Organic Chemistry Revision Crude Oil What is it













- Slides: 18

Organic Chemistry Revision

Crude Oil What is it? How is it formed? Where is it found?

Fractional Distillation – making crude oil useful Crude oil is heated to make it vaporise Vapour goes into the fractioning column The vapour is cooled Different fractions of the oil condense and are collected at different temperatures As the length of the hydrocarbon chain increases, the boiling point and viscosity also increase. Flammability and volatility decrease.


Alkanes are a homologous series. Members of a homologous series have similar properties and can be represented by a general formula For alkanes, the formula is Cn. H 2 n+2


Properties of alkanes The first four (methane – butane) are gases at RTP As the number of carbon atoms increases, the melting points, boiling points and densities increase 5 -17 C atoms = liquids 18+ C atoms = solids C-C bonds and C-H bonds are very strong, so alkanes are not very reactive But – will combust. Very exothermic!

Complete and incomplete combustion Complete: Hydrocarbon + oxygen carbon dioxide + water Happens when there is plenty of air. Incomplete: Hydrocarbon + oxygen carbon monoxide + carbon + water Happens when air is restricted

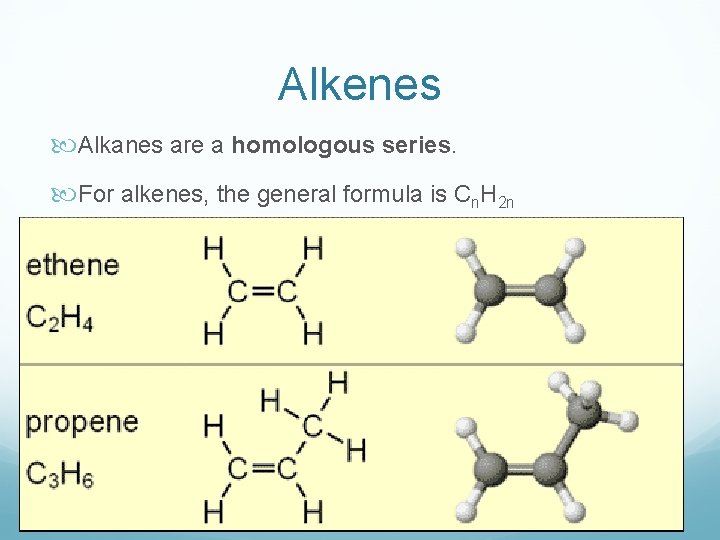
Alkenes Alkanes are a homologous series. For alkenes, the general formula is Cn. H 2 n

Properties of alkenes Have a carbon-carbon double bond As the number of carbon atoms increases, the melting points, boiling points and densities increase Combust like alkanes but burn with sootier flames due to their higher carbon : hydrogen ratio More reactive than alkanes – why? Unsaturated

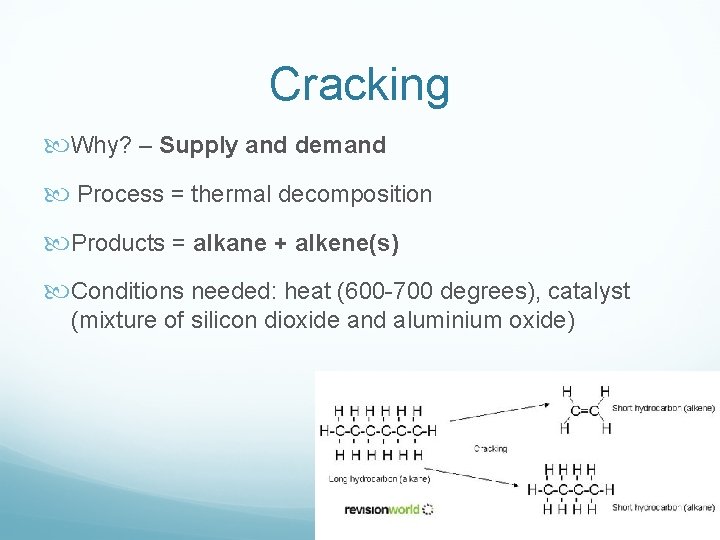
Cracking Why? – Supply and demand Process = thermal decomposition Products = alkane + alkene(s) Conditions needed: heat (600 -700 degrees), catalyst (mixture of silicon dioxide and aluminium oxide)

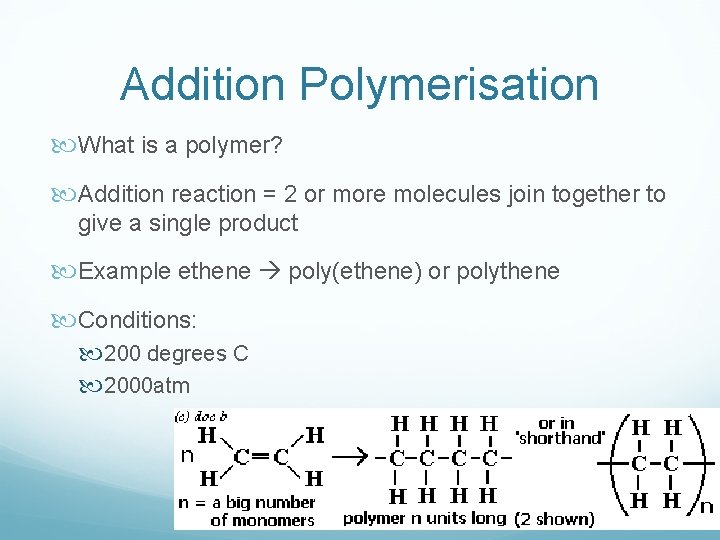
Addition Polymerisation What is a polymer? Addition reaction = 2 or more molecules join together to give a single product Example ethene poly(ethene) or polythene Conditions: 200 degrees C 2000 atm

