Organic Chemistry Crude oil is a finite resource


































- Slides: 38

Organic Chemistry


• Crude oil is a finite resource found in rocks (it will run out) • Crude oil is the remains of an ancient biomass consisting mainly of plankton that was buried in mud

Why is it so important to us?


Crude oil is a mixture of different compounds called hydrocarbons. A hydrocarbon is a compound made of carbon and hydrogen atoms only. The hydrocarbons in crude oil are mostly alkanes.


• General formula • Complete questions 1 -4

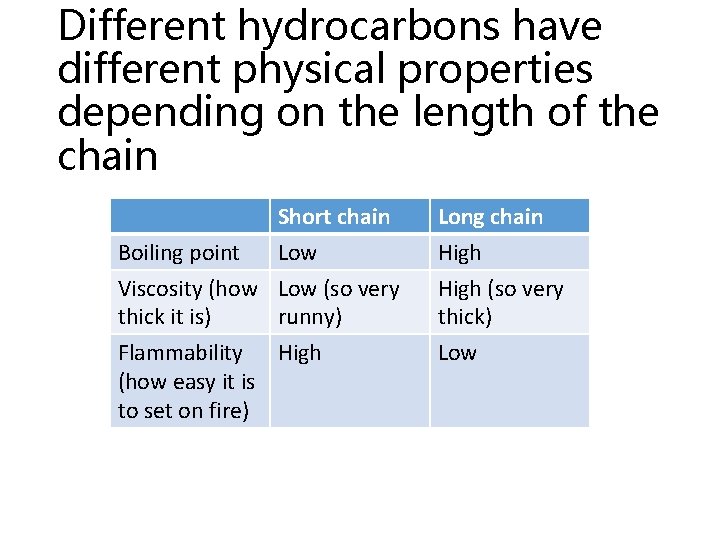
Different hydrocarbons have different physical properties depending on the length of the chain Boiling point Short chain Low Long chain High Viscosity (how Low (so very thick it is) runny) High (so very thick) Flammability High (how easy it is to set on fire) Low

Complete questions 5 -10

Boiling points and separation

Complete question 11 -26


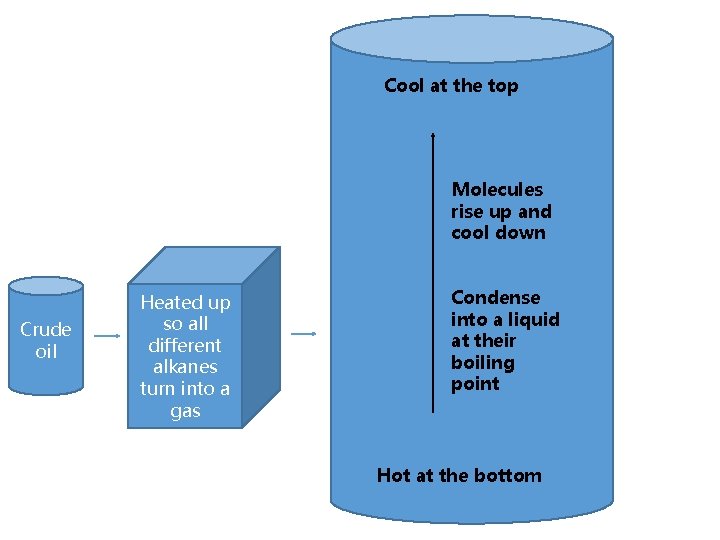
Fractional distillation

Cool at the top Molecules rise up and cool down Crude oil Heated up so all different alkanes turn into a gas Condense into a liquid at their boiling point Hot at the bottom

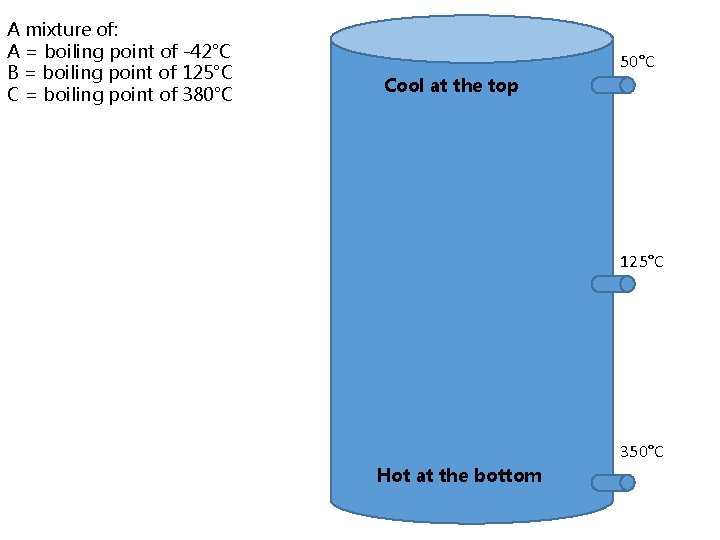
A mixture of: A = boiling point of -42°C B = boiling point of 125°C C = boiling point of 380°C 50°C Cool at the top 125°C 350°C Hot at the bottom


Combustion

• Combustion reactions are when a hydrocarbon reacts with oxygen • Complete combustion always produces carbon dioxide and water • The reaction releases energy which can be used

E. g. complete combustion of methane • Word equation • Symbol equation • Note that carbon and hydrogen have been oxidised (had oxygen added to them)

Incomplete combustion • Incomplete combustion occurs when there is not enough oxygen • Incomplete combustion produces carbon monoxide (which is a toxic gas) • Methane and ethane example

Cracking • There are long hydrocarbons and short ones • The shorter ones are more useful • Used as fuels and to help make polymers and other useful chemicals • Cracking turns the long ones into shorter ones • One way is to pass over a hot catalyst • Another way is to mix with steam and heat to a high temperature • Produce shorter alkanes and alkenes • Alkenes are useful substances that are more reactive than alkanes

Demo

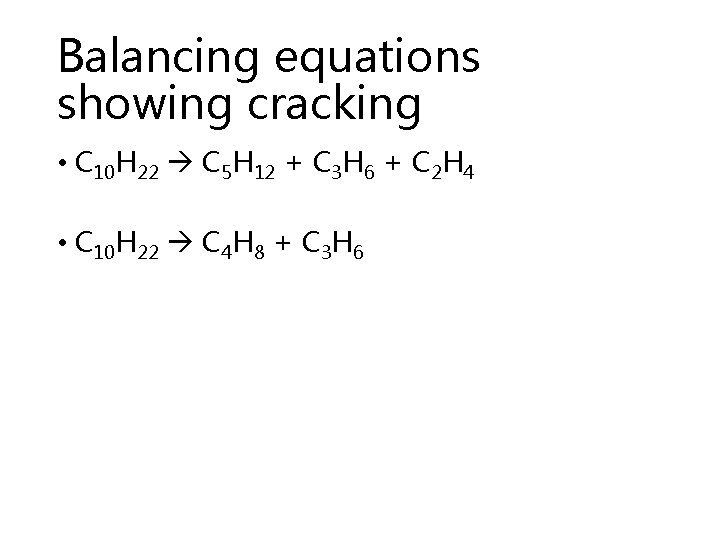
Balancing equations showing cracking • C 10 H 22 C 5 H 12 + C 3 H 6 + C 2 H 4 • C 10 H 22 C 4 H 8 + C 3 H 6

• Test for unsaturation • Answer questions 37 -46

Further organic (triple only)

Alkenes Triple only • Double bond • Unsaturation • First four

• Questions 1 - 3 • Start reading page 158 -159

Reactions of alkenes • Combustion • Same as alkanes but more often incomplete combustion • Smoky flame • Less energy

• Questions 4 -6 • Read page 158 -159

Reactions • Additions with halogen • Addition with hydrogen • Addition with water (steam)

Alcohols • Structure • Functional group • Homologous series • Combustion • Q 9 -14 • Start reading page 162 -163

Alcohols with sodium • Sodium + ethanol sodium ethoxide + hydrogen • 2 Na + 2 C 2 H 5 OH 2 C 2 H 5 ONa + H 2 • Questions 15 -19

Alcohols in water • Smaller alcohols dissolve readily in water • Can be used as solvents

Alcohol with oxidising agent • Turn into carboxylic acids • Can occur naturally in the air • Can be done in the lab

Alcohol formation • Alkene with steam • Fermentation of sugar

Carboxylic acids • Formation from alcohols • Notation for oxidising agent • Reaction with carbonates • Q 20 -27

Esters • Formed from the reaction between an alcohol and a carboxylic acid • Sweet smelling, used in flavourings • Only need to know ethyl ethanoate • Q 28 -35
 Functional groups ib chemistry
Functional groups ib chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Mnemonic for fractional distillation of crude oil
Mnemonic for fractional distillation of crude oil How is crude oil formed
How is crude oil formed Synthetic crude oil
Synthetic crude oil Crude oil contains
Crude oil contains Crude oil
Crude oil Naphthenes in crude oil
Naphthenes in crude oil Petroleum product
Petroleum product Finite subordinate clauses
Finite subordinate clauses Finite verb
Finite verb Learning objectives of non finite verbs
Learning objectives of non finite verbs Finite and non-finite verb
Finite and non-finite verb Non finite forms of the verb qayda
Non finite forms of the verb qayda Titan oil recovery share price
Titan oil recovery share price Preparation of emulsion
Preparation of emulsion Founder of organic chemistry
Founder of organic chemistry Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Structure of pentanoic acid
Structure of pentanoic acid Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Pericyclic
Pericyclic David klein organic chemistry
David klein organic chemistry Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Benzene naming priority
Benzene naming priority Objective lab report example
Objective lab report example Alkane organic chemistry
Alkane organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Introduction to organic chemistry
Introduction to organic chemistry Organic chemistry wade
Organic chemistry wade Crash course chemistry naming compounds
Crash course chemistry naming compounds How is cracking done
How is cracking done Organic biochemistry
Organic biochemistry Organic chemistry myanmar
Organic chemistry myanmar Propagation organic chemistry
Propagation organic chemistry