Organic Chemistry What Is Organic Chemistry Organic chemistry
























- Slides: 87

Organic Chemistry

What Is Organic Chemistry? • Organic chemistry is a branch of chemistry that focuses on compounds that contain carbon. üExcept CO, CO 2, carbonates, and carbides. • Even though organic compounds only contain a few elements, the unique ways carbon atoms can attach together to form molecules leads to millions of different organic compounds. 2

The Chemistry of Life • Life as we know it is because of organic chemistry. • Organic molecules can be very large and complex; this allows the complex functions of the cells to occur. • When chemists tried to classify compounds in the 1700’s, they found vast differences between the compounds found in living and nonliving things. 3

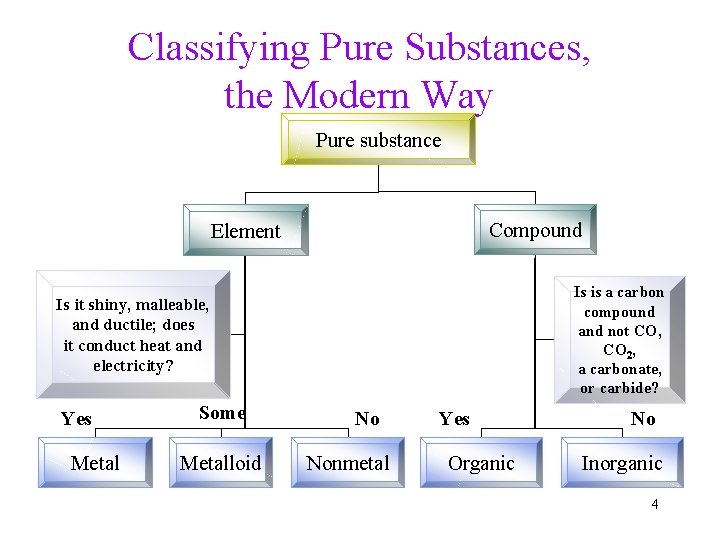
Classifying Pure Substances, the Modern Way Pure substance Compound Element Is is a carbon compound and not CO, CO 2, a carbonate, or carbide? Is it shiny, malleable, and ductile; does it conduct heat and electricity? Yes Metal Some Metalloid No Nonmetal Yes Organic No Inorganic 4

What’s Special About Organic Compounds? • Organic compounds tend to be molecular. • Mainly composed of just six nonmetallic elements. ü C, H, O, N, S, and P. • Compounds found in all three states. ü Solids, liquids, and gases. ü Solids tend to have low melting points. • Solubility in water varies depending on which of the other elements are attached to C and how many there are. ü CH 3 OH is miscible with water; C 10 H 21 OH is insoluble. 5

What’s So Special About Carbon? • Carbon atoms can do some unique things that other atoms cannot. • Carbon can bond to as many as four other atoms. • Bonds to carbon are very strong and nonreactive. • Carbon atoms can attach together in long chains. • Carbon atoms can attach together to form rings. • Carbon atoms can form single, double, or triple bonds. 6

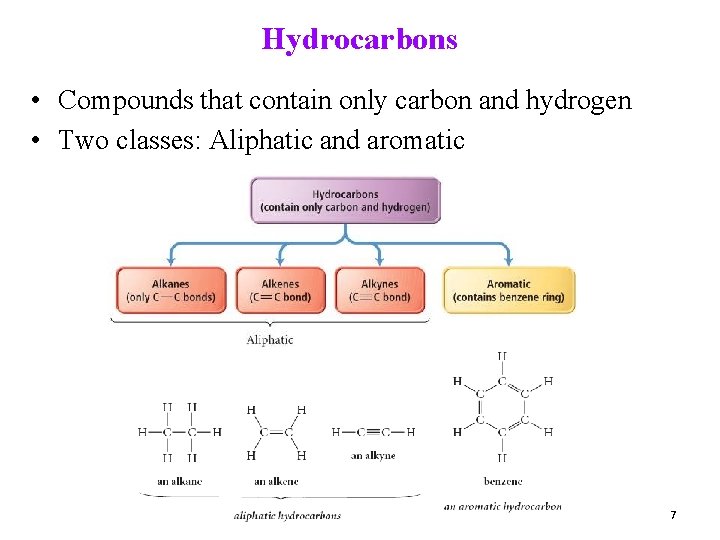
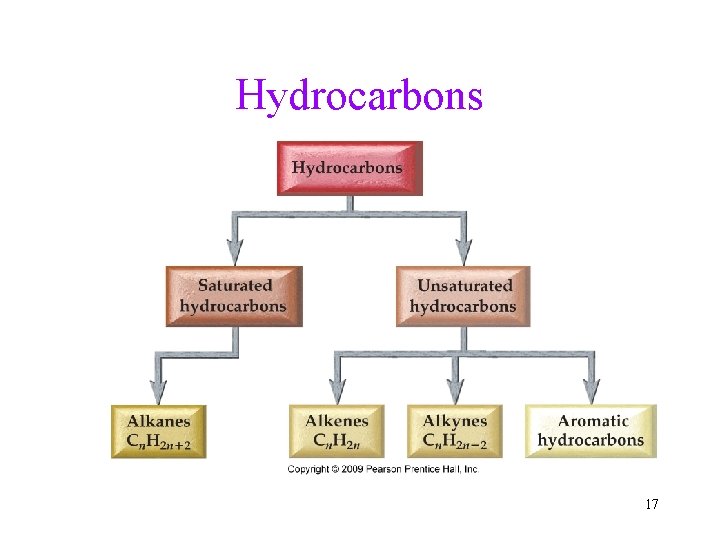
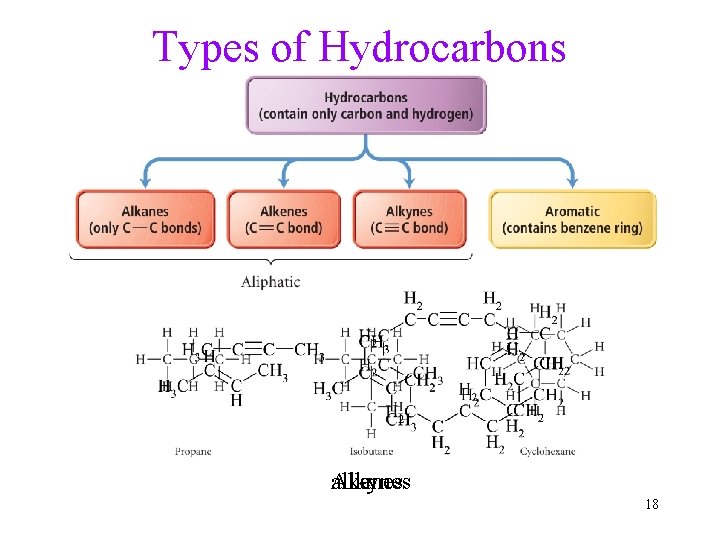
Hydrocarbons • Compounds that contain only carbon and hydrogen • Two classes: Aliphatic and aromatic 7

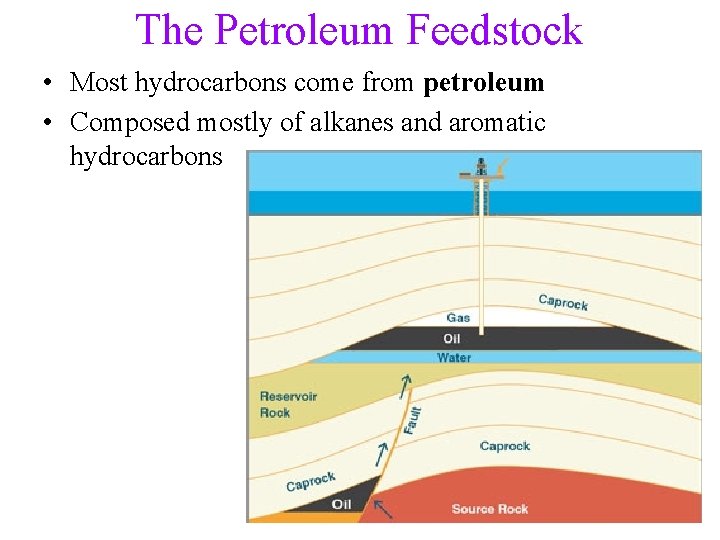
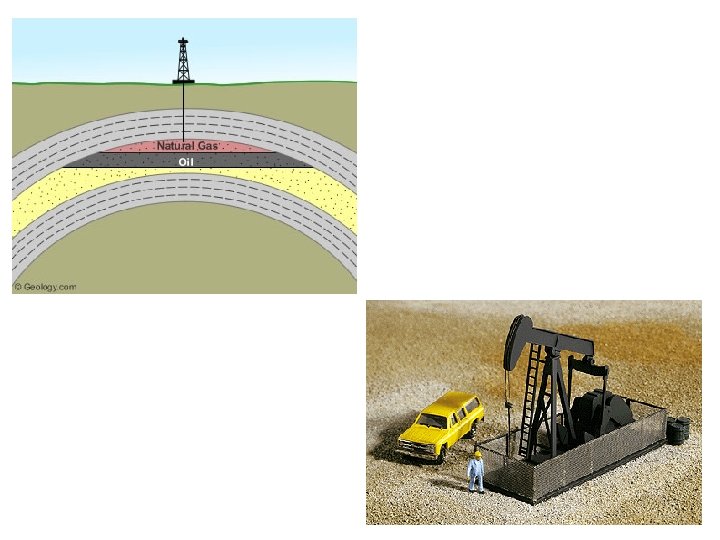
The Petroleum Feedstock • Most hydrocarbons come from petroleum • Composed mostly of alkanes and aromatic hydrocarbons 8

9

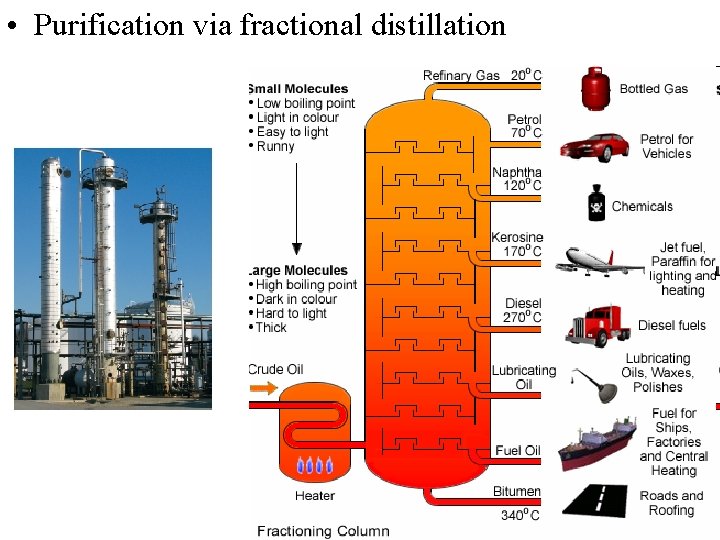
• Purification via fractional distillation 10

Hydrocarbons • Hydrocarbons contain only C and H. • Two classes of hydrocarbons: saturated or unsaturated. • Insoluble in water. ü No polar bonds to attract water molecules. • May be chains or rings. üRing molecules have less H than chain so that the ends can join. üChains may be straight or branched. üRings may be aliphatic or aromatic. 11

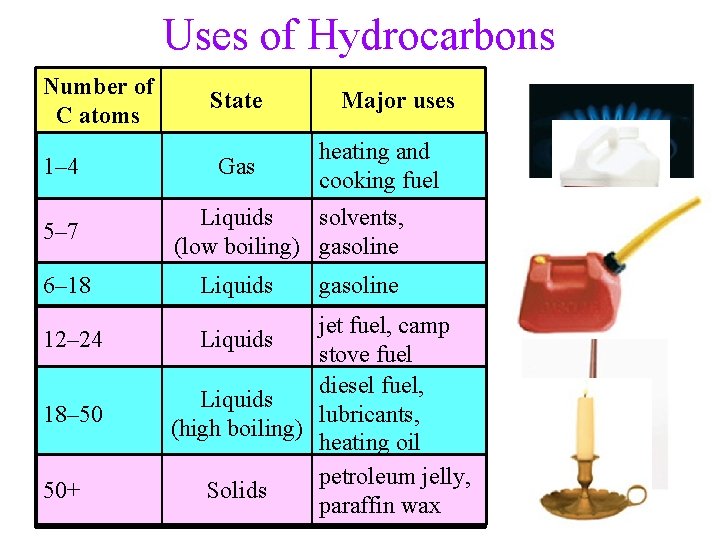
Uses of Hydrocarbons Number of C atoms 1– 4 5– 7 6– 18 12– 24 18– 50 50+ State Major uses heating and gas Gas cooking fuel liquids, solvents, Liquids (low boiling) gasoline liquids Liquids gasoline jet fuel; fuel, camp liquids Liquids stove fuel diesel fuel, Liquids liquids, lubricants, (high boiling) heating oil petroleum jelly, petroleum Solids solids paraffin wax paraffin

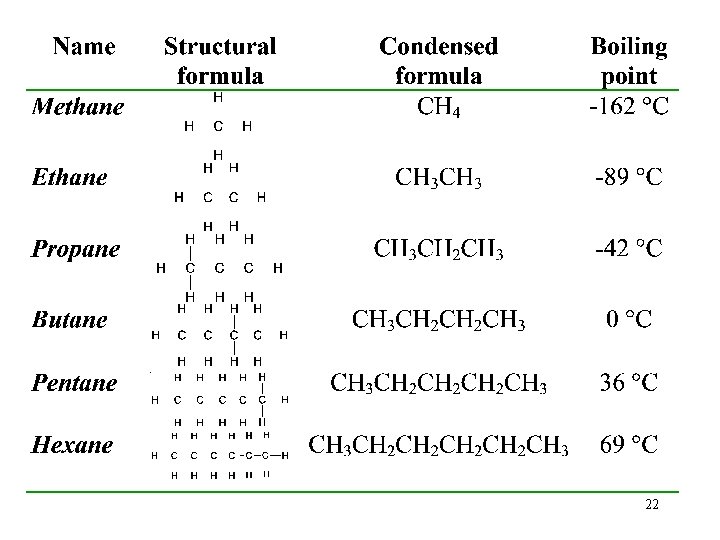
Saturated Hydrocarbons • A saturated hydrocarbon has all C—C single bonds. üIt is saturated with hydrogens. • Saturated hydrocarbons are called alkanes. • Chain alkanes have the general formula Cn. H 2 n+2. ü Chains may be straight or branched. ü Ring alkanes have two fewer Hs per ring than the corresponding chain isomer. 13

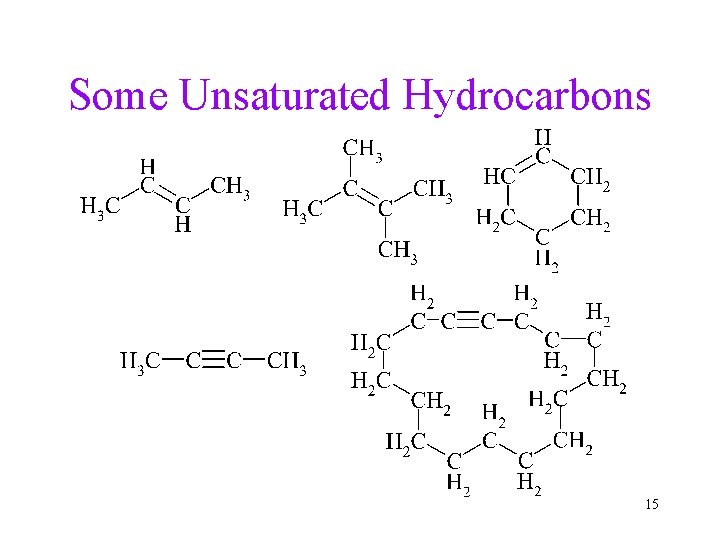
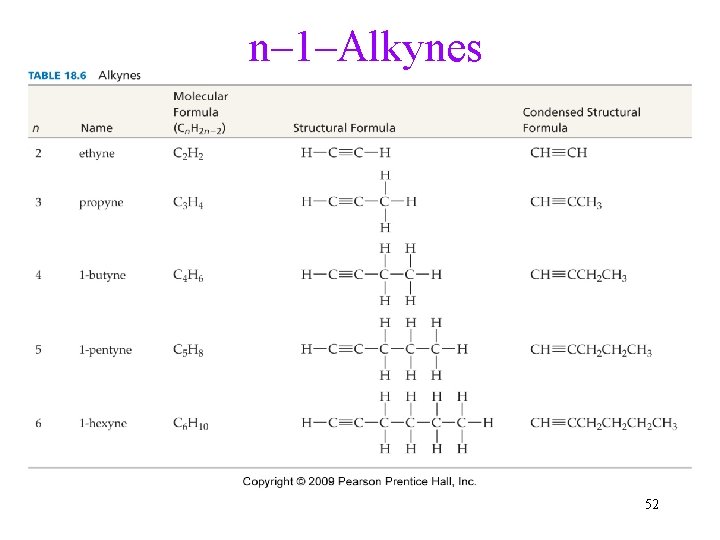
Unsaturated Hydrocarbons • Unsaturated hydrocarbons have one or more C=C double bonds, C C triple bonds, or aromatic rings. • Unsaturated hydrocarbons that contain C=C are called alkenes. ü The general formula of a monounsaturated chain alkene is Cn. H 2 n. • Unsaturated hydrocarbons that contain C C are called alkynes. ü The general formula of a monounsaturated chain alkyne is Cn. H 2 n-2. 14

Some Unsaturated Hydrocarbons 15

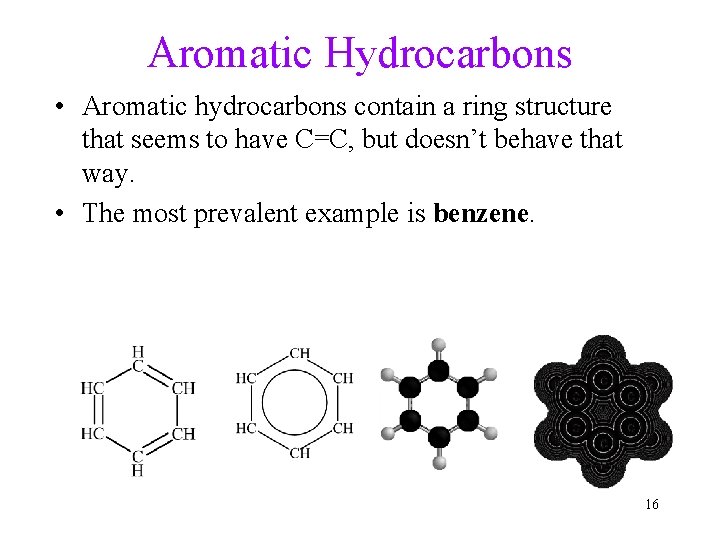
Aromatic Hydrocarbons • Aromatic hydrocarbons contain a ring structure that seems to have C=C, but doesn’t behave that way. • The most prevalent example is benzene. 16

Hydrocarbons 17

Types of Hydrocarbons Alkynes alkenes alkanes 18

Formulas • Molecular formulas just tell you what kinds of atoms are in the molecule, but they don’t tell you how they are attached. • Structural formulas show you the attachment pattern in the molecule. • Models not only show you the attachment pattern, but give you an idea about the shape of the molecule. 19

Carbon Skeleton Formulas • A. k. a. line-angle formulas. • Used very often with ring structures. • Each angle, and its beginning and end represent a C atom. • H omitted on C, but included on functional groups. • Multiple bonds indicated. Double line is double bond; triple line is triple bond. 20

Tro's Introductory Chemistry, Chapter 18 21

22

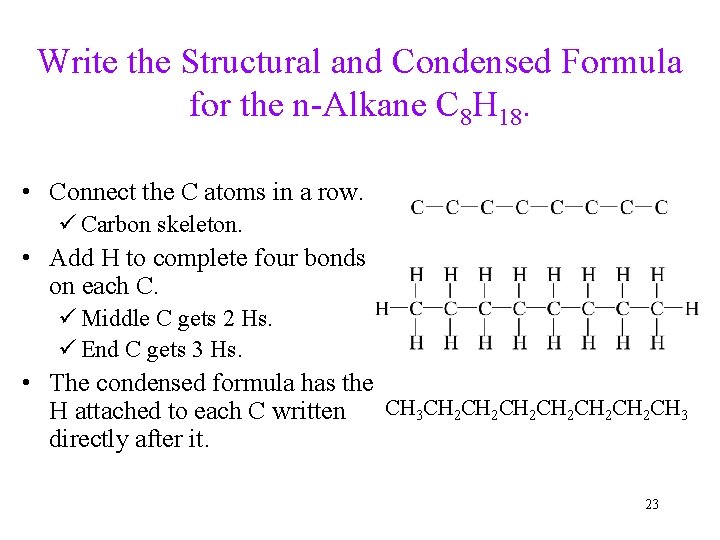
Write the Structural and Condensed Formula for the n-Alkane C 8 H 18. • Connect the C atoms in a row. ü Carbon skeleton. • Add H to complete four bonds on each C. ü Middle C gets 2 Hs. ü End C gets 3 Hs. • The condensed formula has the CH 3 CH 2 CH 2 CH 2 CH 3 H attached to each C written directly after it. 23

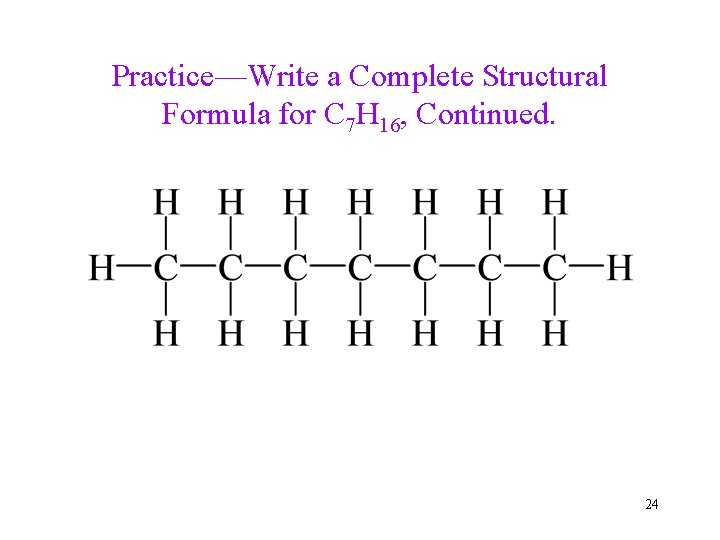
Practice—Write a Complete Structural Formula for C 7 H 16, Continued. 24

Alkanes • • • Also know as paraffins. Aliphatic, saturated. General formula Cn. H 2 n+2 for chains. Very unreactive. CH 3 groups at ends of chains; CH 2 groups in the middle. • Chains may be straight or branched. ün-Alkane = straight chain. 25

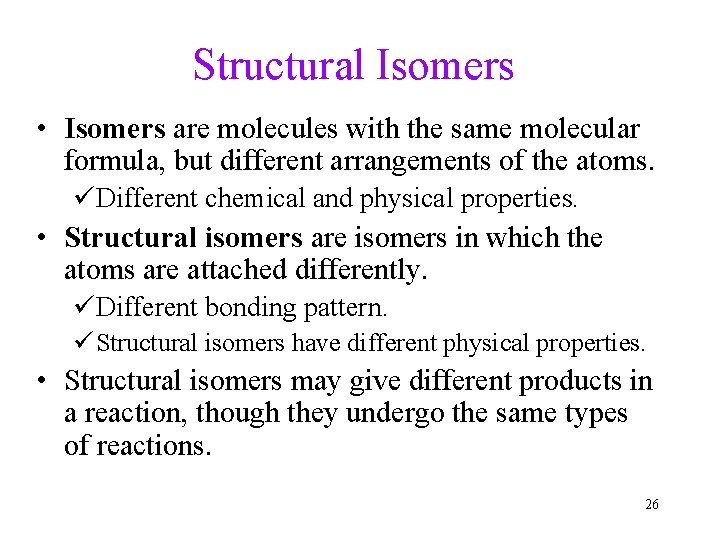
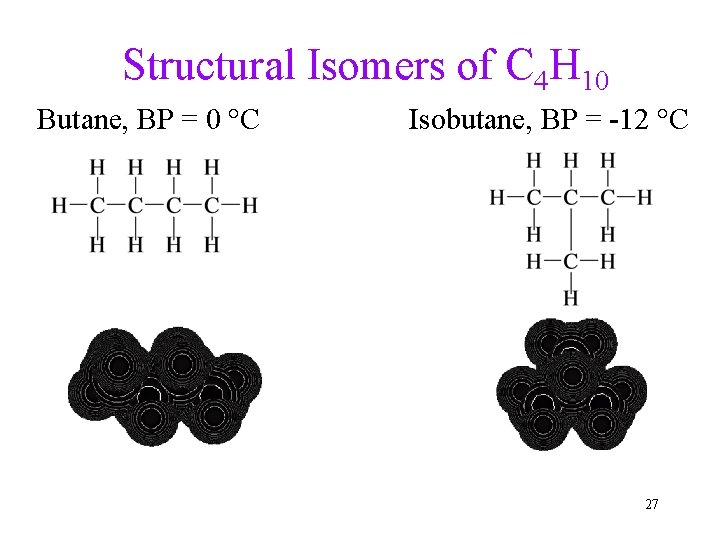
Structural Isomers • Isomers are molecules with the same molecular formula, but different arrangements of the atoms. üDifferent chemical and physical properties. • Structural isomers are isomers in which the atoms are attached differently. üDifferent bonding pattern. ü Structural isomers have different physical properties. • Structural isomers may give different products in a reaction, though they undergo the same types of reactions. 26

Structural Isomers of C 4 H 10 Butane, BP = 0 °C Isobutane, BP = -12 °C 27

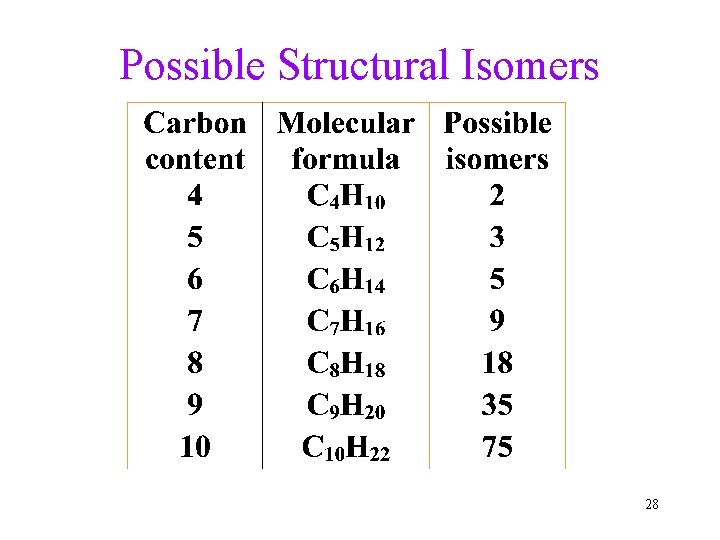
Possible Structural Isomers 28

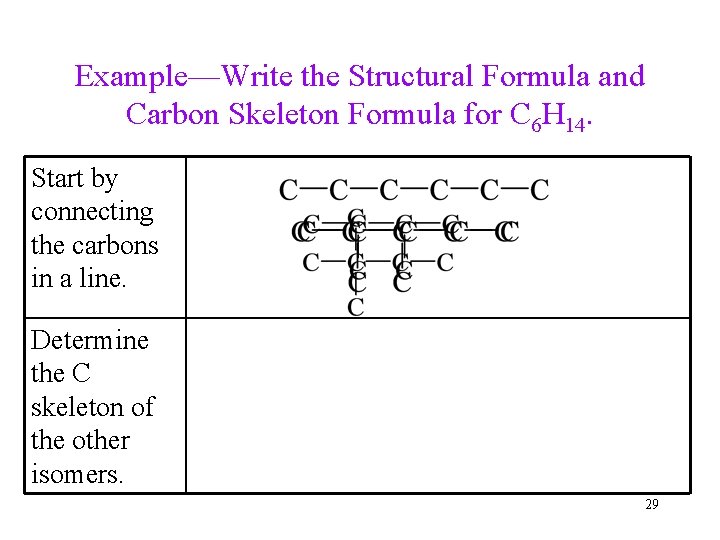
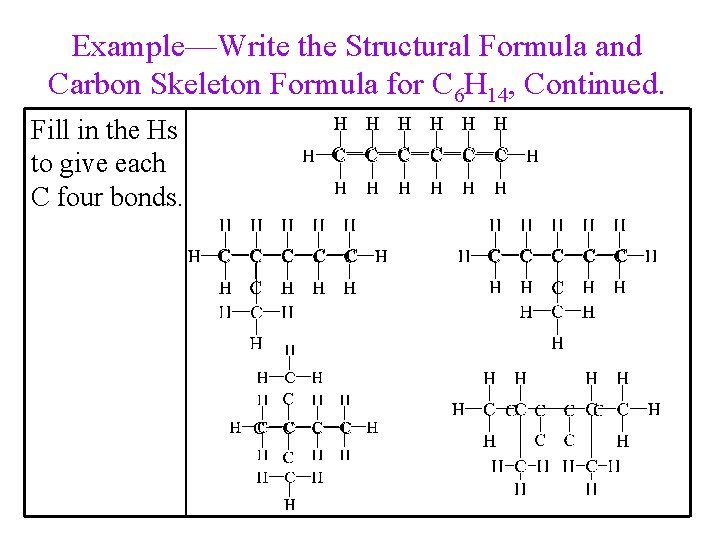
Example—Write the Structural Formula and Carbon Skeleton Formula for C 6 H 14. Start by connecting the carbons in a line. Determine the C skeleton of the other isomers. 29

Example—Write the Structural Formula and Carbon Skeleton Formula for C 6 H 14, Continued. Fill in the Hs to give each C four bonds.

• Physical Properties of Solubility: Hydrocarbons üTend to be insoluble in water üGood preservative for reactive metals • Density: üTend to have lower densities than water Ø 0. 6 g/m. L – 0. 8 g/m. L 32

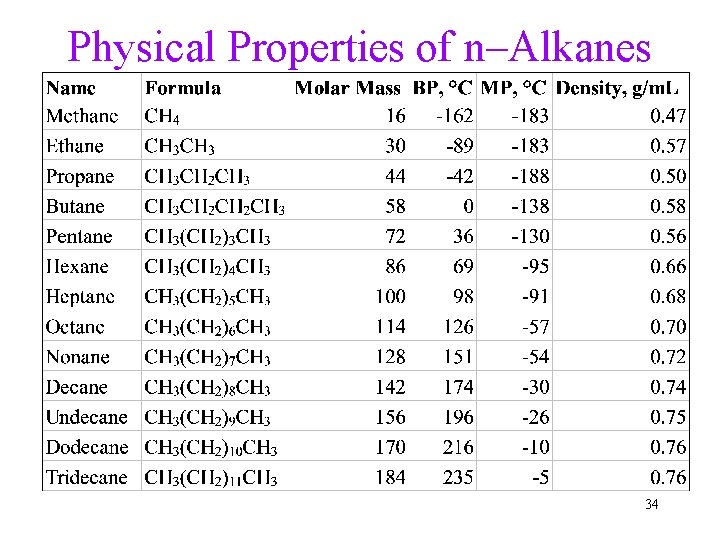
Alkanes—Physical Properties • Nonpolar molecules, intermolecular attractions are London dispersion forces due to momentary dipoles only. • Both boiling points and melting points generally increase as the size of the molecule increases. • Insoluble in water, commonly used as nonpolar solvents. • Less dense than water, density increases with size. 33

Physical Properties of n–Alkanes 34

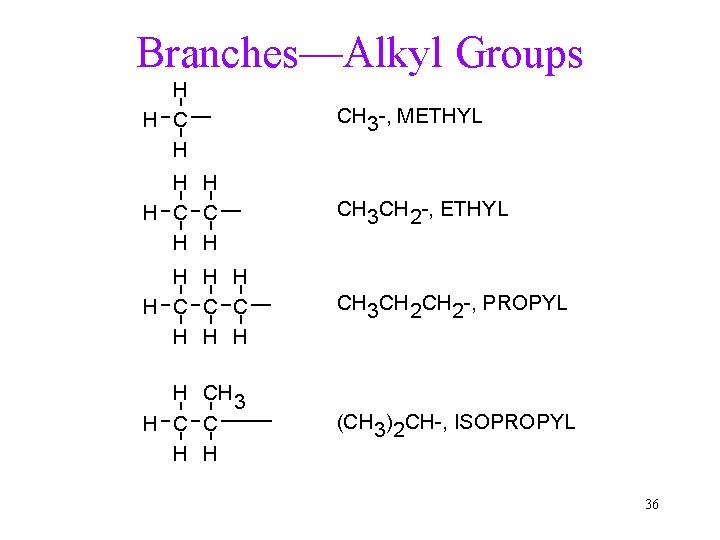
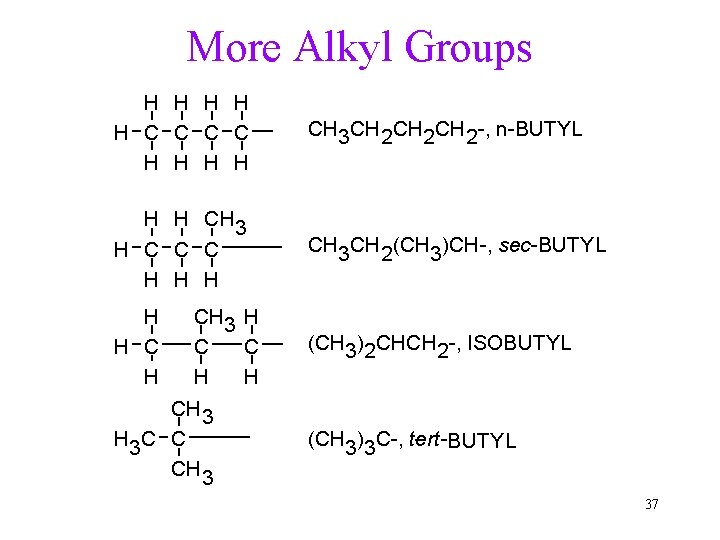
Naming Alkanes 1. Find the longest, continuous carbon chain. 2. Number the chain from the end closest to a branch. ü If first branches equal distance, use the next in. 3. Name branches as alkyl groups. ü Locate each branch by preceding its name with the carbon number on the chain. 4. List branches alphabetically. ü Do not count n-, sec-, t-, but count iso. 5. Use prefix if more than one of the same group is present. ü di-, tri-, tetra-, penta-, hexa-. ü Do not count in alphabetizing. 35

Branches—Alkyl Groups H H CH 3 -, METHYL H H H C C H H CH 3 CH 2 -, ETHYL H H C C C H H H CH 3 CH 2 -, PROPYL H CH 3 H C C H H (CH 3)2 CH-, ISOPROPYL 36

More Alkyl Groups H H H C C H H CH 3 CH 2 CH 2 -, n-BUTYL H H CH 3 H C C C H H H CH 3 CH 2(CH 3)CH-, sec-BUTYL H H C H (CH 3)2 CHCH 2 -, ISOBUTYL CH 3 H C C H H CH 3 H 3 C C CH 3 (CH 3)3 C-, tert- BUTYL 37

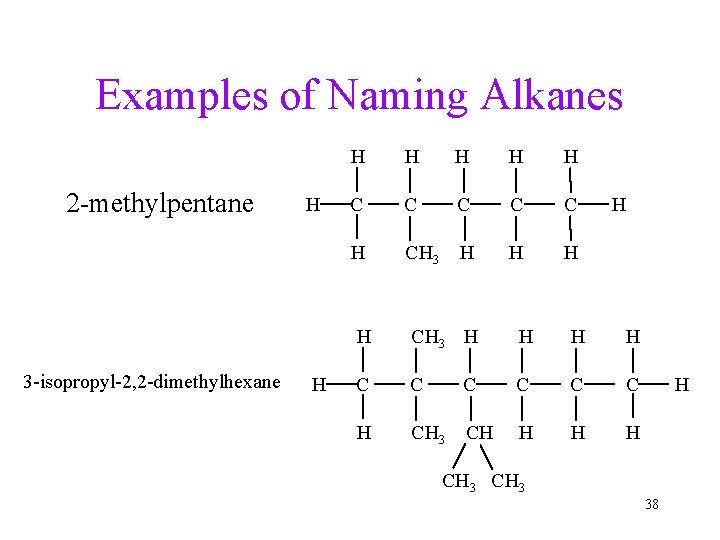
Examples of Naming Alkanes 2 -methylpentane 3 -isopropyl-2, 2 -dimethylhexane H H H H C C C H CH 3 CH H H CH 3 38

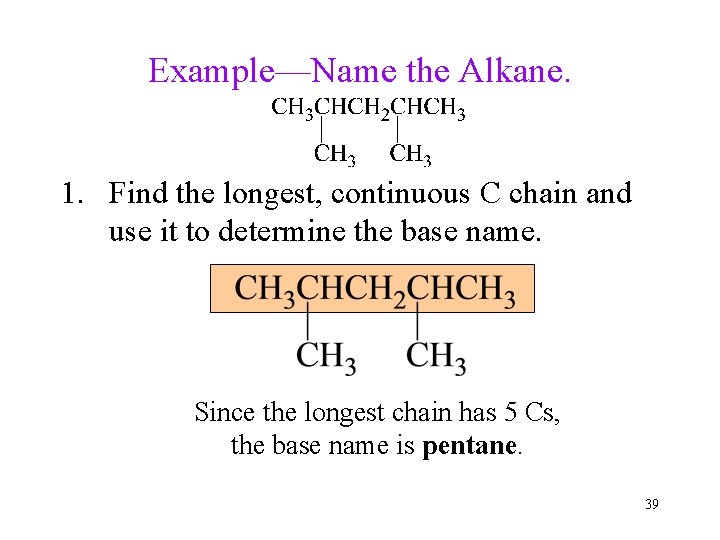
Example—Name the Alkane. 1. Find the longest, continuous C chain and use it to determine the base name. Since the longest chain has 5 Cs, the base name is pentane. 39

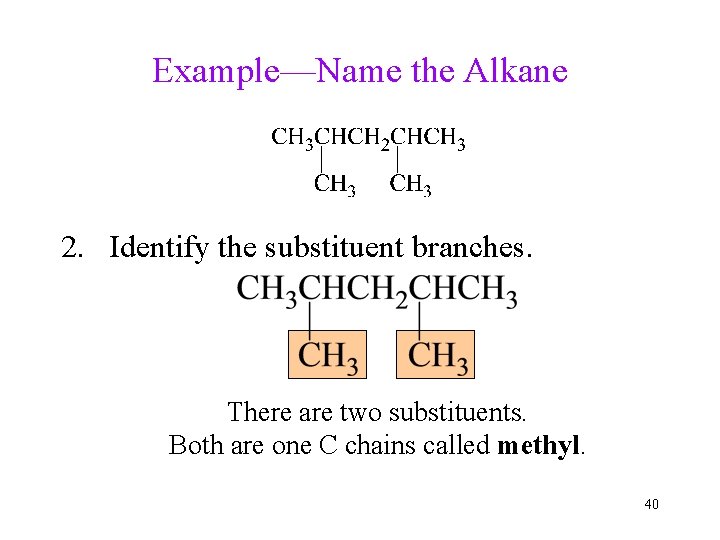
Example—Name the Alkane 2. Identify the substituent branches. There are two substituents. Both are one C chains called methyl. 40

Example—Name the Alkane 3. Number the chain from the end closest to a substituent branch. ü If first substituents equidistant from end, go to next substituent in. Then assign numbers to each substituent based on the number of the main chain C to which its attached. 1 2 3 4 5 Both substituents are equidistant from the end. 2 4 41

Example—Name the Alkane 4. Write the name in the following order: a. Substituent number of first alphabetical substituent followed by dash. b. Substituent name of first alphabetical substituent followed by dash. ü If it’s the last substituent listed, no dash. ü Use prefixes to indicate multiple identical substituents. c. Repeat for other substituents alphabetically. d. Name of main chain. 2, 4 – dimethylpentane 2 4 42

Practice—Name the Following: 43

Practice—Name the Following, Continued: 3 -ethyl-2 -methylpentane 44

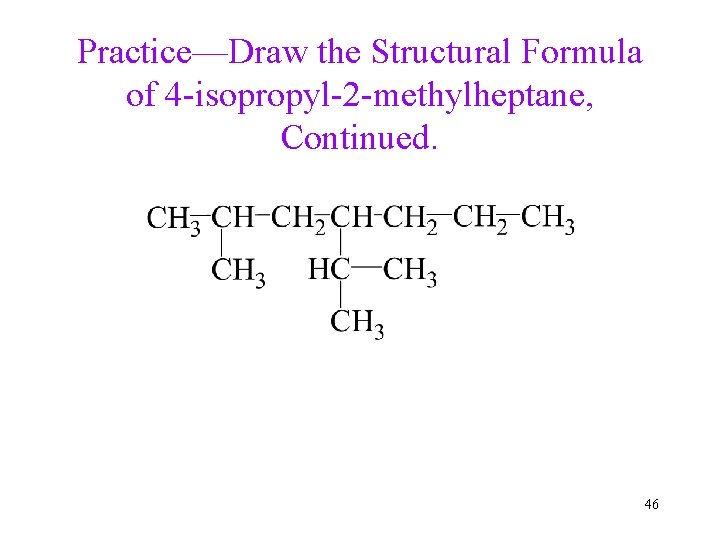
Practice—Draw the Structural Formula of 4 -isopropyl-2 -methylheptane. 45

Practice—Draw the Structural Formula of 4 -isopropyl-2 -methylheptane, Continued. 46

Alkenes • Also known as olefins. • Aliphatic, unsaturated. ü C=C double bonds. • Formula for one double bond = Cn. H 2 n. ü Subtract two Hs from alkane for each double bond. • Trigonal shape around C. ü Flat. • Much more reactive than alkanes. • Polyunsaturated = many double bonds. 47

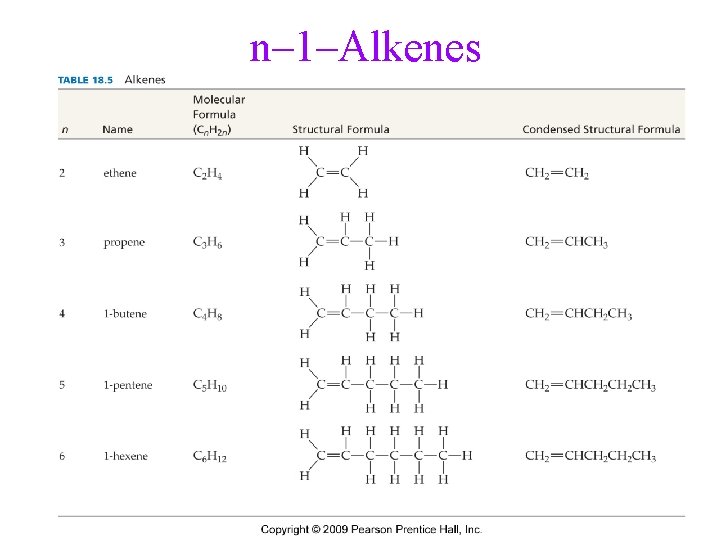
n– 1–Alkenes

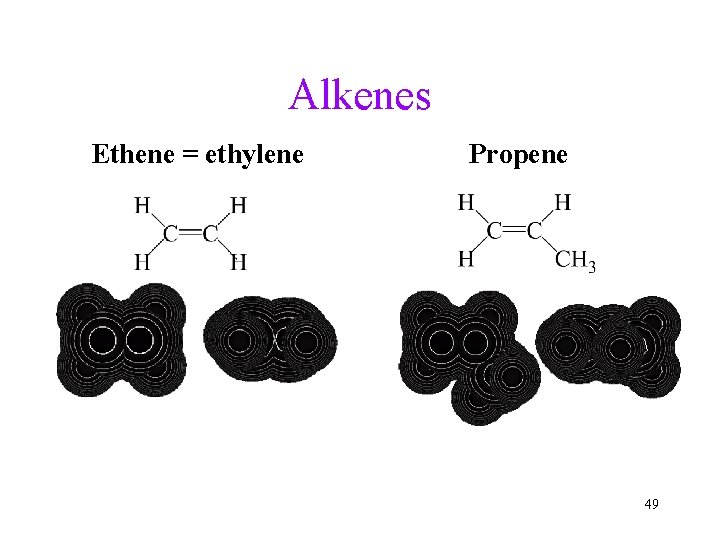
Alkenes Ethene = ethylene Propene 49

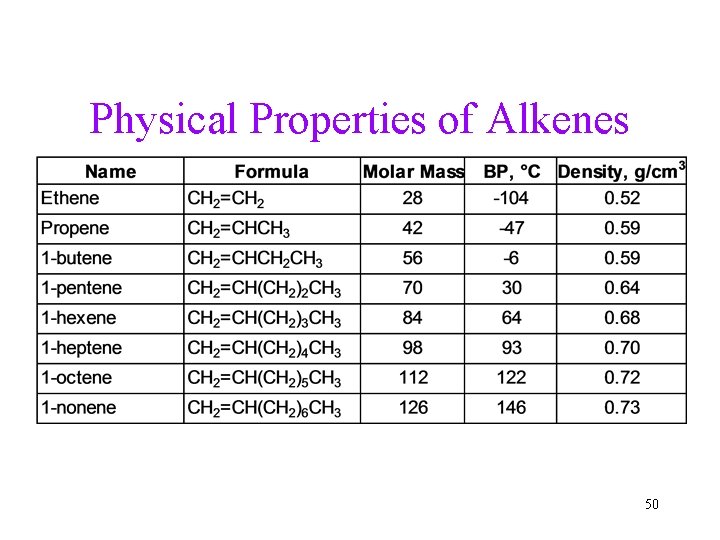
Physical Properties of Alkenes 50

Alkynes • • Also known as acetylenes. Aliphatic, unsaturated. C C triple bond. Formula for one triple bond = Cn. H 2 n-2. ü Subtract four Hs from alkane for each triple bond. • Linear shape. • More reactive than alkenes. 51

n– 1–Alkynes 52

Alkynes Ethyne = acetylene Propyne 53

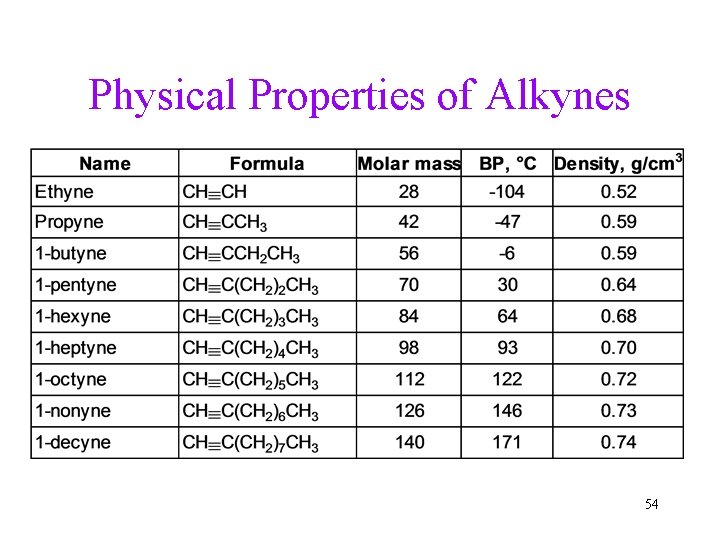
Physical Properties of Alkynes 54

Naming Alkenes and Alkynes • Find longest chain containing multiple bond. üAlkene takes precedence over alkyne. • Change suffix on main name from -ane to -ene for base name of alkene, or to –yne for the base name of the alkyne. • Number chain from end closest to multiple bond. • Number in front of main name indicates first carbon of multiple bond. 55

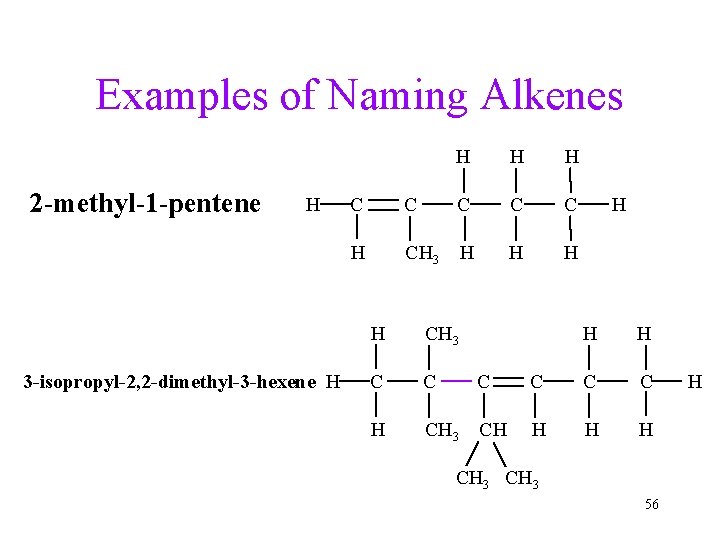
Examples of Naming Alkenes 2 -methyl-1 -pentene H 3 -isopropyl-2, 2 -dimethyl-3 -hexene H H C C C H CH 3 H H CH 3 C C C H CH 3 CH H C C C H H H CH 3 56 H

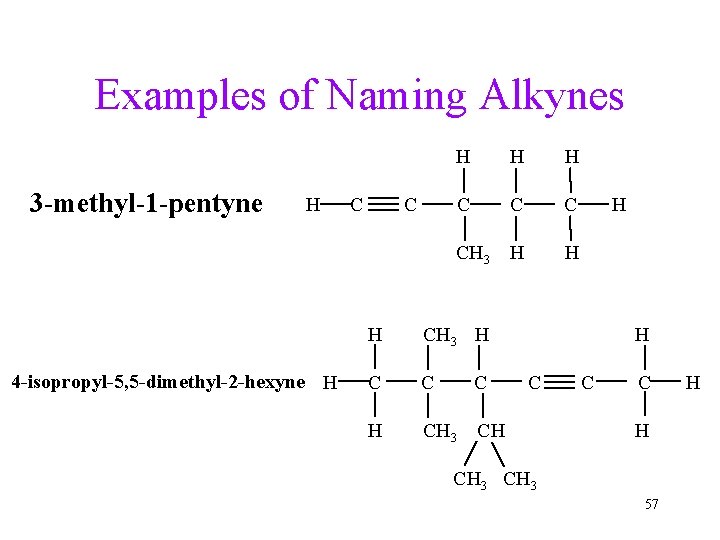
Examples of Naming Alkynes 3 -methyl-1 -pentyne H 4 -isopropyl-5, 5 -dimethyl-2 -hexyne H C C H H H C CH 3 H H H CH 3 H C C C H CH 3 CH H H C C C H CH 3 57 H

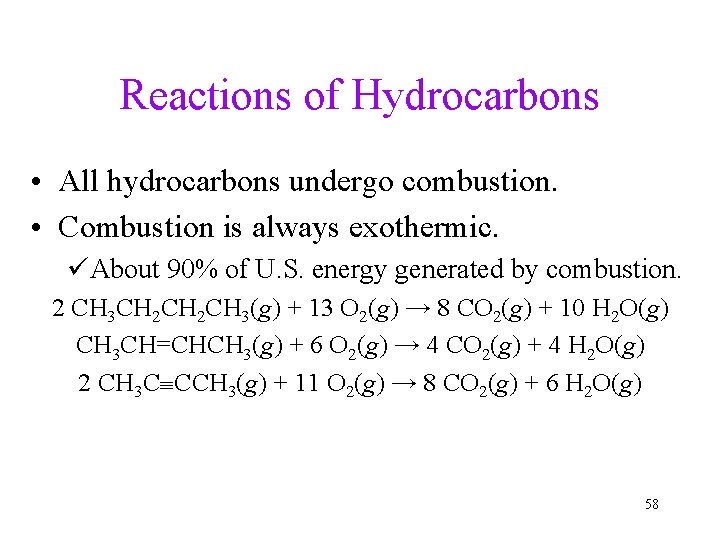
Reactions of Hydrocarbons • All hydrocarbons undergo combustion. • Combustion is always exothermic. üAbout 90% of U. S. energy generated by combustion. 2 CH 3 CH 2 CH 3(g) + 13 O 2(g) → 8 CO 2(g) + 10 H 2 O(g) CH 3 CH=CHCH 3(g) + 6 O 2(g) → 4 CO 2(g) + 4 H 2 O(g) 2 CH 3 C CCH 3(g) + 11 O 2(g) → 8 CO 2(g) + 6 H 2 O(g) 58

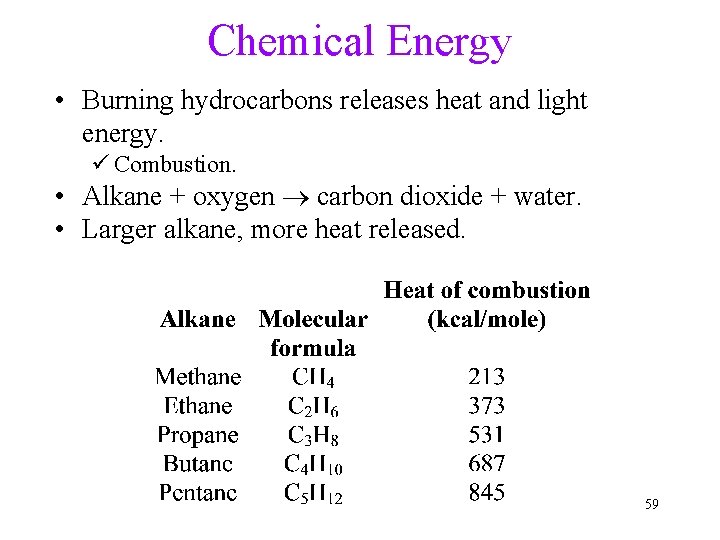
Chemical Energy • Burning hydrocarbons releases heat and light energy. ü Combustion. • Alkane + oxygen ® carbon dioxide + water. • Larger alkane, more heat released. 59

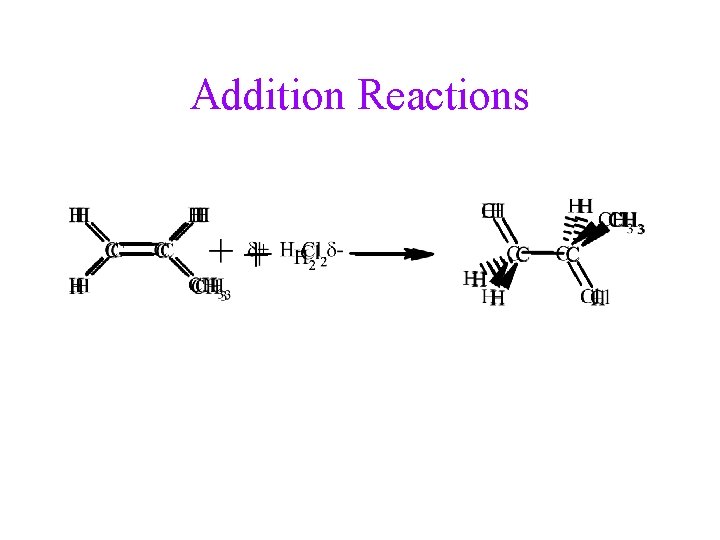
Addition Reactions

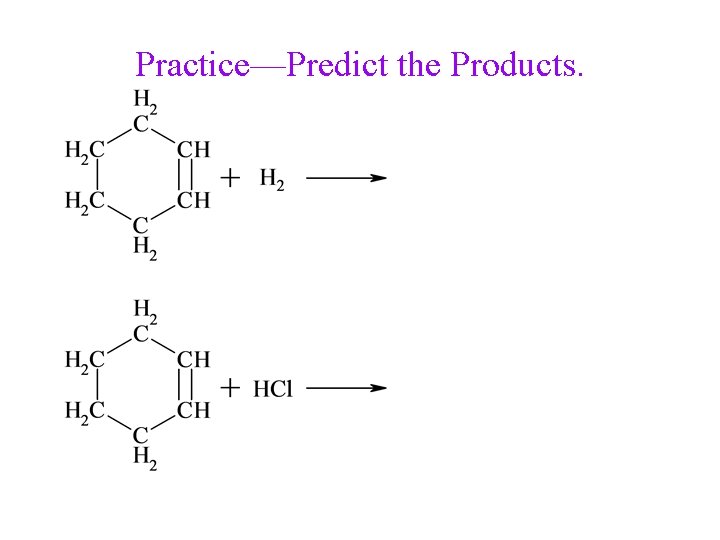
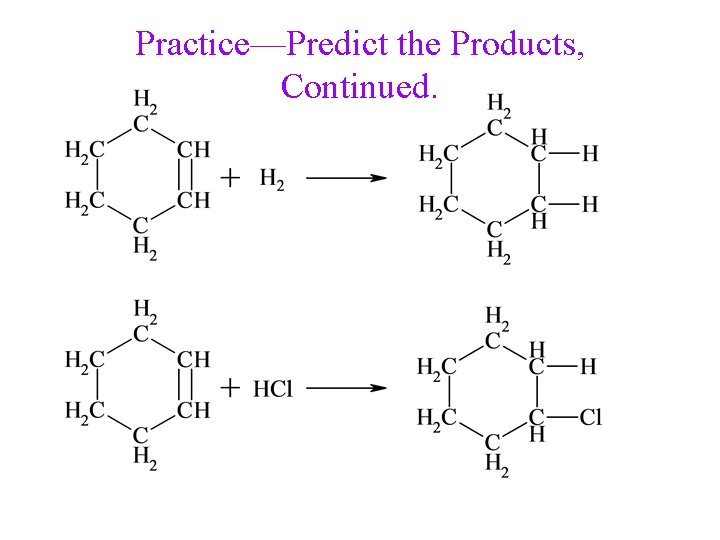
Practice—Predict the Products.

Practice—Predict the Products, Continued.

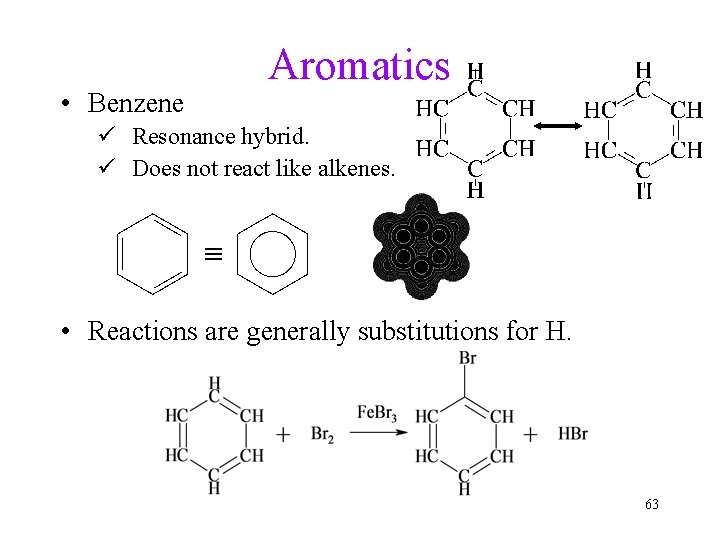
Aromatics • Benzene ü Resonance hybrid. ü Does not react like alkenes. • Reactions are generally substitutions for H. 63

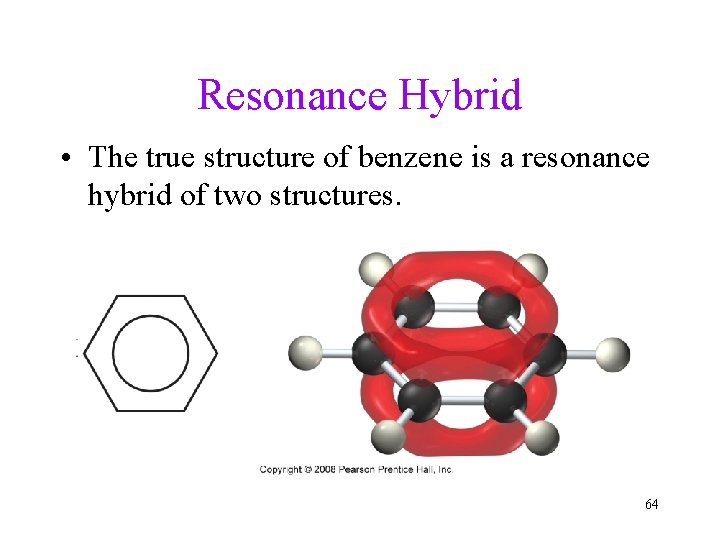
Resonance Hybrid • The true structure of benzene is a resonance hybrid of two structures. 64

Naming Monosubstituted Benzene Derivatives • (Name of substituent) benzene. ü Halogen substituent = change ending to “o. ” fluorobenzene propylbenzene • Or name of a common derivative. 65

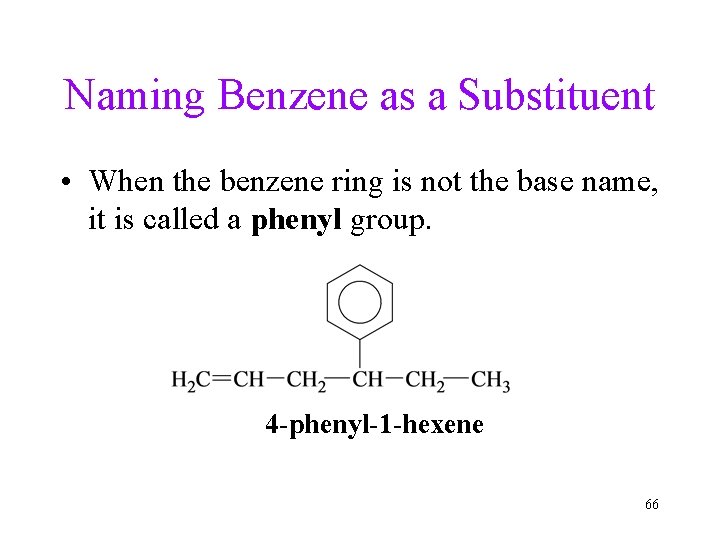
Naming Benzene as a Substituent • When the benzene ring is not the base name, it is called a phenyl group. 4 -phenyl-1 -hexene 66

Naming Disubstituted Benzene Derivatives • Number the ring starting at attachment for first substituent, then move toward second. üOrder substituents alphabetically. üUse “di-” if both substituents are the same. 3 2 1 1 -bromo-3 -fluorobenzene 1 2 1, 2 -dimethylbenzene 67

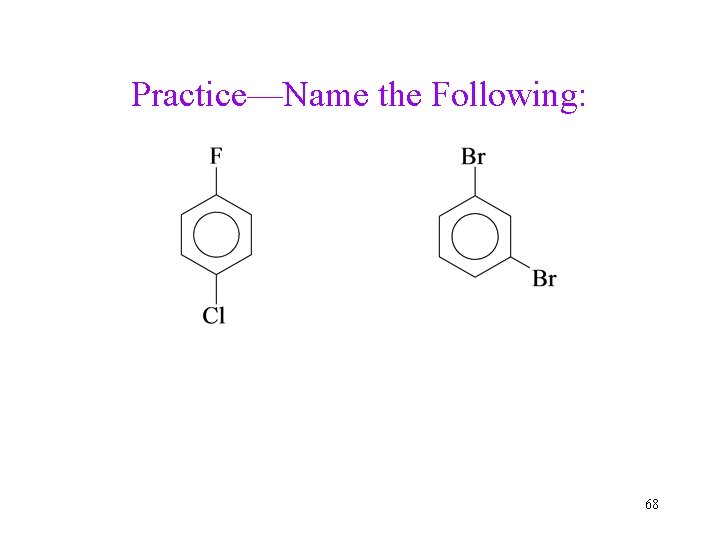
Practice—Name the Following: 68

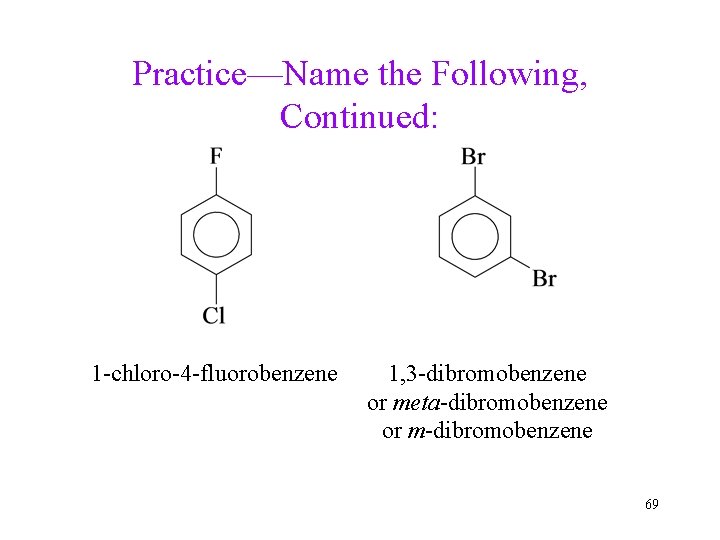
Practice—Name the Following, Continued: 1 -chloro-4 -fluorobenzene 1, 3 -dibromobenzene or meta-dibromobenzene or m-dibromobenzene 69

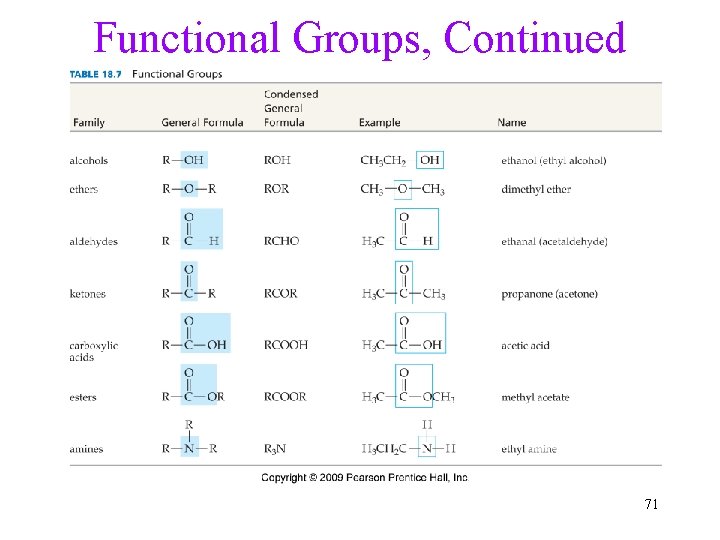
Functional Groups • Other organic compounds are hydrocarbons in which functional groups have been substituted for hydrogens. • A functional group is a group of atoms that show a characteristic influence on the properties of the molecule. ü Generally, the reactions that a compound will perform are determined by what functional groups it has. ü Since the kind of hydrocarbon chain is irrelevant to the reactions, it may be indicated by the general symbol R. R group CH 3—OH Functional group 70

Functional Groups, Continued 71

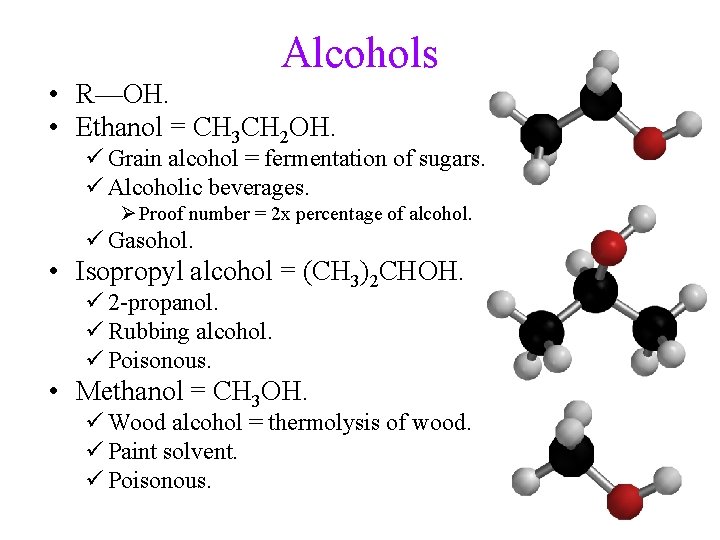
Alcohols • R—OH. • Ethanol = CH 3 CH 2 OH. ü Grain alcohol = fermentation of sugars. ü Alcoholic beverages. Ø Proof number = 2 x percentage of alcohol. ü Gasohol. • Isopropyl alcohol = (CH 3)2 CHOH. ü 2 -propanol. ü Rubbing alcohol. ü Poisonous. • Methanol = CH 3 OH. ü Wood alcohol = thermolysis of wood. ü Paint solvent. ü Poisonous.

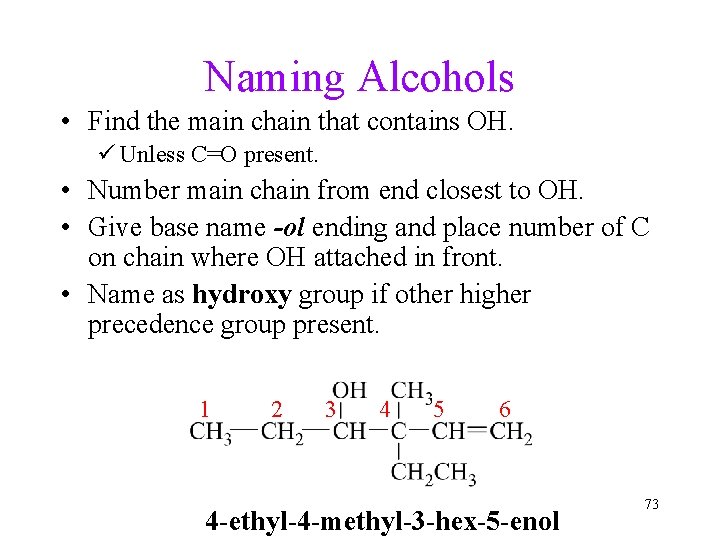
Naming Alcohols • Find the main chain that contains OH. ü Unless C=O present. • Number main chain from end closest to OH. • Give base name -ol ending and place number of C on chain where OH attached in front. • Name as hydroxy group if other higher precedence group present. 1 2 3 4 5 6 4 -ethyl-4 -methyl-3 -hex-5 -enol 73

Practice—Draw a Structural Formula for 3 -ethyl-2, 4 -dimethyl-2 -pent-4 -enol. 74

Ethers • R–O–R. • Ether = diethyl ether = CH 3 CH 2 OCH 2 CH 3. üAnesthetic. • To name ethers, name each alkyl group attached to the O, then add the word ether to the end. 75

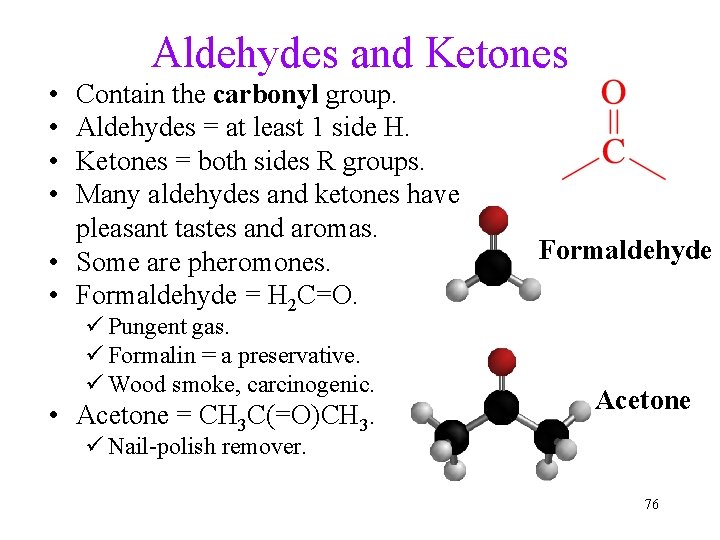
Aldehydes and Ketones • • Contain the carbonyl group. Aldehydes = at least 1 side H. Ketones = both sides R groups. Many aldehydes and ketones have pleasant tastes and aromas. • Some are pheromones. • Formaldehyde = H 2 C=O. ü Pungent gas. ü Formalin = a preservative. ü Wood smoke, carcinogenic. • Acetone = CH 3 C(=O)CH 3. Formaldehyde Acetone ü Nail-polish remover. 76

Aldehyde Odors and Flavors • Butanal = butter. • Vanillin = vanilla. • Benzaldehyde = almonds. • Cinnamaldehyde = cinnamon. 77

Ketone Odors and Flavors • Acetophenone = pistachio. • Carvone = spearmint. • Ionone = raspberries. • Muscone = musk. 78

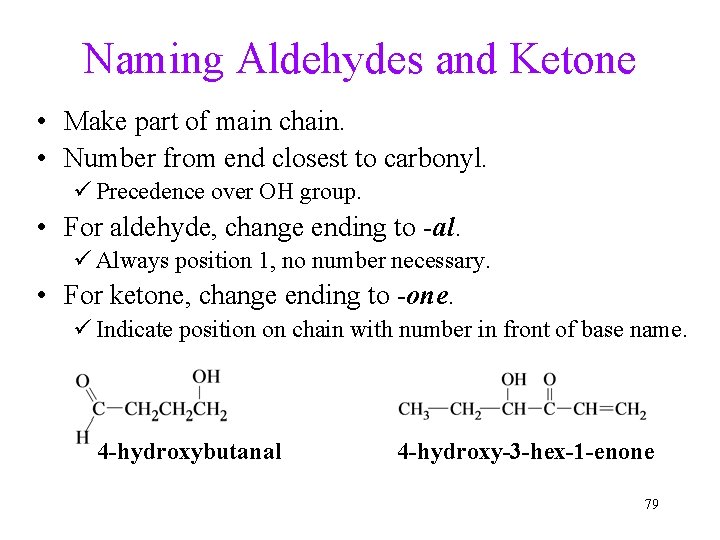
Naming Aldehydes and Ketone • Make part of main chain. • Number from end closest to carbonyl. ü Precedence over OH group. • For aldehyde, change ending to -al. ü Always position 1, no number necessary. • For ketone, change ending to -one. ü Indicate position on chain with number in front of base name. 4 -hydroxybutanal 4 -hydroxy-3 -hex-1 -enone 79

Carboxylic Acids • • RCOOH. Sour tasting. Weak acids. Citric acid. üFound in citrus fruit. • Ethanoic acid = acetic acid. üVinegar. • Methanoic acid = formic acid. üInsect bites and stings. 80

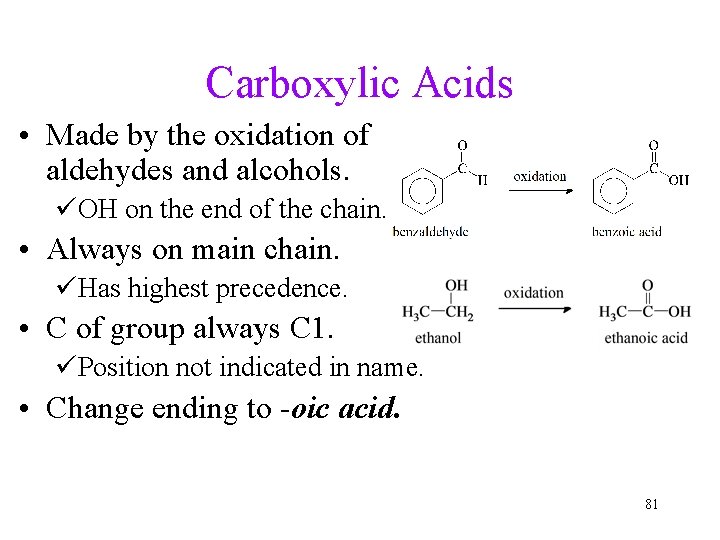
Carboxylic Acids • Made by the oxidation of aldehydes and alcohols. üOH on the end of the chain. • Always on main chain. üHas highest precedence. • C of group always C 1. üPosition not indicated in name. • Change ending to -oic acid. 81

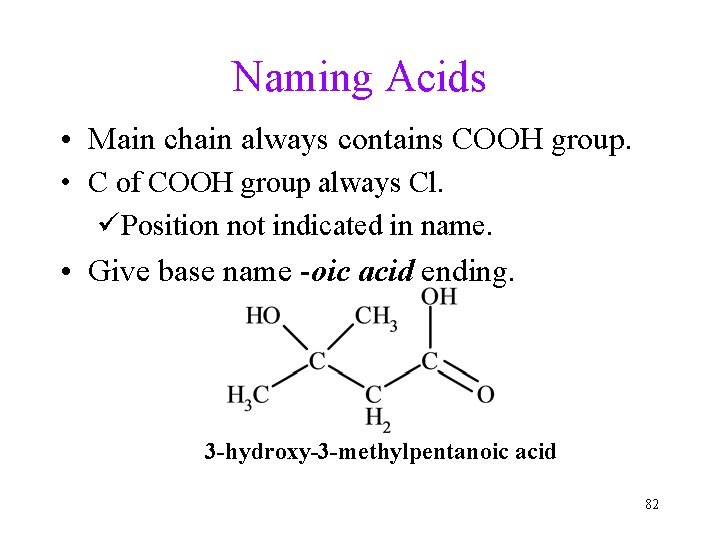
Naming Acids • Main chain always contains COOH group. • C of COOH group always Cl. üPosition not indicated in name. • Give base name -oic acid ending. 3 -hydroxy-3 -methylpentanoic acid 82

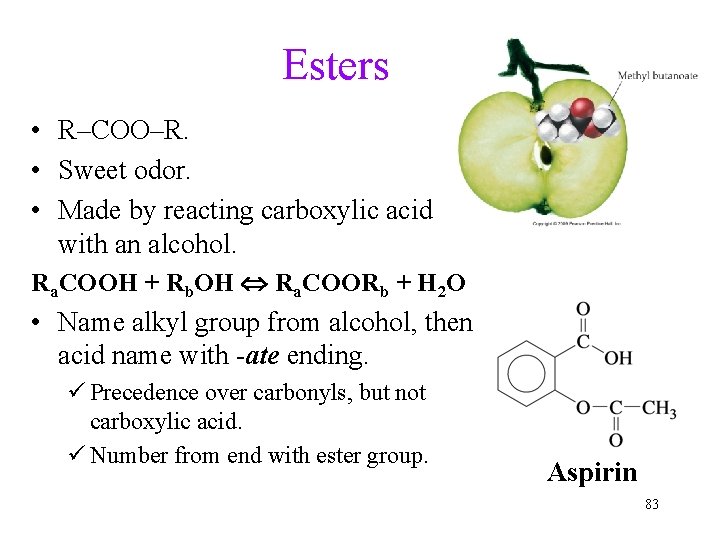
Esters • R–COO–R. • Sweet odor. • Made by reacting carboxylic acid with an alcohol. Ra. COOH + Rb. OH Ra. COORb + H 2 O • Name alkyl group from alcohol, then acid name with -ate ending. ü Precedence over carbonyls, but not carboxylic acid. ü Number from end with ester group. Aspirin 83

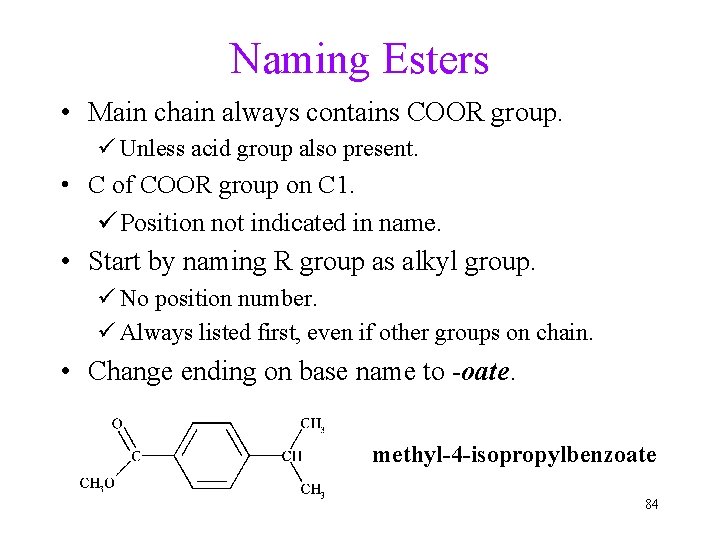
Naming Esters • Main chain always contains COOR group. ü Unless acid group also present. • C of COOR group on C 1. ü Position not indicated in name. • Start by naming R group as alkyl group. ü No position number. ü Always listed first, even if other groups on chain. • Change ending on base name to -oate. methyl-4 -isopropylbenzoate 84

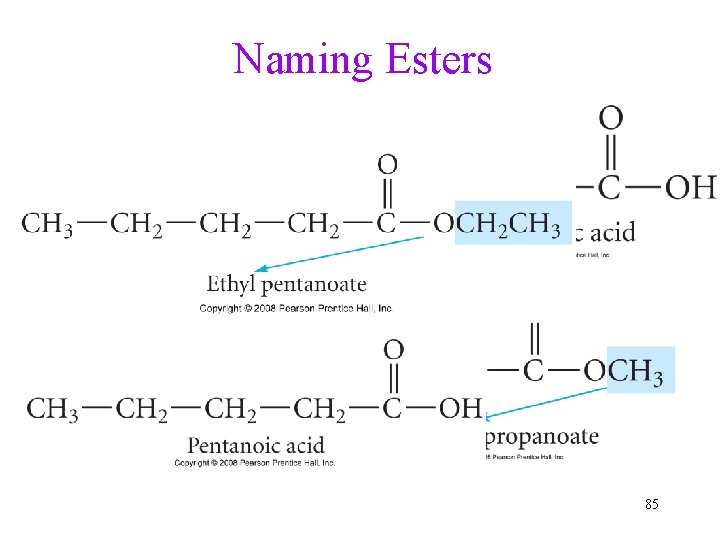
Naming Esters 85

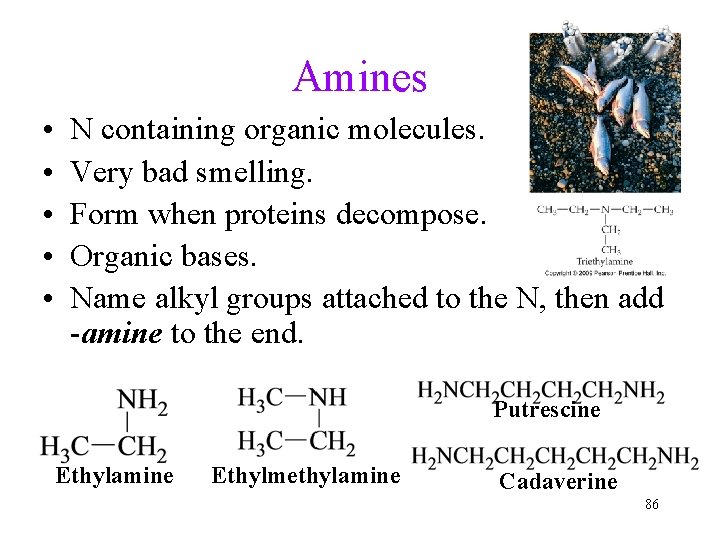
Amines • • • N containing organic molecules. Very bad smelling. Form when proteins decompose. Organic bases. Name alkyl groups attached to the N, then add -amine to the end. Putrescine Ethylamine Ethylmethylamine Cadaverine 86

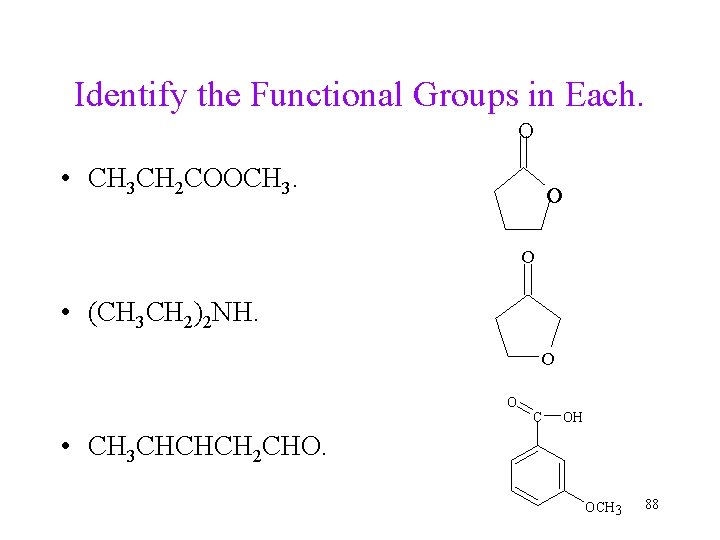
Identify the Functional Groups in Each. O • CH 3 CH 2 COOCH 3. O O • (CH 3 CH 2)2 NH. O O C OH • CH 3 CHCHCH 2 CHO. OCH 3 88

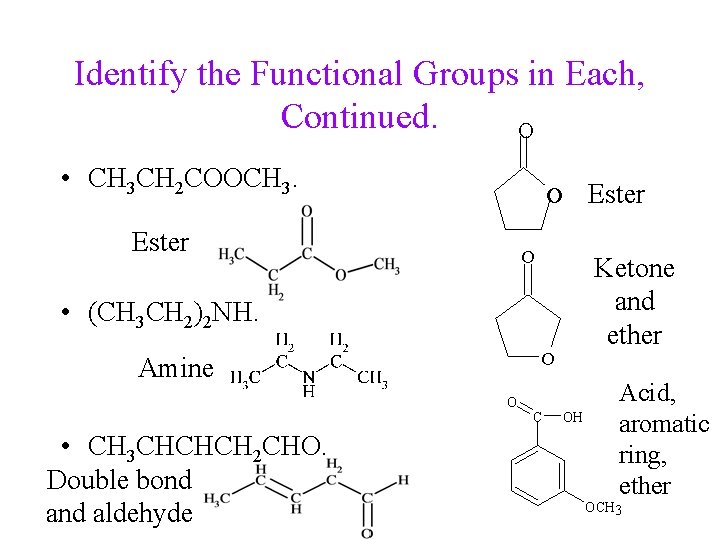
Identify the Functional Groups in Each, Continued. O • CH 3 CH 2 COOCH 3. O Ester O Ketone and ether • (CH 3 CH 2)2 NH. O Amine O • CH 3 CHCHCH 2 CHO. Double bond aldehyde C Ester OH Acid, aromatic ring, ether OCH 3