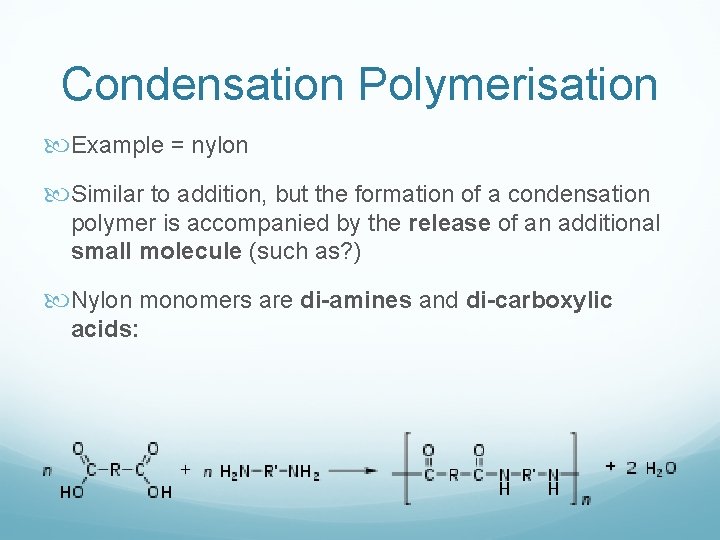
Condensation Polymerisation Example = nylon Similar to addition, but the formation of a condensation polymer is accompanied by the release of an additional small molecule (such as? ) Nylon monomers are di-amines and di-carboxylic acids:

Manufacture of ethanol By the reaction of steam and ethene: Conditions: High temp (300 degrees C) Catalyst = phosphoric acid 60 atm

Advantages and disadvantages Fast and efficient Continuous Product is relatively pure Some countries may have local oil supply But – crude oil is non-renewable

Manufacture of ethanol By the fermentation of sugars: Glucose ethanol + carbon dioxide Conditions: ENZYME Slightly above room temperature The carbon dioxide is allowed to escape and air prevented from getting in, to stop the oxidisation of the alcohol.

Advantages and disadvantages Cheap Renewable resource (sugar cane) Cheap labour in the countries where sugar cane is grown But – Reaction is slow Inefficient batch process Poor quality product Alcohol must be distilled from the fermentation mixture (costly)

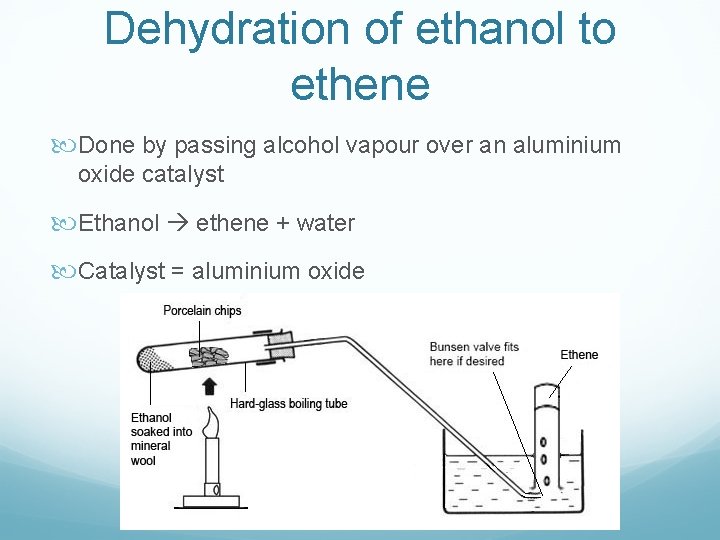
Dehydration of ethanol to ethene Done by passing alcohol vapour over an aluminium oxide catalyst Ethanol ethene + water Catalyst = aluminium oxide
 Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Crude oil contains
Crude oil contains Petroleum processing
Petroleum processing Crude oil
Crude oil How is crude oil formed
How is crude oil formed Catalytic cracking diagram
Catalytic cracking diagram Canadian oil and gas trusts
Canadian oil and gas trusts Products derived from oil
Products derived from oil Passive revision
Passive revision Titan oil recovery
Titan oil recovery Creaming is a.......... process
Creaming is a.......... process Organic chemistry
Organic chemistry Numbering carbon chains
Numbering carbon chains Hhcchh
Hhcchh Ir spectroscopy
Ir spectroscopy Organic chemistry lab report format
Organic chemistry lab report format Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry