Crude oil To state that crude oil is

- Slides: 15

Crude oil * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

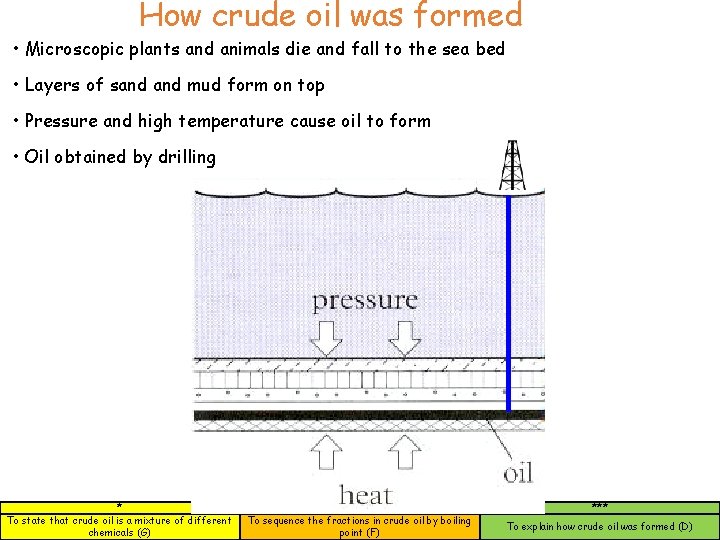
How crude oil was formed • Microscopic plants and animals die and fall to the sea bed • Layers of sand mud form on top • Pressure and high temperature cause oil to form • Oil obtained by drilling * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

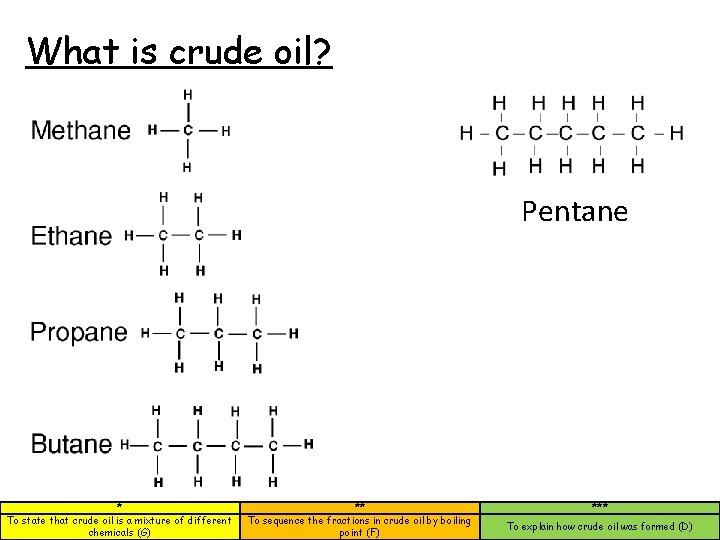
What is crude oil? Pentane * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

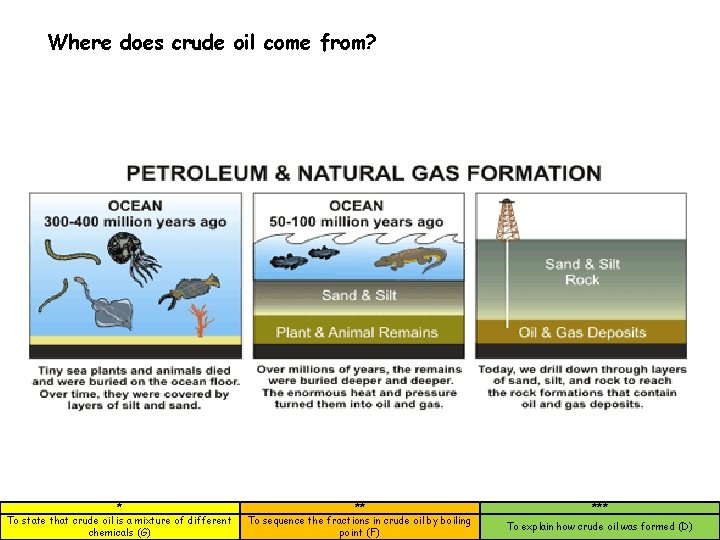
Where does crude oil come from? * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

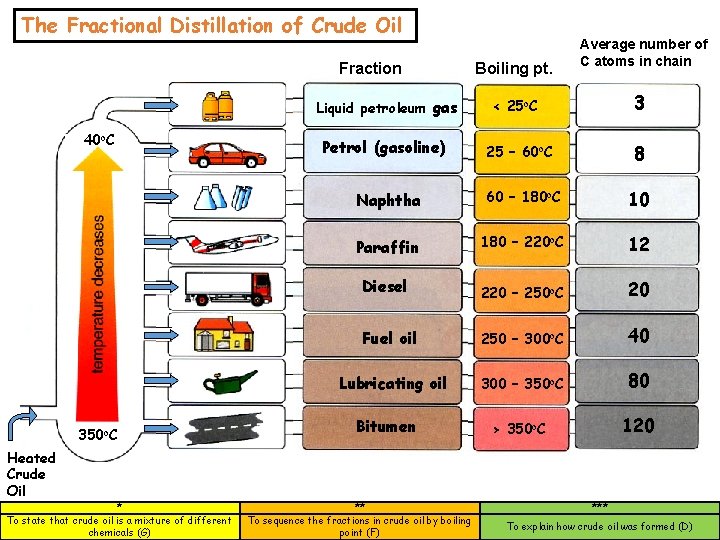
The Fractional Distillation of Crude Oil Fraction Liquid petroleum gas 40 o. C 350 o. C Heated Crude Oil * To state that crude oil is a mixture of different chemicals (G) Petrol (gasoline) Boiling pt. Average number of C atoms in chain 3 < 25 o. C 8 25 – 60 o. C Naphtha 60 – 180 o. C 10 Paraffin 180 – 220 o. C 12 Diesel 220 – 250 o. C 20 Fuel oil 250 – 300 o. C 40 Lubricating oil 300 – 350 o. C 80 > 350 o. C 120 Bitumen ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

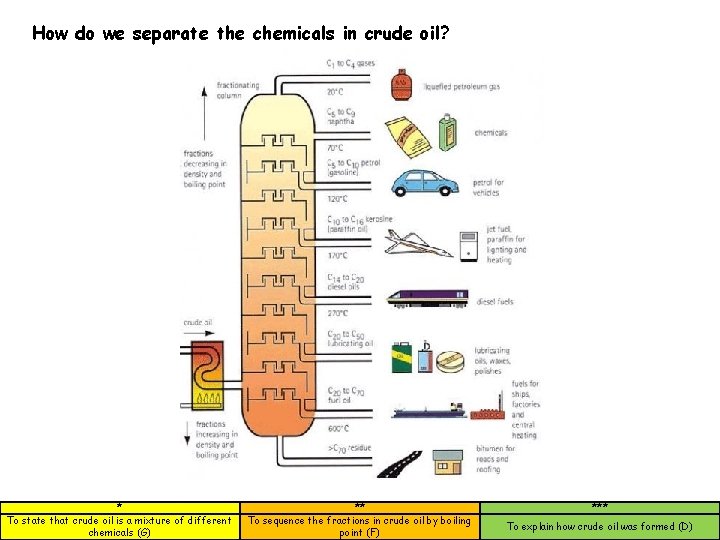
How do we separate the chemicals in crude oil? * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

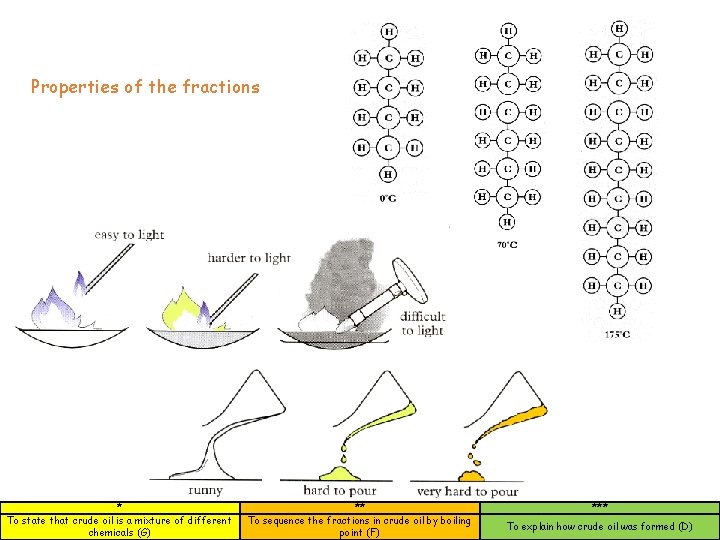
Properties of the fractions * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

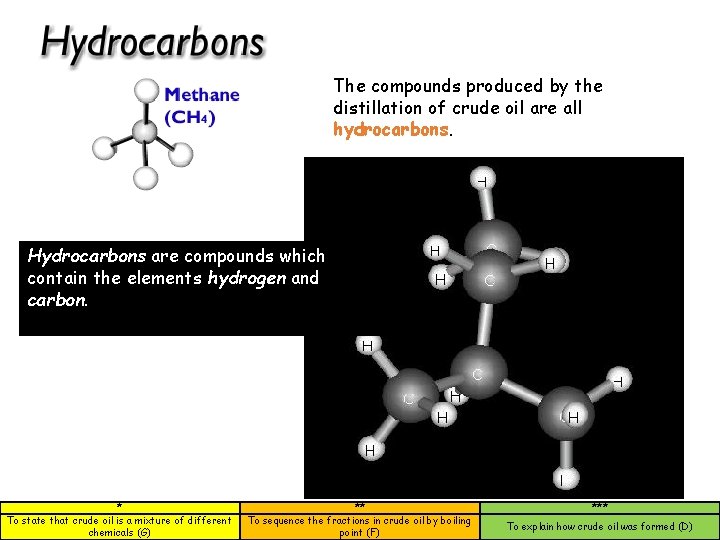
The compounds produced by the distillation of crude oil are all hydrocarbons. Hydrocarbons are compounds which contain the elements hydrogen and carbon. * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

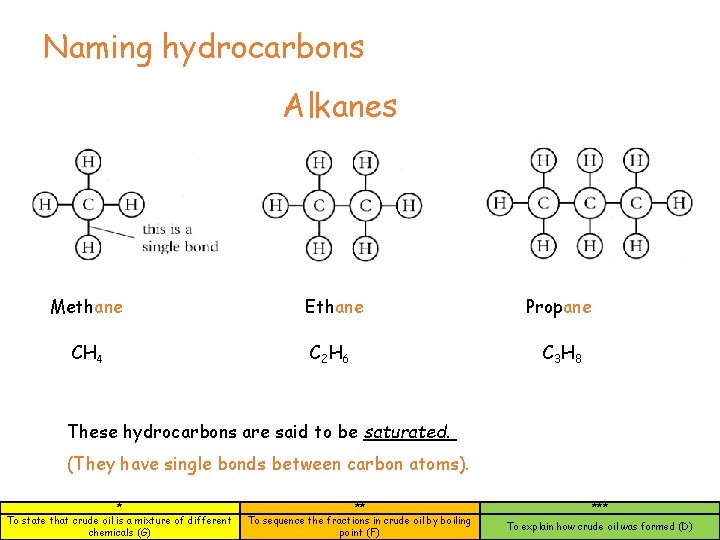
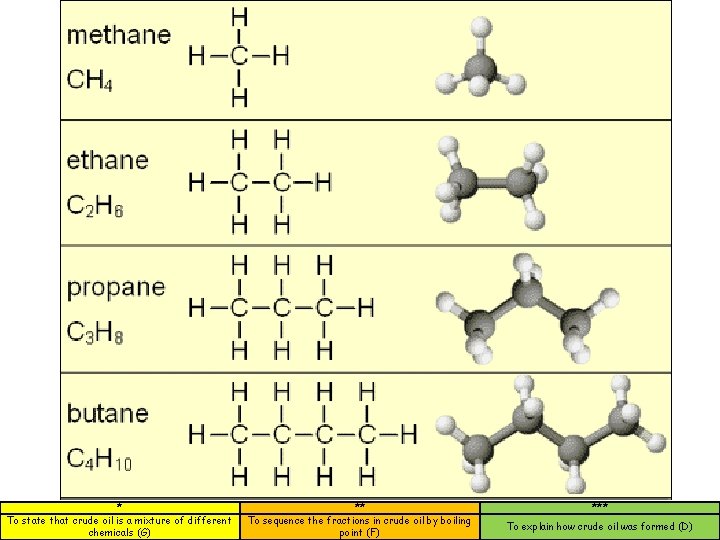
Naming hydrocarbons Alkanes Methane Ethane Propane CH 4 C 2 H 6 C 3 H 8 These hydrocarbons are said to be saturated. (They have single bonds between carbon atoms). * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

* To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

Fuels Most alkanes are used as fuels to produce useful forms of energy. When completely burned alkanes form carbon dioxide and water. * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

