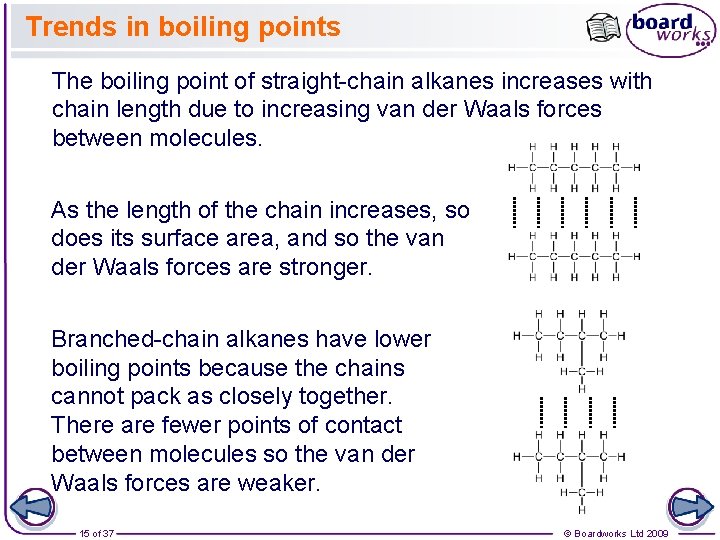
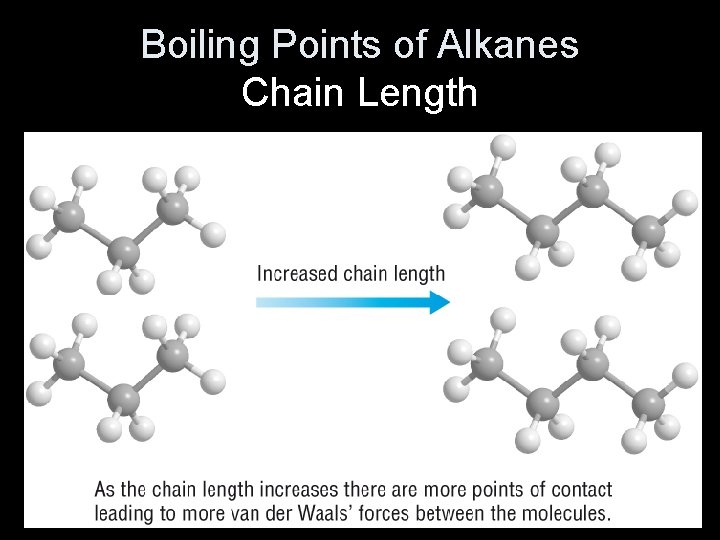
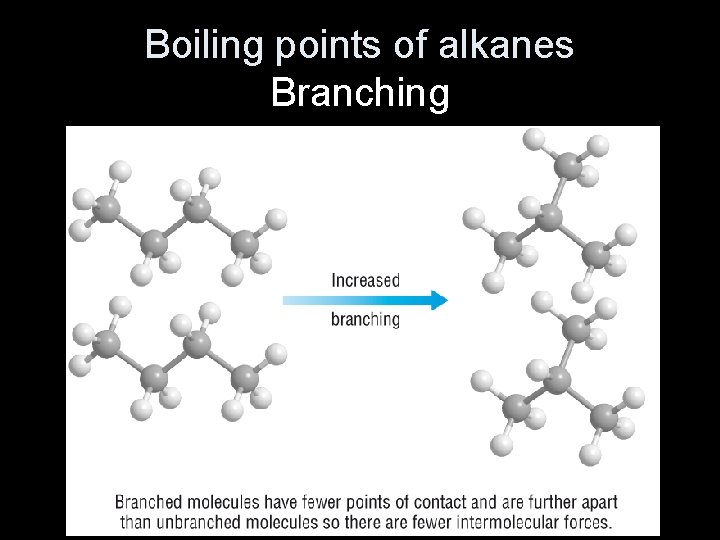
Trends in boiling points The boiling point of straight-chain alkanes increases with chain length due to increasing van der Waals forces between molecules. As the length of the chain increases, so does its surface area, and so the van der Waals forces are stronger. Branched-chain alkanes have lower boiling points because the chains cannot pack as closely together. There are fewer points of contact between molecules so the van der Waals forces are weaker. 15 of 37 © Boardworks Ltd 2009

Boiling Points of Alkanes Chain Length

Boiling points of alkanes Branching

Crude oil and cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe the process of fractional distillation of crude oil • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why alkanes are reformed • Explain why the boiling points of different alkanes are different

Supply and demand The demand for lower boiling point (shorter chain) fractions is greater than the proportion found in crude oil. Crude oil contains more higher boiling point (longer chain) fractions, which are in lower demand are less economically valuable. There is therefore a shortage of shorter chain fractions and a surplus of longer chain ones. 19 of 37 © Boardworks Ltd 2009

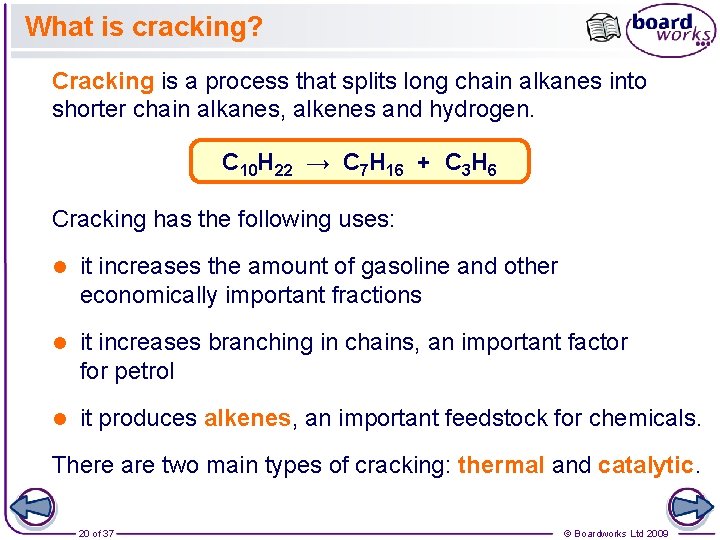
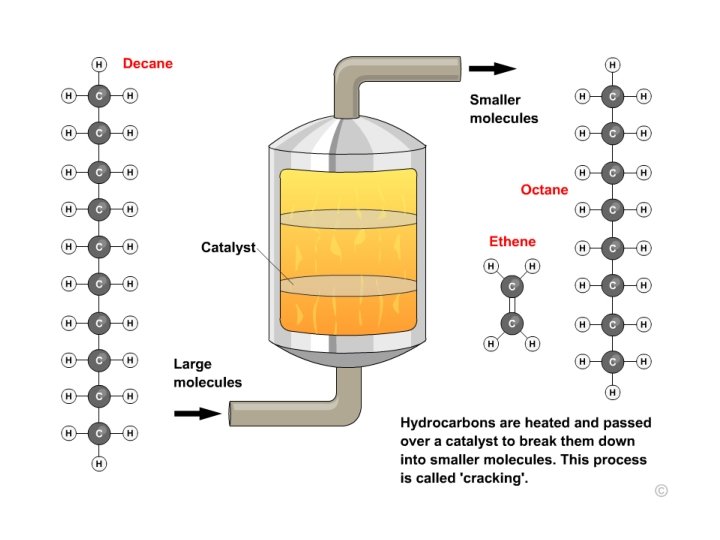
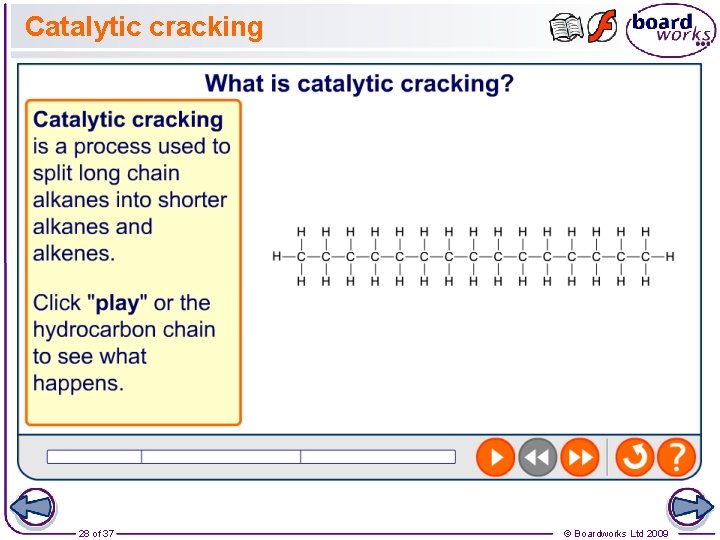
What is cracking? Cracking is a process that splits long chain alkanes into shorter chain alkanes, alkenes and hydrogen. C 10 H 22 → C 7 H 16 + C 3 H 6 Cracking has the following uses: l it increases the amount of gasoline and other economically important fractions l it increases branching in chains, an important factor for petrol l it produces alkenes, an important feedstock for chemicals. There are two main types of cracking: thermal and catalytic. 20 of 37 © Boardworks Ltd 2009

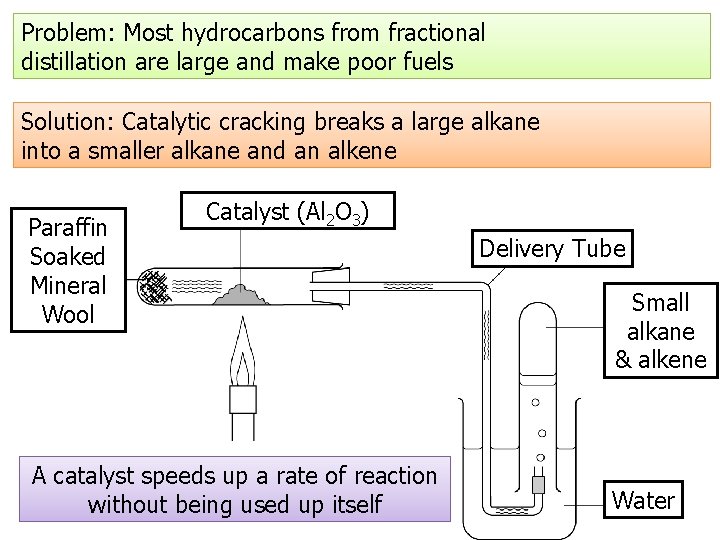
Problem: Most hydrocarbons from fractional distillation are large and make poor fuels Solution: Catalytic cracking breaks a large alkane into a smaller alkane and an alkene Paraffin Soaked Mineral Wool Catalyst (Al 2 O 3) A catalyst speeds up a rate of reaction without being used up itself Delivery Tube Small alkane & alkene Water

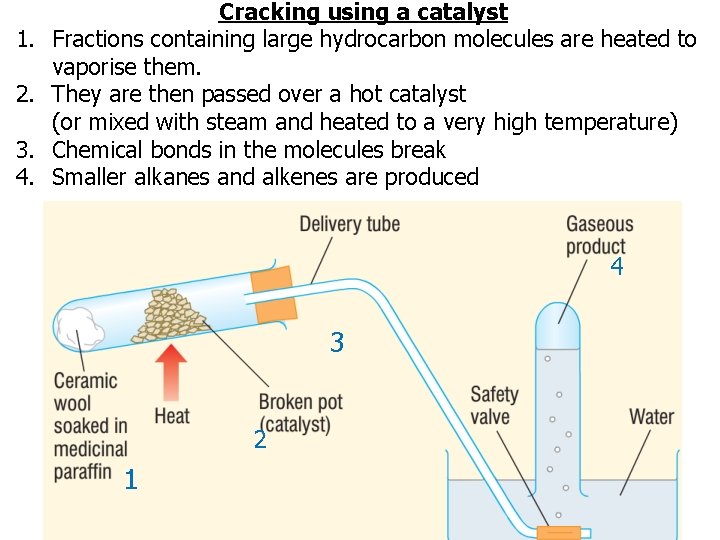
1. 2. 3. 4. Cracking using a catalyst Fractions containing large hydrocarbon molecules are heated to vaporise them. They are then passed over a hot catalyst (or mixed with steam and heated to a very high temperature) Chemical bonds in the molecules break Smaller alkanes and alkenes are produced 4 3 2 1


Fractional distillation question • Starters for 10 page 9 Q 1

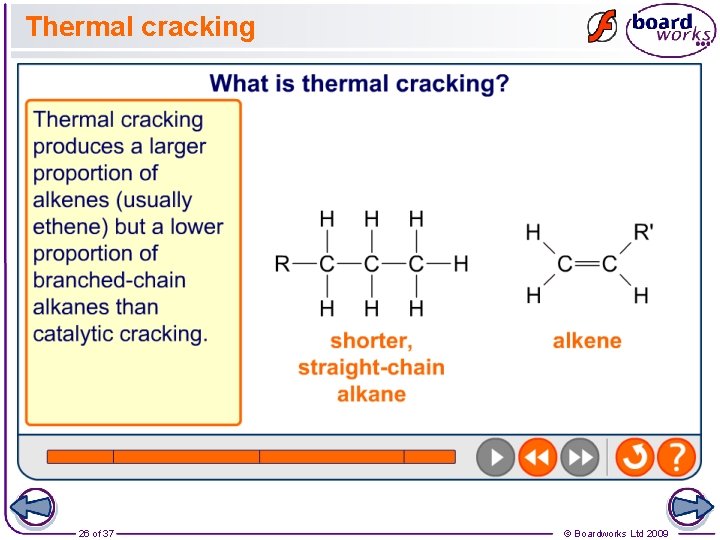
Thermal Cracking • Thermal cracking takes place at high temperature (up to 1000 o. C) and high pressure (up to 70 atm). • It produces a lot of alkenes • These alkenes are used to make heaps of valuable products, like polymers (plastics). A good example is poly(ethene), which is made from ethene.

Thermal cracking 26 of 37 © Boardworks Ltd 2009

Catalytic Cracking • Catalytic cracking uses something called a zeolite catalyst (hydrated aluminosilicate), at a slight pressure and high temperature (about 450 o. C). • It mostly produces aromtatic hydrocarbons and motor fuels. • Using a catalyst cuts costs, because the reaction can be done at a low pressure and a lower temperature. The catalyst also speeds up the reaction, saving time (and time is money).

Catalytic cracking 28 of 37 © Boardworks Ltd 2009

Thermal vs. catalytic cracking Catalytic cracking has several advantages over thermal cracking: l it produces a higher proportion of branched alkanes, which burn more easily than straight-chain alkanes and are therefore an important component of petrol l the use of a lower temperature and pressure mean it is cheaper l it produces a higher proportion of arenes, which are valuable feedstock chemicals. However, unlike thermal cracking, catalytic cracking cannot be used on all fractions, such as bitumen, the supply of which outstrips its demand. 29 of 37 © Boardworks Ltd 2009

Other products from cracking Alkenes such as ethene are always produced in cracking. They are an important feedstock for use in the chemical industry, particularly in the production of plastics. Arenes such as benzene are also produced during catalytic cracking. Benzene is added in small quantities to petrol as a replacement for the lead compounds. It too is now the subject of health concerns, and its use is being reduced. 30 of 37 © Boardworks Ltd 2009

Cracking question • Starters for 10 page 9 Q 2 • Write a possible equation for the cracking of C 12 H 26

Crude oil and cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe the process of fractional distillation of crude oil • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why alkanes are reformed • Explain why the boiling points of different alkanes are different

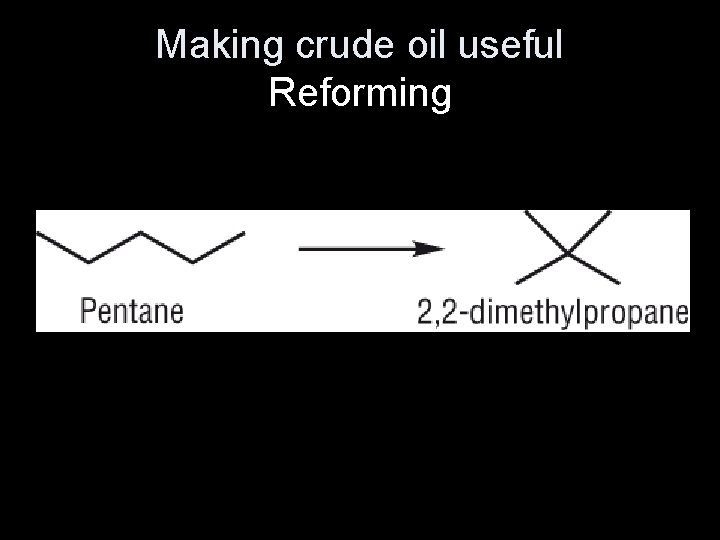
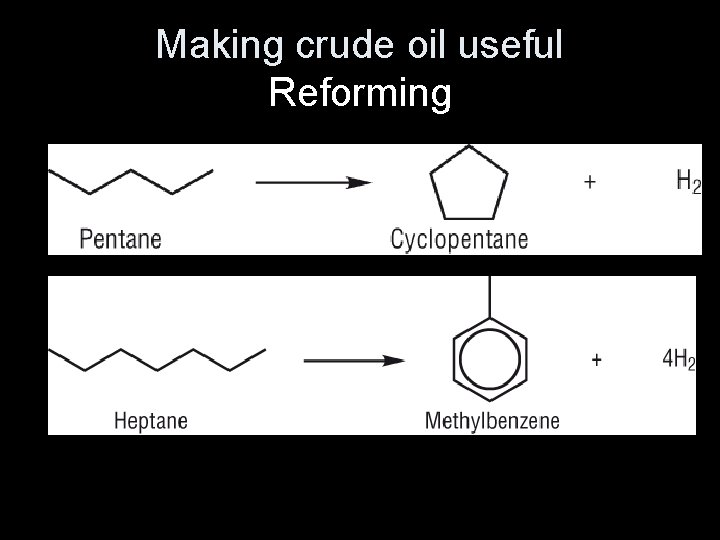
Reforming alkanes • Reforming converts unbranched hydrocarbons, which have lower octane ratings, into branched hydrocarbons and cyclic hydrocarbons. • These have higher octane ratings, so they burn more efficiently in vehicle engines.

