Unit 10 Organic Chemistry What is Organic Chemistry




- Slides: 51

Unit 10 Organic Chemistry

What is Organic Chemistry? Organic chemistry is the study of carbon compounds. Besides carbon, the most common elements in organic compounds are hydrogen, oxygen, nitrogen, sulfur, and the halogens.

Why is it Important? Animals, plants, and other forms of life consist of organic compounds. Organic products are found in foods, drugs, clothing, fuel, and many other products that we use everyday. Organic chemistry is an enormous field. o >90% of all compounds are organic.

History of Organic Chemistry Scientists originally thought organic compounds contained a “vital force” due to their natural origin. Until 1828, when Friedrich Wöhler made urea (found in human urine) in the lab from a mineral. Today, many organic compounds are synthesized in the laboratory.

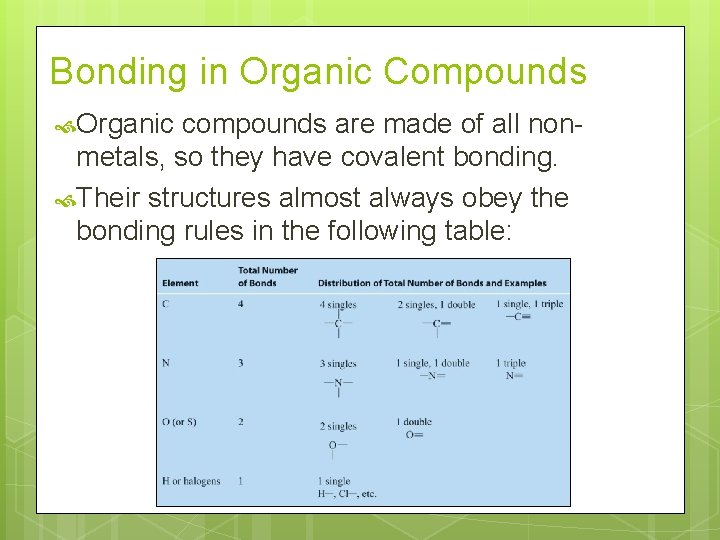
Bonding in Organic Compounds Organic compounds are made of all nonmetals, so they have covalent bonding. Their structures almost always obey the bonding rules in the following table:

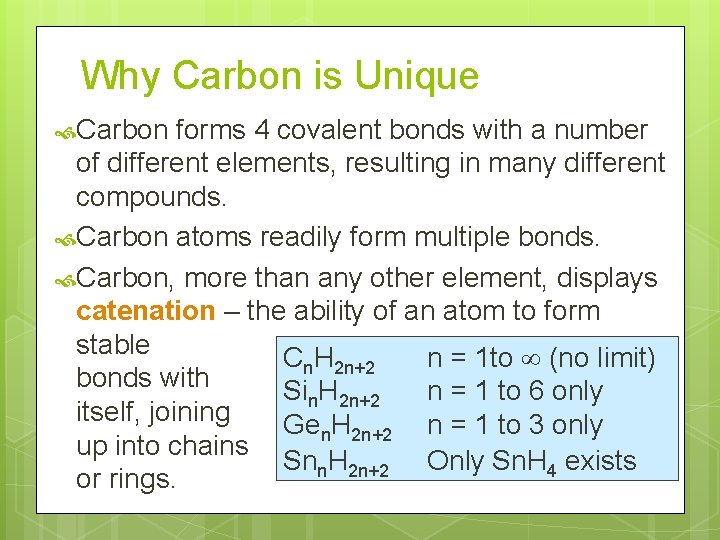
Why Carbon is Unique Carbon forms 4 covalent bonds with a number of different elements, resulting in many different compounds. Carbon atoms readily form multiple bonds. Carbon, more than any other element, displays catenation – the ability of an atom to form stable Cn. H 2 n+2 n = 1 to (no limit) bonds with Sin. H 2 n+2 n = 1 to 6 only itself, joining Gen. H 2 n+2 n = 1 to 3 only up into chains Snn. H 2 n+2 Only Sn. H 4 exists or rings.

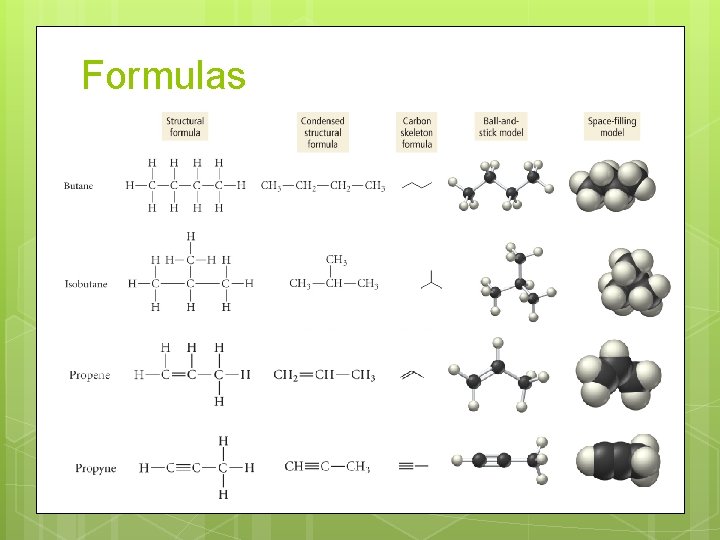
Representing Organic Compounds Structural Formulas show all of the carbon and hydrogen atoms and how they are bonded together. Line-Angle (Carbon Skeleton) formulas do not show the hydrogen atoms that are bonded to carbons. Each angle, and beginning and end of a line, represents a carbon atom. Space-filling and ball-and-stick models are three-dimensional representations of the molecule.

Formulas

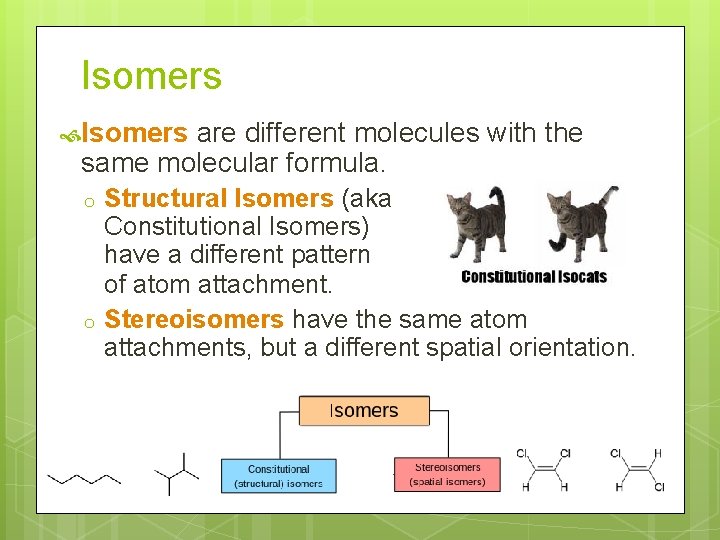
Isomers are different molecules with the same molecular formula. o o Structural Isomers (aka Constitutional Isomers) have a different pattern of atom attachment. Stereoisomers have the same atom attachments, but a different spatial orientation.

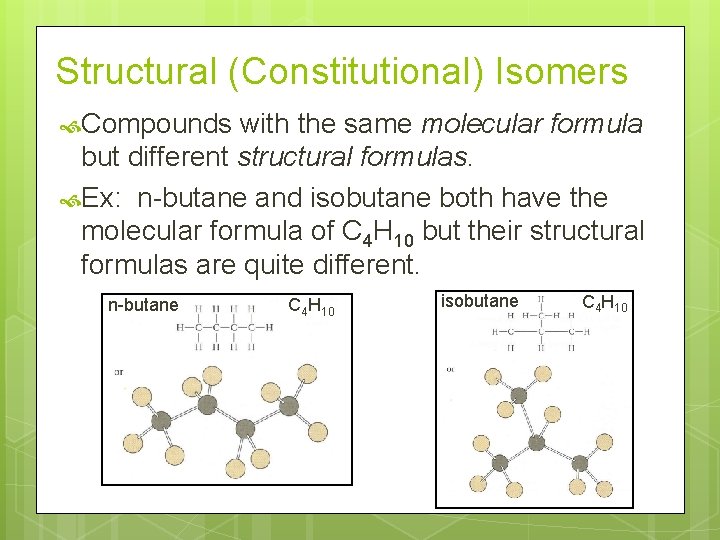
Structural (Constitutional) Isomers Compounds with the same molecular formula but different structural formulas. Ex: n-butane and isobutane both have the molecular formula of C 4 H 10 but their structural formulas are quite different. n-butane C 4 H 10 isobutane C 4 H 10

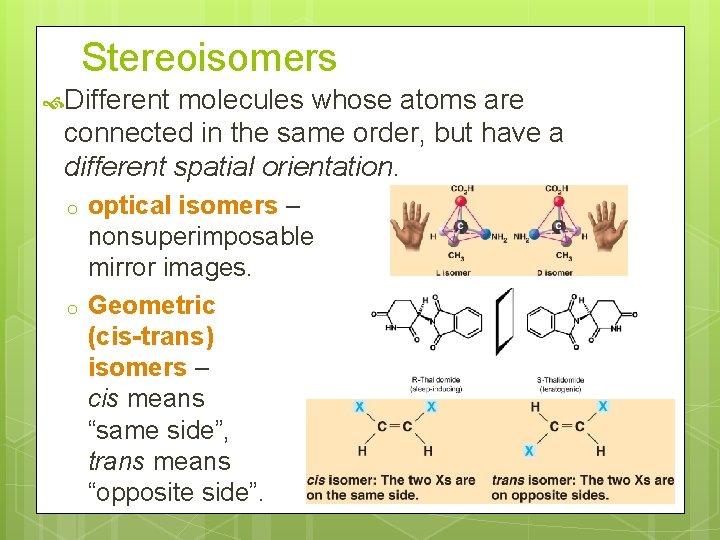
Stereoisomers Different molecules whose atoms are connected in the same order, but have a different spatial orientation. o o optical isomers – nonsuperimposable mirror images. Geometric (cis-trans) isomers – cis means “same side”, trans means “opposite side”.

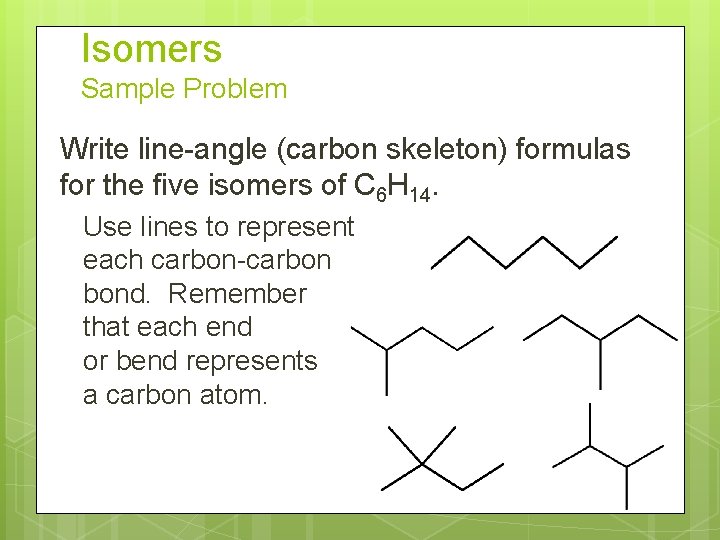
Isomers Sample Problem Write line-angle (carbon skeleton) formulas for the five isomers of C 6 H 14. Use lines to represent each carbon-carbon bond. Remember that each end or bend represents a carbon atom.

Hydrocarbons are the simplest organic compounds, containing only carbon and hydrogen. However, due to the uniqueness of carbon, there are many different kinds of hydrocarbons. Hydrocarbons are commonly used as fuels, and are the starting materials in the synthesis of many consumer products.

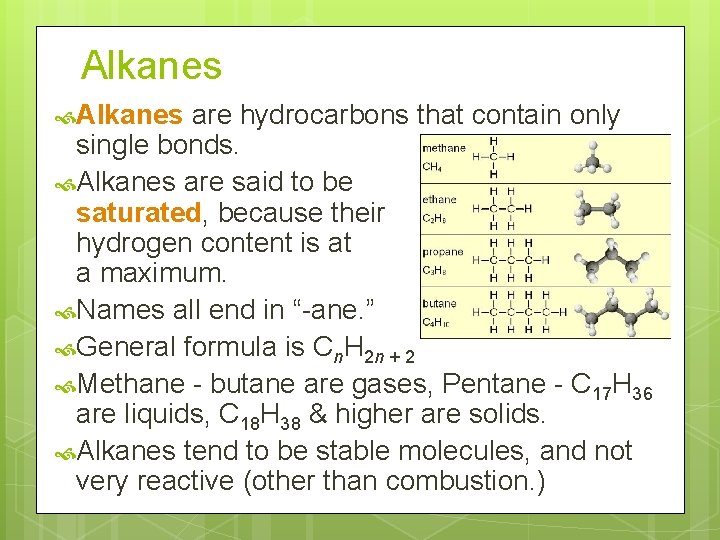
Alkanes are hydrocarbons that contain only single bonds. Alkanes are said to be saturated, because their hydrogen content is at a maximum. Names all end in “-ane. ” General formula is Cn. H 2 n + 2 Methane - butane are gases, Pentane - C 17 H 36 are liquids, C 18 H 38 & higher are solids. Alkanes tend to be stable molecules, and not very reactive (other than combustion. )

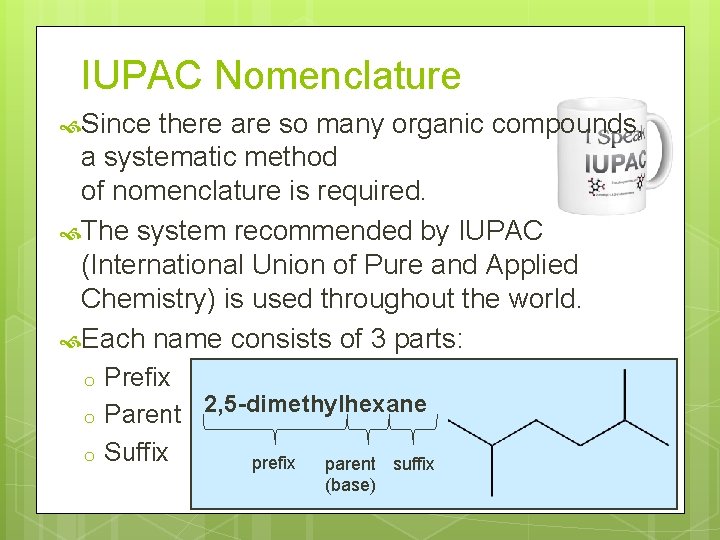
IUPAC Nomenclature Since there are so many organic compounds, a systematic method of nomenclature is required. The system recommended by IUPAC (International Union of Pure and Applied Chemistry) is used throughout the world. Each name consists of 3 parts: o o o Prefix Parent 2, 5 -dimethylhexane Suffix prefix parent suffix (base)

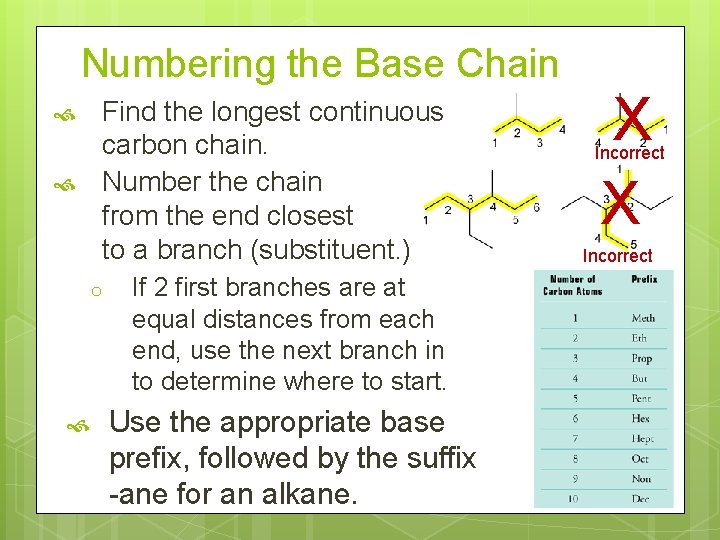
Numbering the Base Chain Find the longest continuous carbon chain. Number the chain from the end closest to a branch (substituent. ) o If 2 first branches are at equal distances from each end, use the next branch in to determine where to start. Use the appropriate base prefix, followed by the suffix -ane for an alkane. X X Incorrect

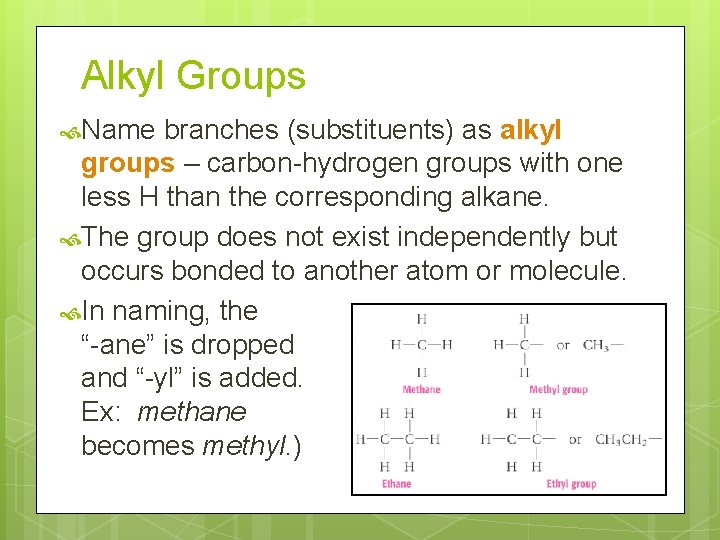
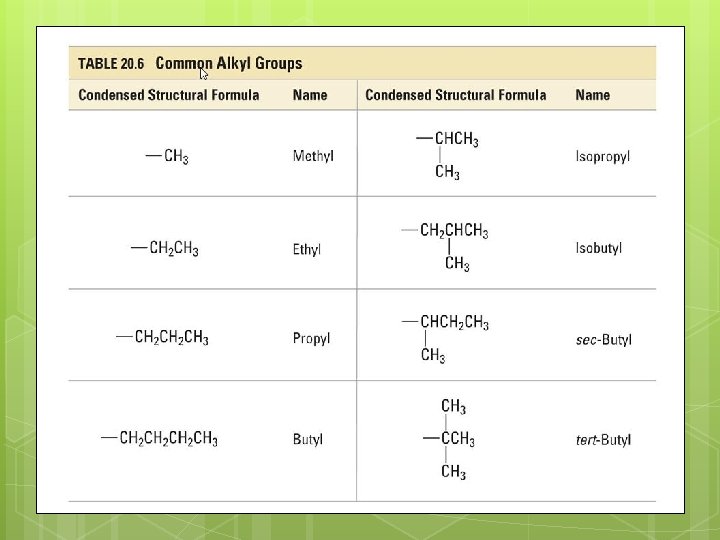
Alkyl Groups Name branches (substituents) as alkyl groups – carbon-hydrogen groups with one less H than the corresponding alkane. The group does not exist independently but occurs bonded to another atom or molecule. In naming, the “-ane” is dropped and “-yl” is added. Ex: methane becomes methyl. )


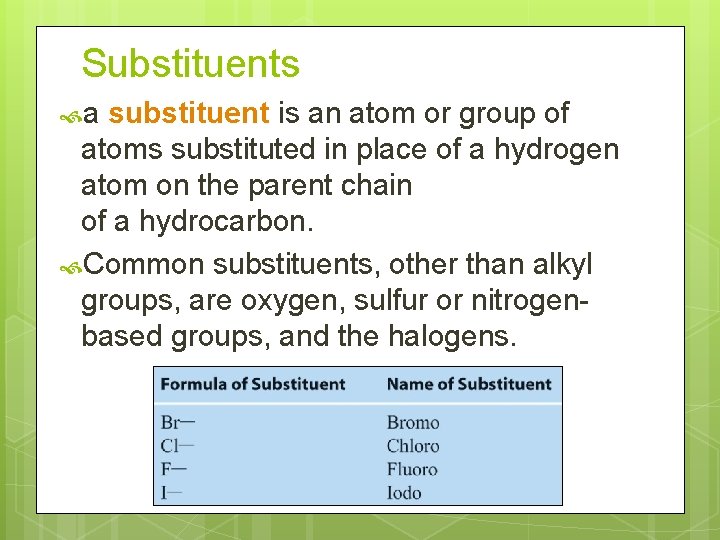
Substituents a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon. Common substituents, other than alkyl groups, are oxygen, sulfur or nitrogenbased groups, and the halogens.

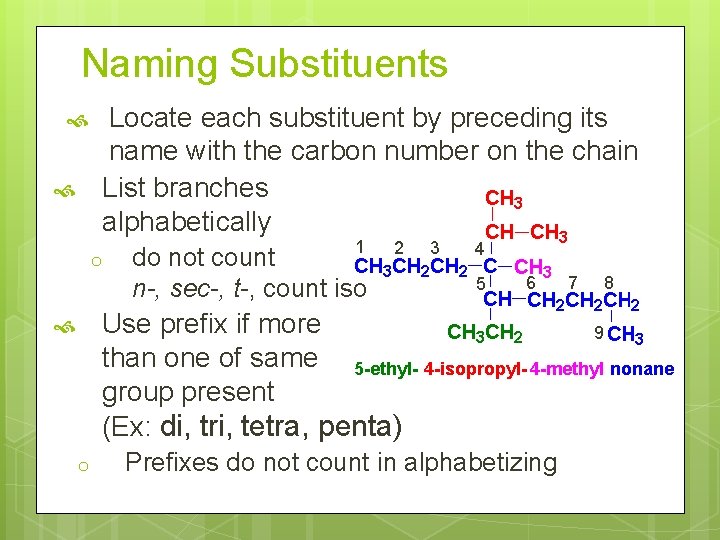
Naming Substituents Locate each substituent by preceding its name with the carbon number on the chain List branches alphabetically o 1 do not count n-, sec-, t-, count iso 2 3 4 5 6 7 8 Use prefix if more 9 than one of same 5 -ethyl- 4 -isopropyl- 4 -methyl nonane group present (Ex: di, tri, tetra, penta) o Prefixes do not count in alphabetizing

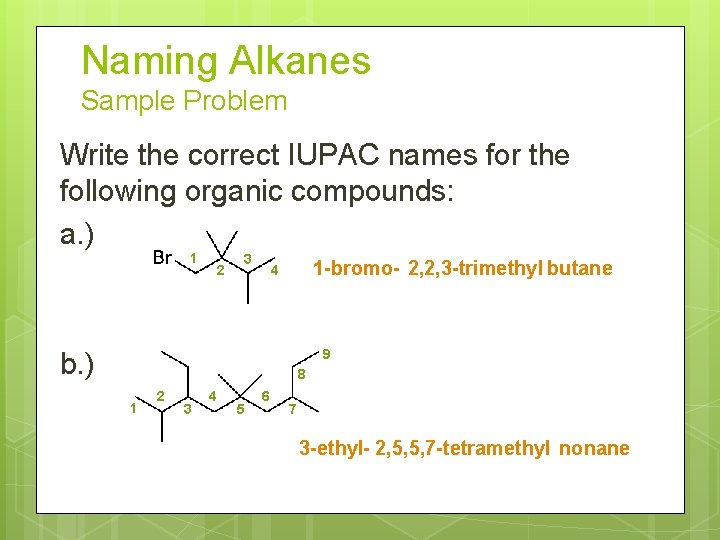
Naming Alkanes Sample Problem Write the correct IUPAC names for the following organic compounds: a. ) 1 2 3 1 -bromo- 2, 2, 3 -trimethyl butane 4 b. ) 9 8 1 2 3 4 5 6 7 3 -ethyl- 2, 5, 5, 7 -tetramethyl nonane

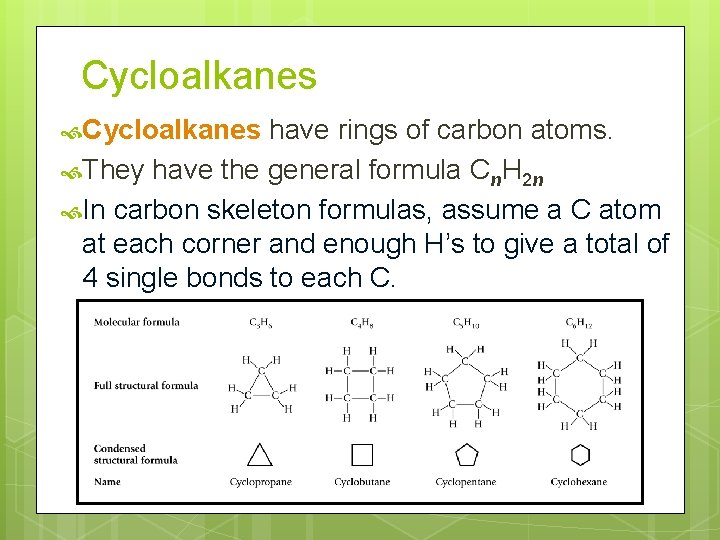
Cycloalkanes have rings of carbon atoms. They have the general formula Cn. H 2 n In carbon skeleton formulas, assume a C atom at each corner and enough H’s to give a total of 4 single bonds to each C.

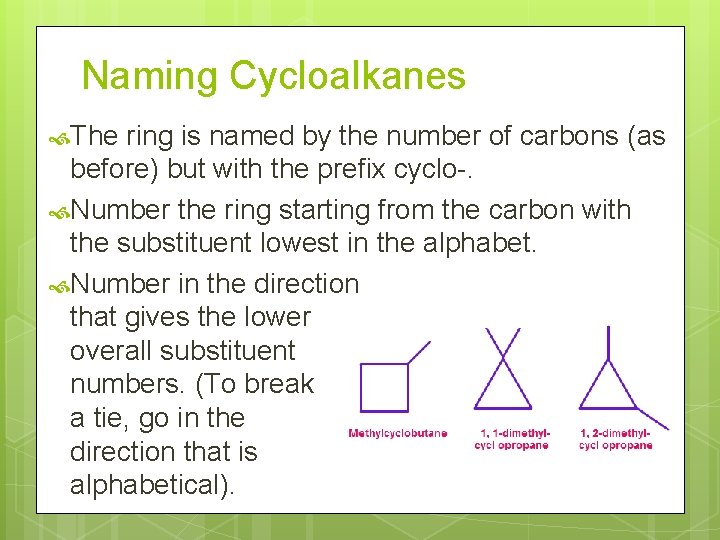
Naming Cycloalkanes The ring is named by the number of carbons (as before) but with the prefix cyclo-. Number the ring starting from the carbon with the substituent lowest in the alphabet. Number in the direction that gives the lower overall substituent numbers. (To break a tie, go in the direction that is alphabetical).

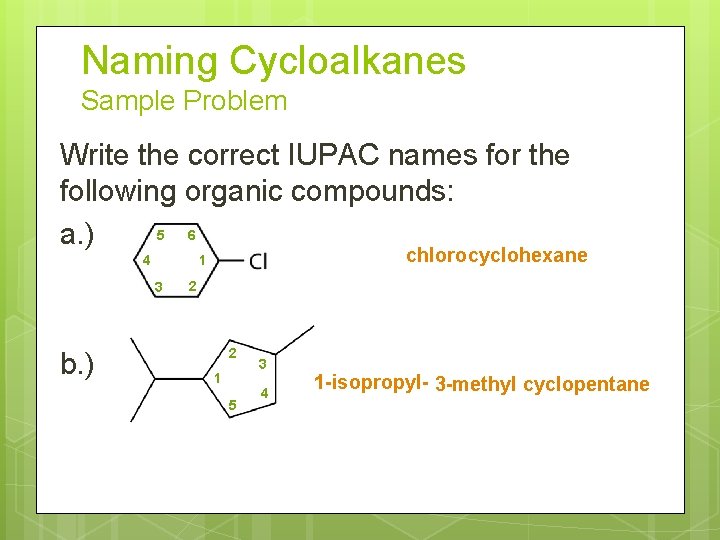
Naming Cycloalkanes Sample Problem Write the correct IUPAC names for the following organic compounds: 5 6 a. ) 3 b. ) chloro cyclohexane 1 4 2 2 1 5 3 4 1 -isopropyl- 3 -methyl cyclopentane

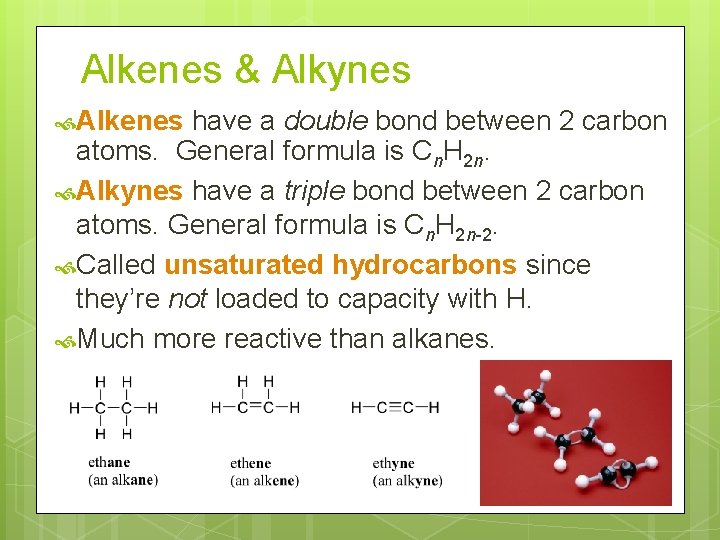
Alkenes & Alkynes Alkenes have a double bond between 2 carbon atoms. General formula is Cn. H 2 n. Alkynes have a triple bond between 2 carbon atoms. General formula is Cn. H 2 n-2. Called unsaturated hydrocarbons since they’re not loaded to capacity with H. Much more reactive than alkanes.

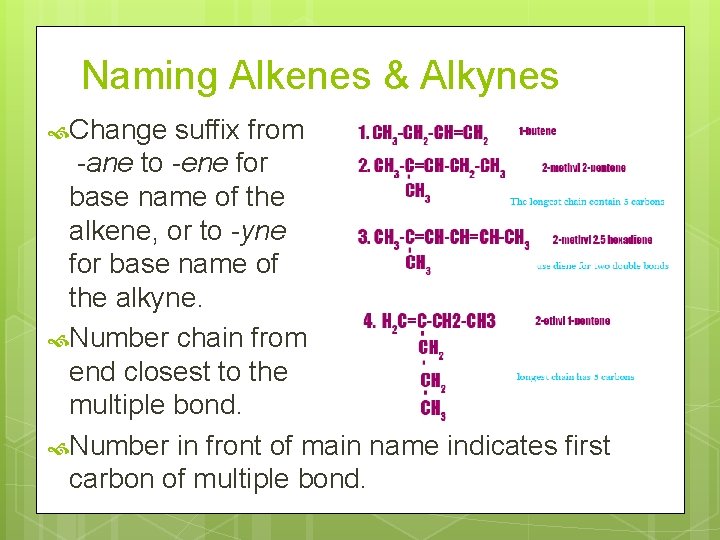
Naming Alkenes & Alkynes Change suffix from -ane to -ene for base name of the alkene, or to -yne for base name of the alkyne. Number chain from end closest to the multiple bond. Number in front of main name indicates first carbon of multiple bond.

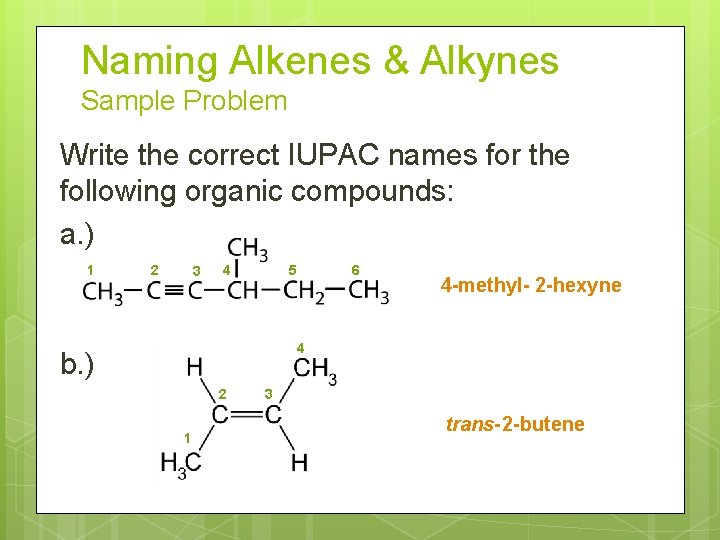
Naming Alkenes & Alkynes Sample Problem Write the correct IUPAC names for the following organic compounds: a. ) 1 2 3 4 5 6 4 -methyl- 2 -hexyne 4 b. ) 2 1 3 trans- 2 -butene

Classification of Hydrocarbons All of the hydrocarbons we have looked at so far are called aliphatic. There is another classification, called aromatic, which includes any hydrocarbon containing a benzene ring.

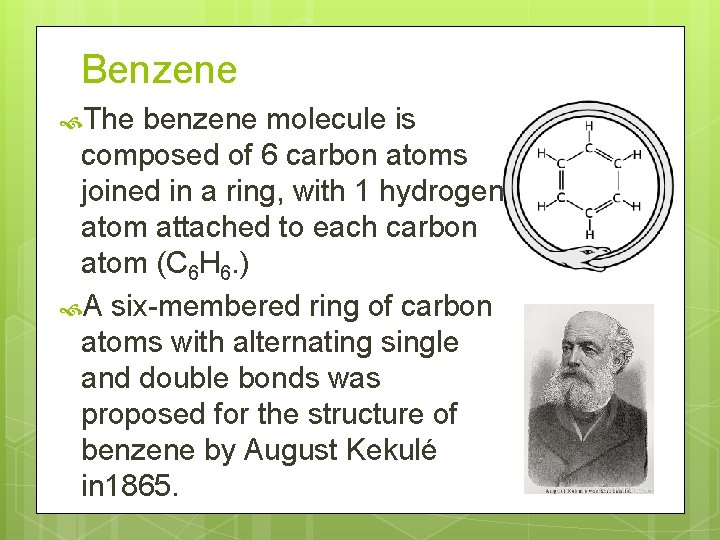
Benzene The benzene molecule is composed of 6 carbon atoms joined in a ring, with 1 hydrogen atom attached to each carbon atom (C 6 H 6. ) A six-membered ring of carbon atoms with alternating single and double bonds was proposed for the structure of benzene by August Kekulé in 1865.

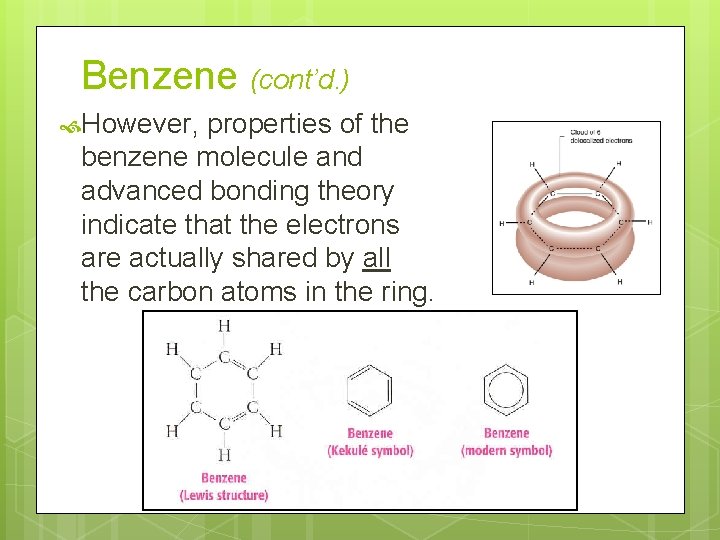
Benzene (cont’d. ) However, properties of the benzene molecule and advanced bonding theory indicate that the electrons are actually shared by all the carbon atoms in the ring.

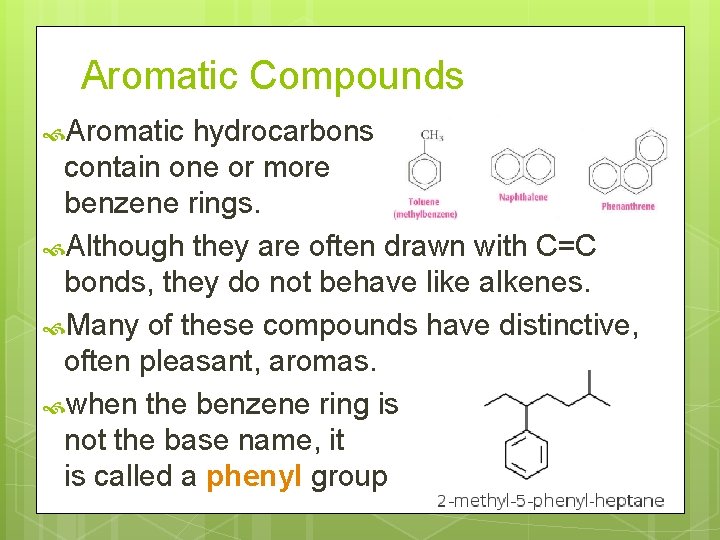
Aromatic Compounds Aromatic hydrocarbons contain one or more benzene rings. Although they are often drawn with C=C bonds, they do not behave like alkenes. Many of these compounds have distinctive, often pleasant, aromas. when the benzene ring is not the base name, it is called a phenyl group

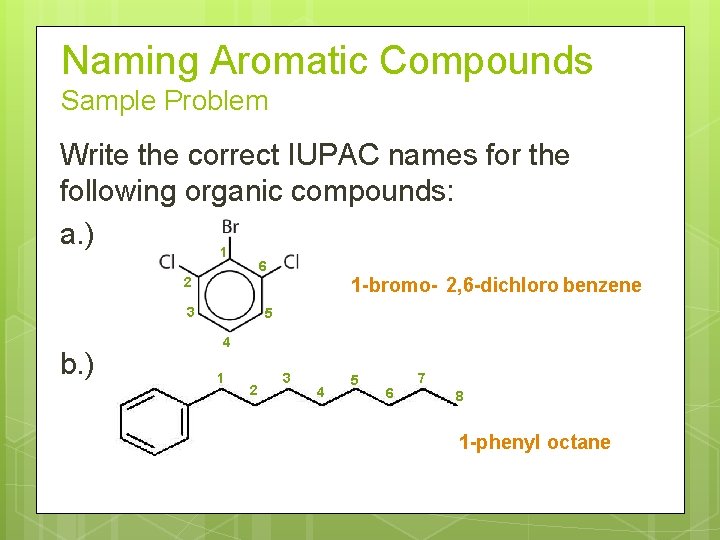
Naming Aromatic Compounds Sample Problem Write the correct IUPAC names for the following organic compounds: a. ) 1 6 1 -bromo- 2, 6 -dichloro benzene 2 3 b. ) 5 4 1 2 3 4 5 6 7 8 1 -phenyl octane

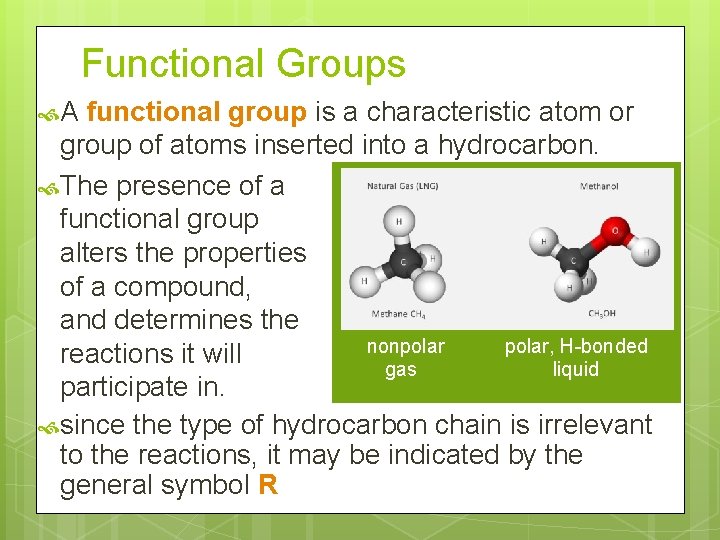
Functional Groups A functional group is a characteristic atom or group of atoms inserted into a hydrocarbon. The presence of a functional group alters the properties of a compound, and determines the nonpolar, H-bonded reactions it will gas liquid participate in. since the type of hydrocarbon chain is irrelevant to the reactions, it may be indicated by the general symbol R