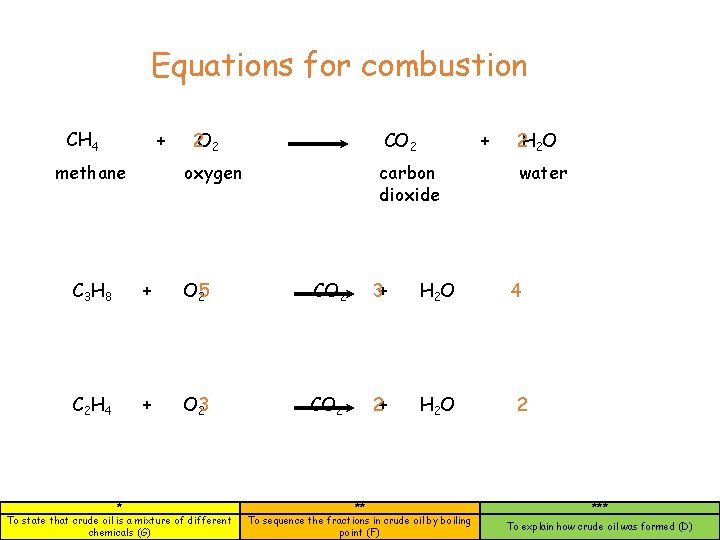
Equations for combustion CH 4 + methane 2 O 2 CO 2 oxygen + carbon dioxide C 3 H 8 + O 25 CO 2 3+ H 2 O C 2 H 4 + O 23 CO 2 2+ H 2 O * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) 2 H 2 O water 4 2 *** To explain how crude oil was formed (D)

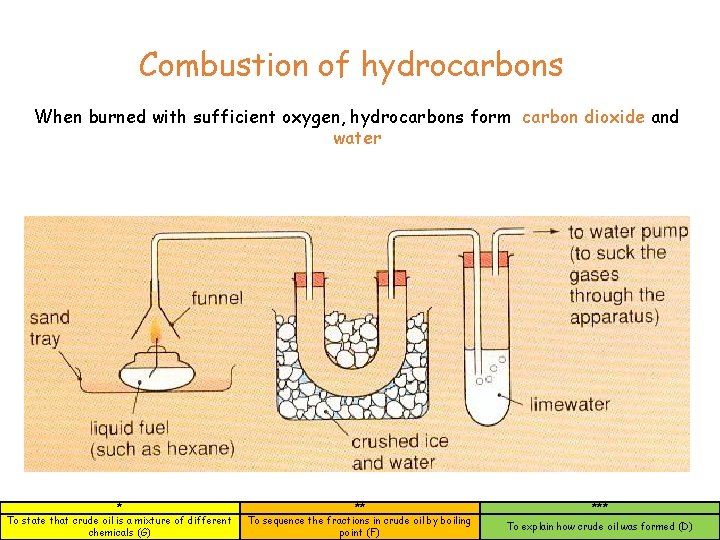
Combustion of hydrocarbons When burned with sufficient oxygen, hydrocarbons form carbon dioxide and water * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

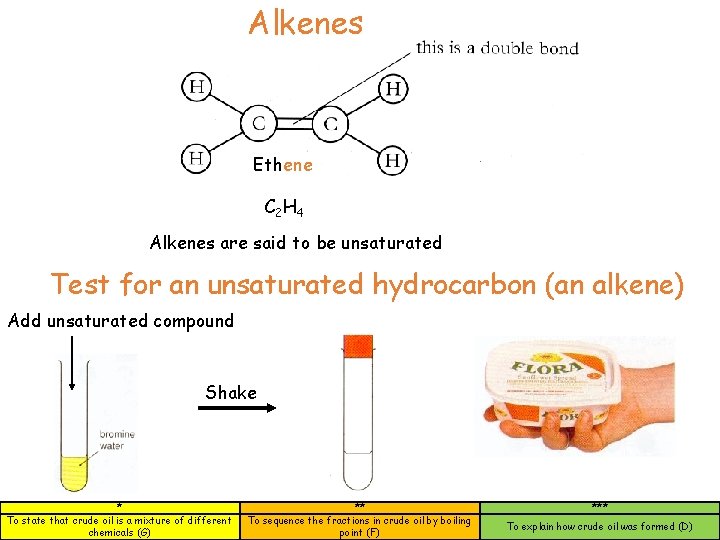
Alkenes Ethene C 2 H 4 Alkenes are said to be unsaturated Test for an unsaturated hydrocarbon (an alkene) Add unsaturated compound Shake * To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)

* To state that crude oil is a mixture of different chemicals (G) ** To sequence the fractions in crude oil by boiling point (F) *** To explain how crude oil was formed (D)
 Refining of petroleum
Refining of petroleum Crude oil
Crude oil What is crude oil made of
What is crude oil made of Catalytic cracking diagram
Catalytic cracking diagram Synthetic crude oil
Synthetic crude oil Petroleum product
Petroleum product Crude oil contains
Crude oil contains Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Premature atrial contraction
Premature atrial contraction Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều