Revision Functional Groups and Organic Chemistry IB Chemistry

Revision Functional Groups and Organic Chemistry
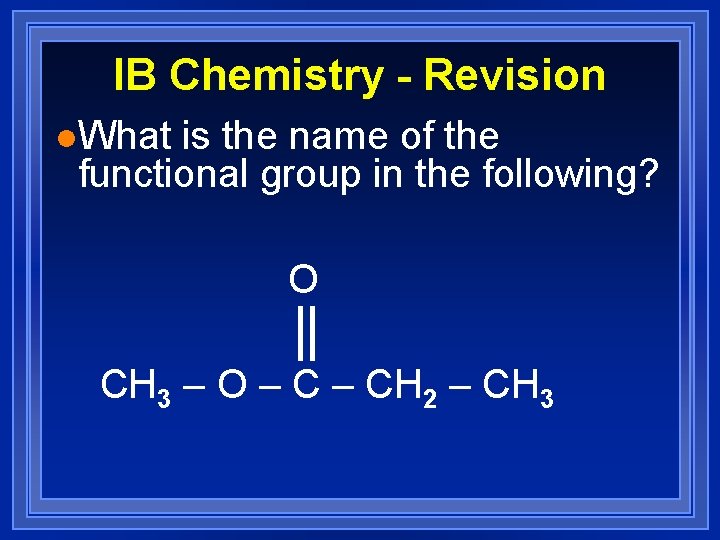
IB Chemistry - Revision l. What is the name of the functional group in the following? O CH 3 – O – CH 2 – CH 3
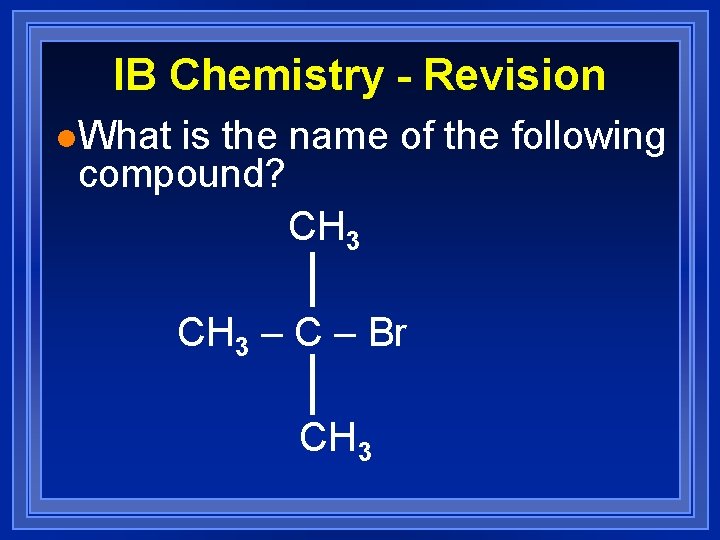
IB Chemistry - Revision l. What is the name of the following compound? CH 3 – C – Br CH 3

answer 2 bromo-methyl propane
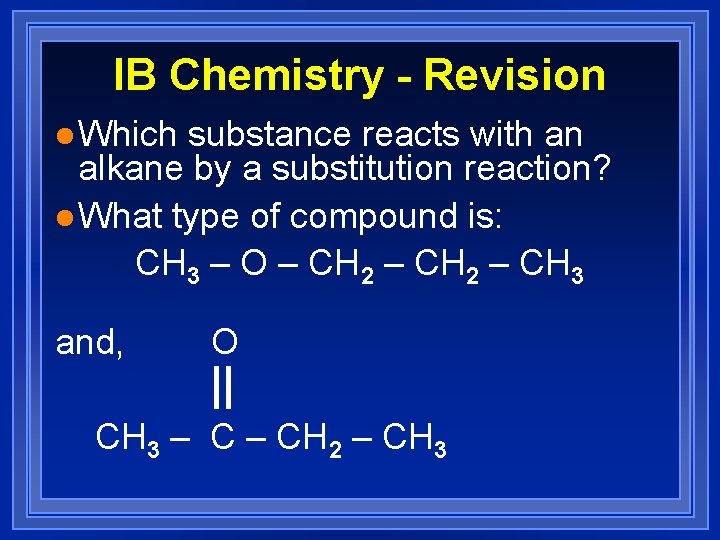
IB Chemistry - Revision l Which substance reacts with an alkane by a substitution reaction? l What type of compound is: CH 3 – O – CH 2 – CH 3 and, O CH 3 – CH 2 – CH 3

IB Chemistry - Revision l. The most important way to classify organic compounds is by ______. l. Which of the following halogenoalkanes has the highest boiling point: a) 1 -chloropropane, or b) 1, 2, 3 -trichloropropane?
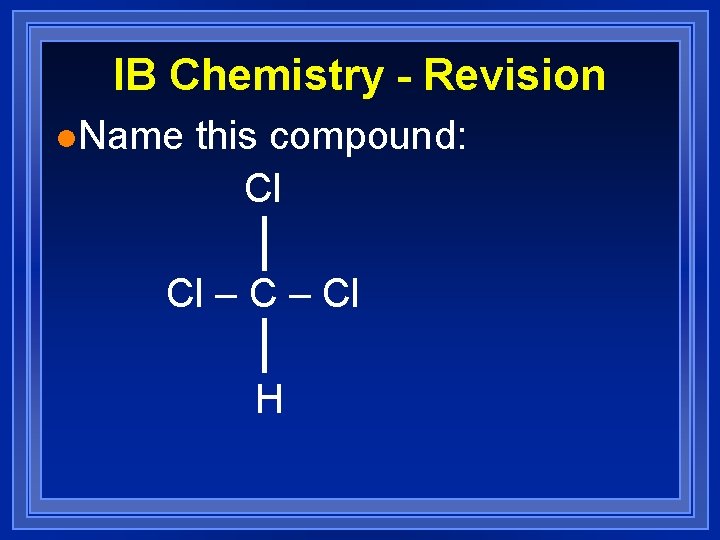
IB Chemistry - Revision l. Name this compound: Cl Cl – Cl H

IB Chemistry - Revision l. Give the common name for: OH CH 3 -CH 2 -CH-CH 3 l. What substance is used to test for degree of unsaturation?
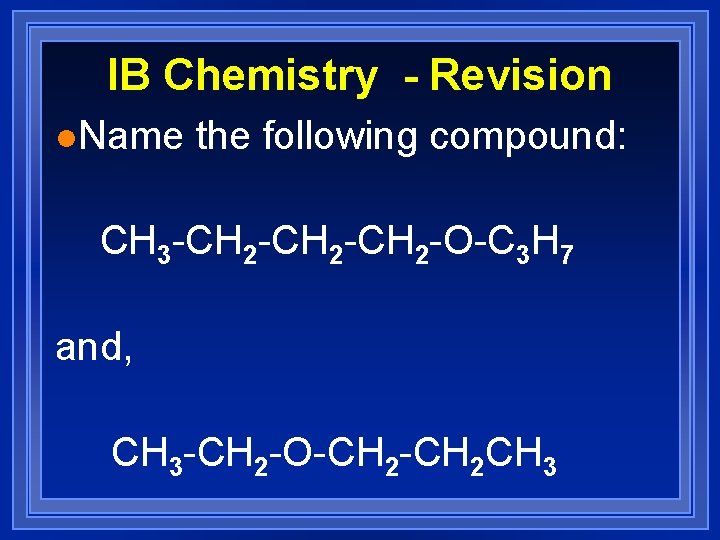
IB Chemistry - Revision l. Name the following compound: CH 3 -CH 2 -CH 2 -O-C 3 H 7 and, CH 3 -CH 2 -O-CH 2 CH 3

IB Chemistry - Revision l. What type of alcohol is: OH CH 3 -CH 2 -CH-CH 3

IB Chemistry - Revision l. Which of the following would you expect to be most soluble in water: a) CH 3 CH 2 Cl or, b) CH 3 CH 2 OH l. Phenols are characterized by having a(n) _______.

IB Chemistry - Revision l. What is glycerol? type of alcohol is a main breakdown product of fats and oils?

IB Chemistry - Revision l. What is the alcohol used in antifreeze? l. Based on your knowledge of intermolecular forces, which of these would you expect to have the highest boiling point: a) propanone, or b) 1 -propanol

IB Chemistry - Revision l. Show the mechanism of the reaction between C 3 H 6 + HBr. l. In an addition reaction, which bond of the reactant is broken? l. What type of compound is the following: C-C-C-O-C-C-C l. Which has the lowest boiling point: a) 4 -octanol, or b) ethoxyethane

IB Chemistry - Revision l. What is the name of the following compound: O CH 3 – CH 2 – CH 3
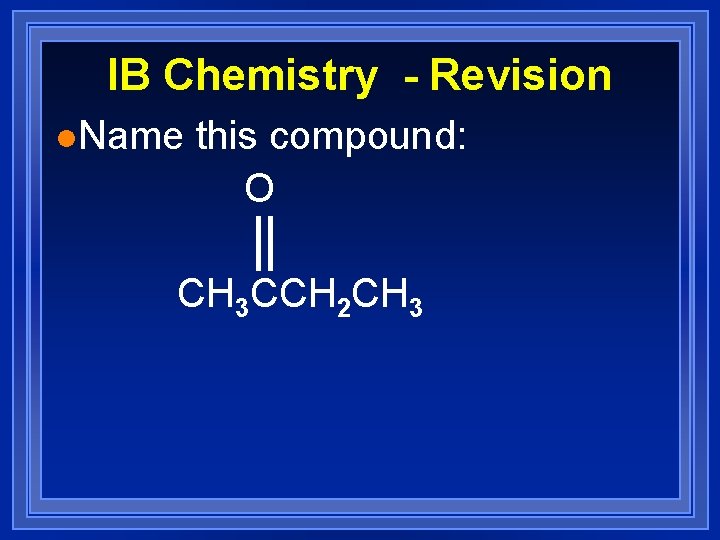
IB Chemistry - Revision l. Name this compound: O CH 3 CCH 2 CH 3
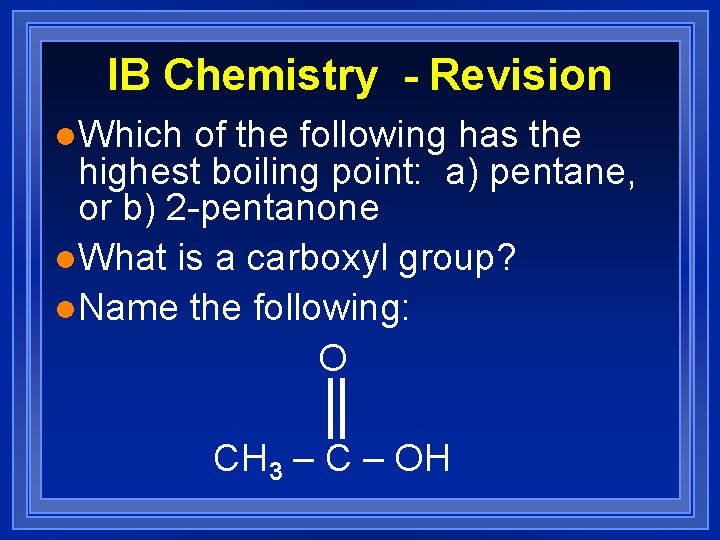
IB Chemistry - Revision l. Which of the following has the highest boiling point: a) pentane, or b) 2 -pentanone l. What is a carboxyl group? l. Name the following: O CH 3 – C – OH

IB Chemistry - Revision l. Carboxylic acids with long hydrocarbon chains are called _______. l. Which of the following compounds is the most soluble in water: a) propanoic acid, or b) propane l. A carboxylic acid with 6 straight carbons would be named ____.

IB Chemistry - Revision l. If an alkanal is oxidized, what type of molecule does it become? l. When an oxygen atom is attached to a carbon atom, the carbon atom becomes more ___. l. Which of the following compounds is most reduced: a) ethene, or b) ethane

IB Chemistry - Revision l. Which of the following compounds will produce the most energy when completely oxidized: a) butane, or b) butanol l. Look up bond energy values and work out the correct answer l. What is a test for alcohols? l. What is the expected product when 1 -propanol is oxidized?

IB Chemistry - Revision l. What type of compound is: O C–C–C–O–C–C l. Which of the following has the highest boiling point: a) butane, or b) butanoic acid

IB Chemistry - Revision l. Esters contribute which property to fruits? l. Which compound reacts with a carboxylic acid to form an ester?
- Slides: 22