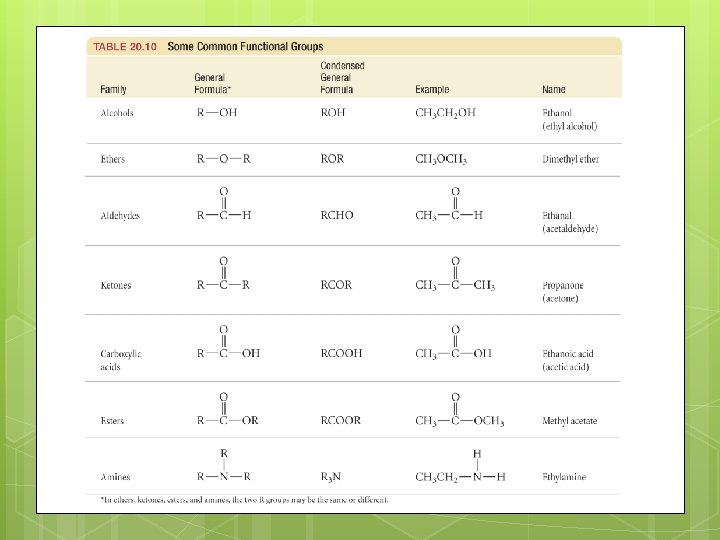
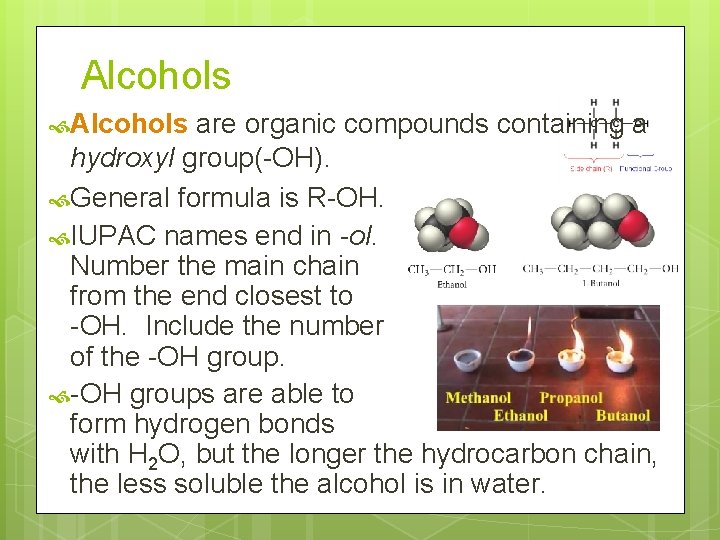
Alcohols are organic compounds containing a hydroxyl group(-OH). General formula is R-OH. IUPAC names end in -ol. Number the main chain from the end closest to -OH. Include the number of the -OH groups are able to form hydrogen bonds with H 2 O, but the longer the hydrocarbon chain, the less soluble the alcohol is in water.

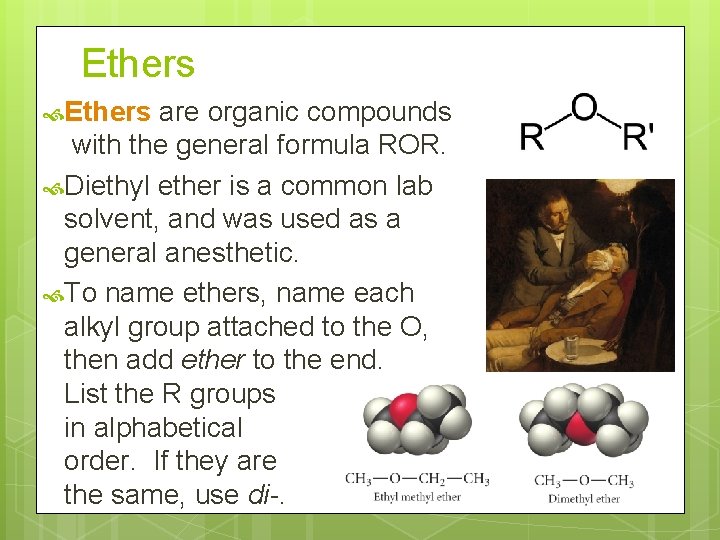
Ethers are organic compounds with the general formula ROR. Diethyl ether is a common lab solvent, and was used as a general anesthetic. To name ethers, name each alkyl group attached to the O, then add ether to the end. List the R groups in alphabetical order. If they are the same, use di-.

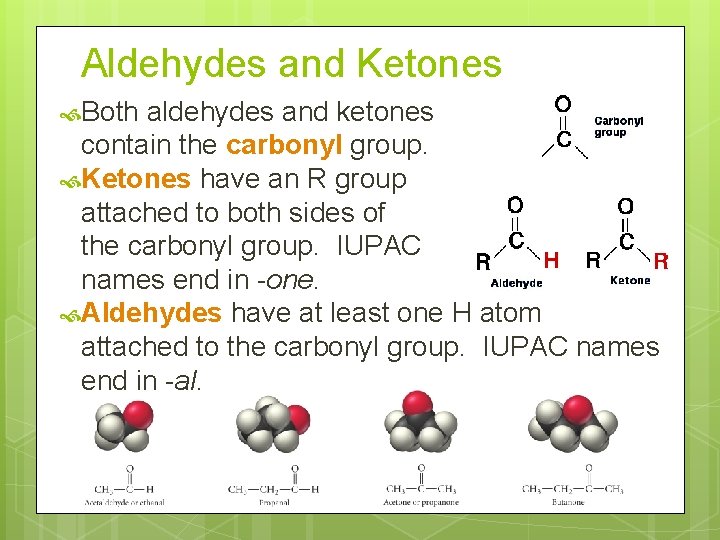
Aldehydes and Ketones Both aldehydes and ketones contain the carbonyl group. Ketones have an R group attached to both sides of the carbonyl group. IUPAC names end in -one. Aldehydes have at least one H atom attached to the carbonyl group. IUPAC names end in -al.

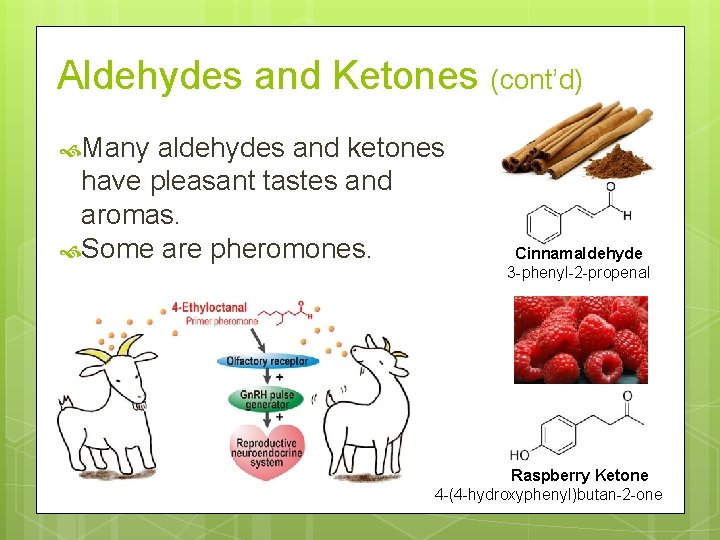
Aldehydes and Ketones (cont’d) Many aldehydes and ketones have pleasant tastes and aromas. Some are pheromones. Cinnamaldehyde 3 -phenyl-2 -propenal Raspberry Ketone 4 -(4 -hydroxyphenyl)butan-2 -one

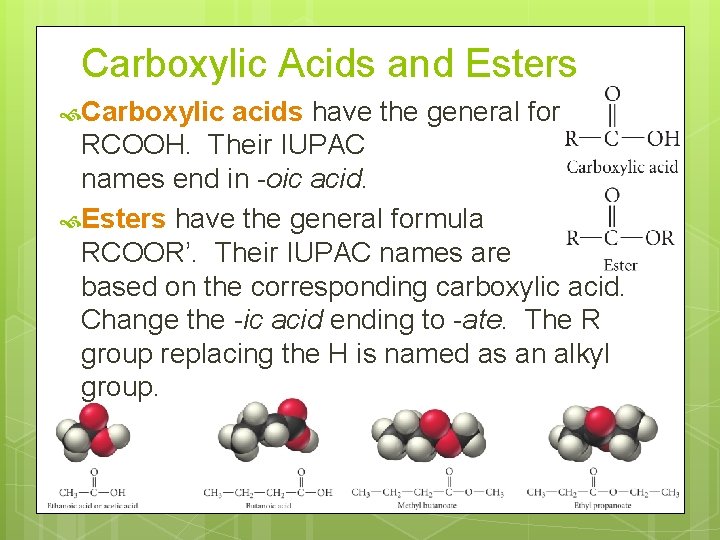
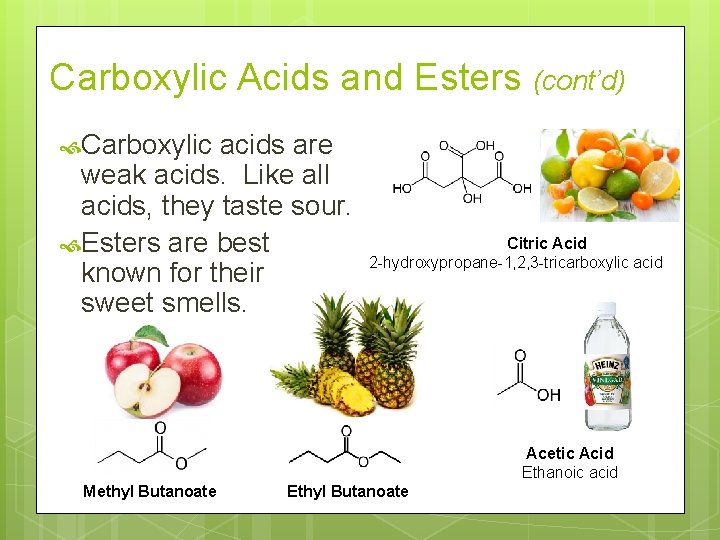
Carboxylic Acids and Esters Carboxylic acids have the general formula RCOOH. Their IUPAC names end in -oic acid. Esters have the general formula RCOOR’. Their IUPAC names are based on the corresponding carboxylic acid. Change the -ic acid ending to -ate. The R group replacing the H is named as an alkyl group.

Carboxylic Acids and Esters (cont’d) Carboxylic acids are weak acids. Like all acids, they taste sour. Esters are best known for their sweet smells. Citric Acid 2 -hydroxypropane-1, 2, 3 -tricarboxylic acid Acetic Acid Ethanoic acid Methyl Butanoate Ethyl Butanoate

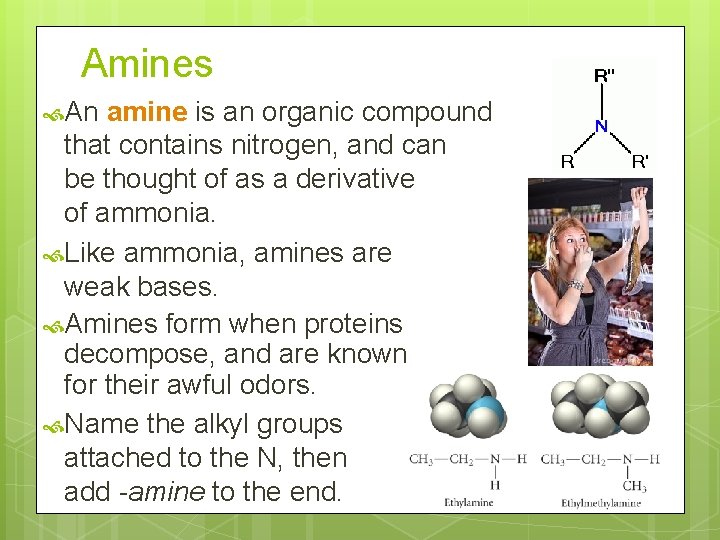
Amines An amine is an organic compound that contains nitrogen, and can be thought of as a derivative of ammonia. Like ammonia, amines are weak bases. Amines form when proteins decompose, and are known for their awful odors. Name the alkyl groups attached to the N, then add -amine to the end.

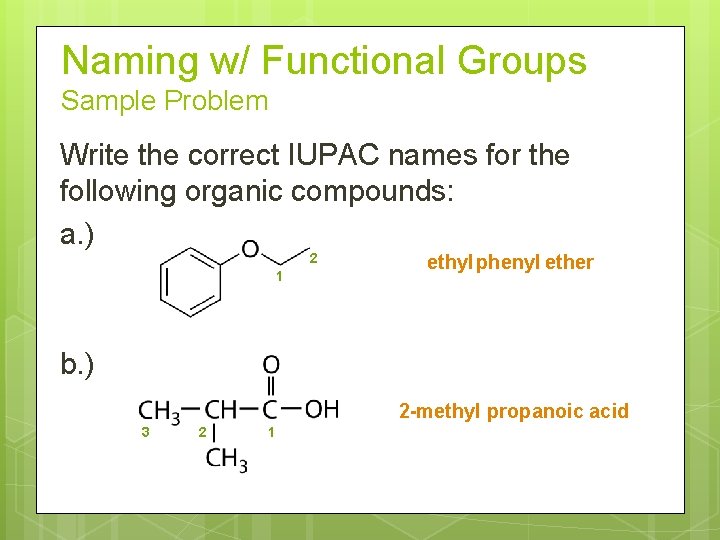
Naming w/ Functional Groups Sample Problem Write the correct IUPAC names for the following organic compounds: a. ) 2 1 ethyl phenyl ether b. ) 2 -methyl propanoic acid 3 2 1

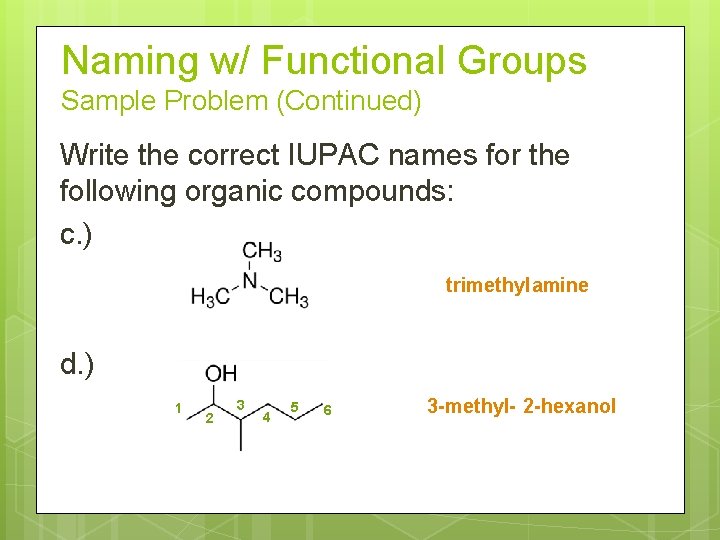
Naming w/ Functional Groups Sample Problem (Continued) Write the correct IUPAC names for the following organic compounds: c. ) trimethyl amine d. ) 1 2 3 4 5 6 3 -methyl- 2 -hexanol

Types of Organic Reactions Organic compounds participate in 3 main types of chemical reactions: o o o Substitution – atom(s) of the starting material are replaced by other atom(s). Elimination - atoms of the starting material are“lost” and a new bond is formed. Addition - atoms are added to the starting material.

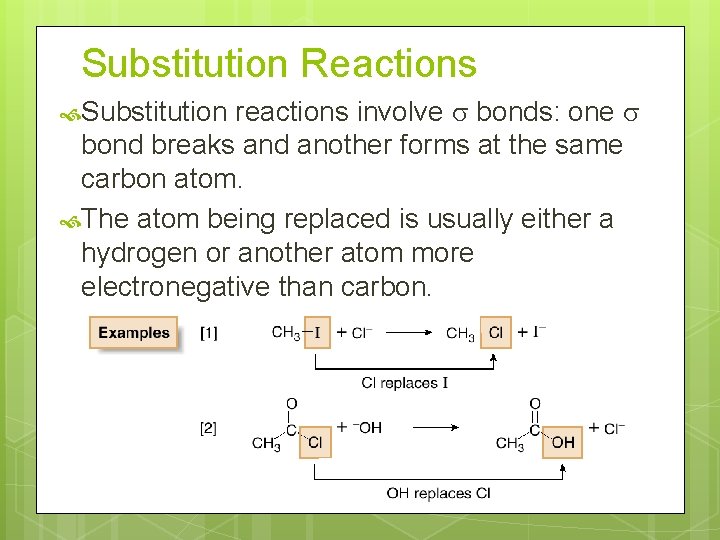
Substitution Reactions reactions involve bonds: one bond breaks and another forms at the same carbon atom. The atom being replaced is usually either a hydrogen or another atom more electronegative than carbon. Substitution

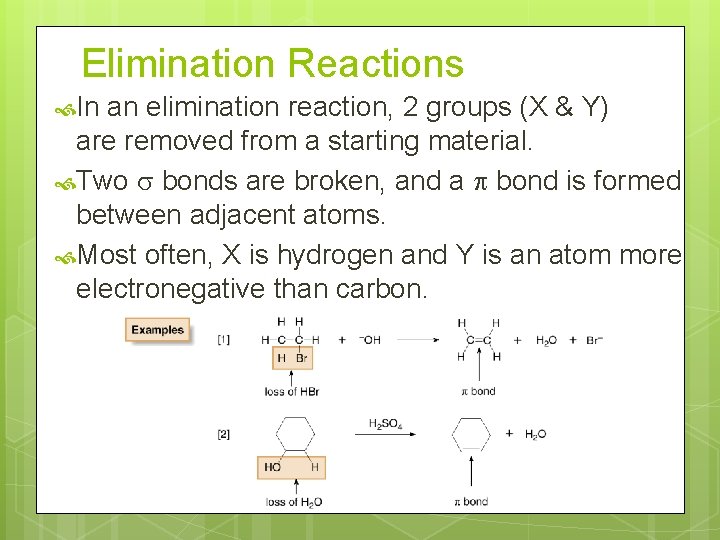
Elimination Reactions In an elimination reaction, 2 groups (X & Y) are removed from a starting material. Two bonds are broken, and a bond is formed between adjacent atoms. Most often, X is hydrogen and Y is an atom more electronegative than carbon.

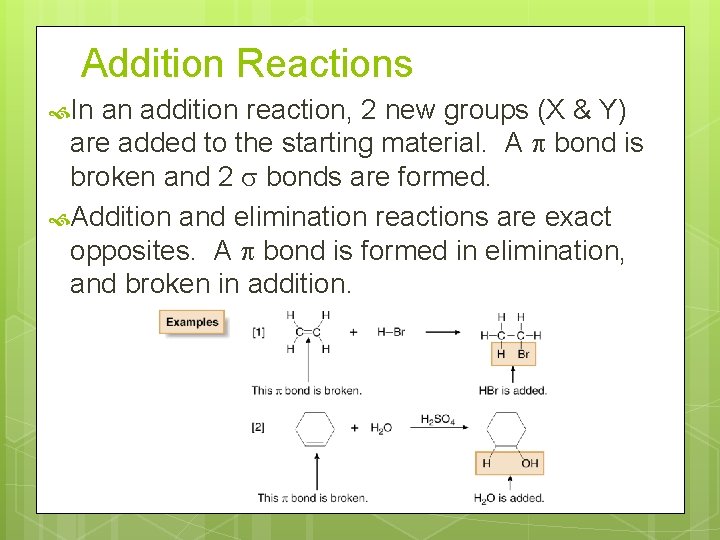
Addition Reactions In an addition reaction, 2 new groups (X & Y) are added to the starting material. A bond is broken and 2 bonds are formed. Addition and elimination reactions are exact opposites. A bond is formed in elimination, and broken in addition.

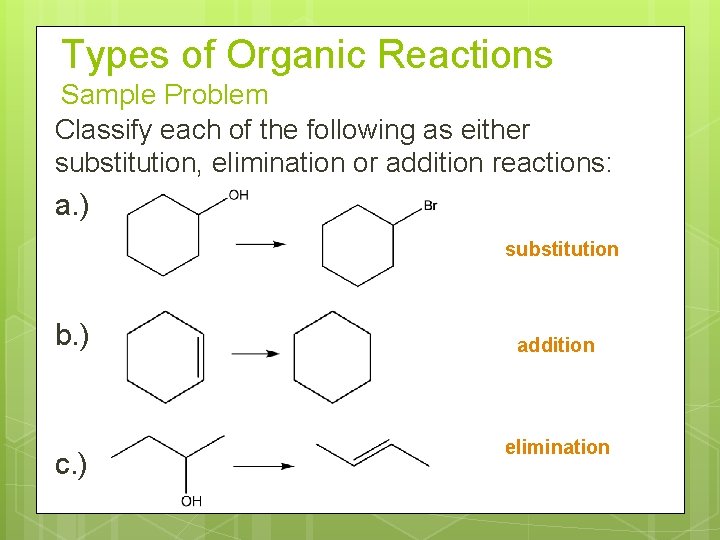
Types of Organic Reactions Sample Problem Classify each of the following as either substitution, elimination or addition reactions: a. ) substitution b. ) c. ) addition elimination

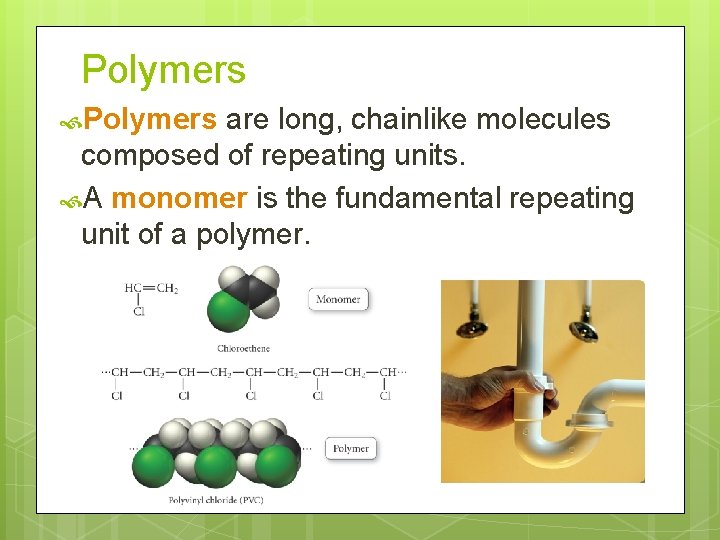
Polymers are long, chainlike molecules composed of repeating units. A monomer is the fundamental repeating unit of a polymer.

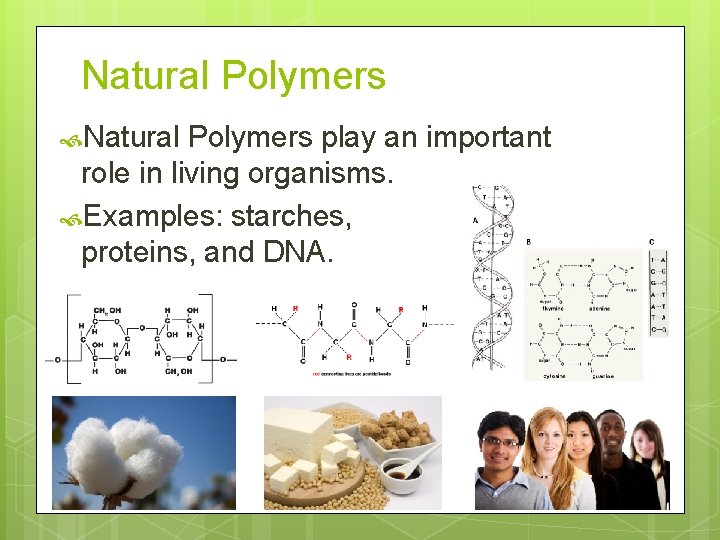
Natural Polymers play an important role in living organisms. Examples: starches, proteins, and DNA.

Synthetic Polymers Synthetics are man-made materials that have no duplicate in nature. The first synthetic polymer was prepared by Leo Baekeland in 1907. Due to the scientific approach, chemists have been able to tailor new molecules for specific purposes.