• Knocking is where alkanes explode of their own accord when the fuel/air mixture in the engine is compressed. • Straight chain alkanes are the most likely hydrocarbons to cause knocking. Adding branched chain and cyclic hydrocarbons to the petrol mixture makes knocking less likely to happen, so combustion is more efficient.

Making crude oil useful Reforming

Making crude oil useful Reforming

Crude oil and cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe the process of fractional distillation of crude oil • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why alkanes are reformed • Explain why the boiling points of different alkanes are different

Oil Refining Question Briefly describe each process in oil refining (fractional distillation, cracking and reforming), and outline why it is carried out. (6 marks) • Fractional distillation involves heating crude oil, then cooling and condensing the vapours at different temperatures (1) • It is carried out to separate the crude oil into fractions (which each contain hydrocarbons with similar boiling points) (1) • Cracking involves passing the vapours from fractions containing larger alkane molecules over a hot (zeolite) catalyst, which produces smaller alkane molecules and alkenes (1) • It is carried out to match the supply of each fraction with its demand (and to produce alkenes to make polymers) (1) • Reforming involves heating fractions in the presence of catalysts to produce branched hydrocarbons and cyclic hydrocarbons (1) • These hydrocarbons burn more efficiently in engines/have higher octane rating (1)

Crude oil and cracking Lesson objective: To understand that alkane fuels are obtained from the fractional distillation, cracking and reforming of crude oil Outcomes: • Describe the process of fractional distillation of crude oil • Describe thermal and catalytic cracking • Describe the reforming of crude oil • Explain why cracking is carried out • Explain why alkanes are reformed • Explain why the boiling points of different alkanes are different
 Cracking organic chemistry
Cracking organic chemistry Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Mnemonic for crude oil fractions
Mnemonic for crude oil fractions How is crude oil formed
How is crude oil formed Oil sands extraction process diagram
Oil sands extraction process diagram Crude oil contains
Crude oil contains Crude oil
Crude oil Naphthenes in crude oil
Naphthenes in crude oil What products are made from oil
What products are made from oil Primary emulsion
Primary emulsion Organic oil recovery
Organic oil recovery Compound lipids definition
Compound lipids definition Octane lewis structure
Octane lewis structure Acetoacetic ester synthesis mechanism
Acetoacetic ester synthesis mechanism Founder of organic chemistry
Founder of organic chemistry Soap organic chemistry
Soap organic chemistry Ester organic chemistry
Ester organic chemistry Structural formula vs displayed formula
Structural formula vs displayed formula Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Leveling effect organic chemistry
Leveling effect organic chemistry List of functional groups in order of priority
List of functional groups in order of priority Organic chemistry lab report example
Organic chemistry lab report example Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Organic chemistry grade 10
Organic chemistry grade 10 Organic chemistry
Organic chemistry What is organic chemistry
What is organic chemistry Organic chemistry wade
Organic chemistry wade Met et prop but pent hex hept oct
Met et prop but pent hex hept oct Meth eth prop
Meth eth prop Organic chemistry myanmar
Organic chemistry myanmar Ethene + hbr
Ethene + hbr M+1 peak
M+1 peak Hono organic chemistry
Hono organic chemistry Father of organic chemistry
Father of organic chemistry