Organic Chemistry Crude oil is a finite resource









































- Slides: 51

Organic Chemistry


• Crude oil is a finite resource found in rocks (it will run out) • Crude oil is the remains of an ancient biomass consisting mainly of plankton that was buried in mud

Why is it so important to us?


Crude oil is a mixture of different compounds called hydrocarbons. A hydrocarbon is a compound made of carbon and hydrogen atoms only. The hydrocarbons in crude oil are mostly alkanes.


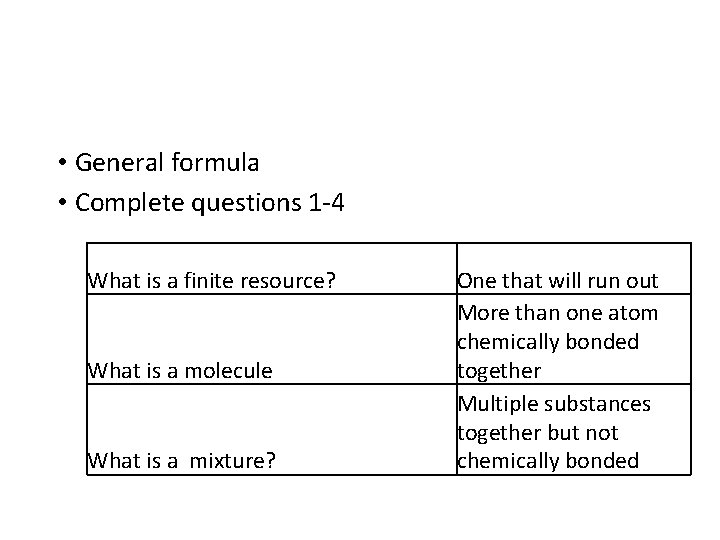
• General formula • Complete questions 1 -4 What is a finite resource? What is a molecule What is a mixture? One that will run out More than one atom chemically bonded together Multiple substances together but not chemically bonded

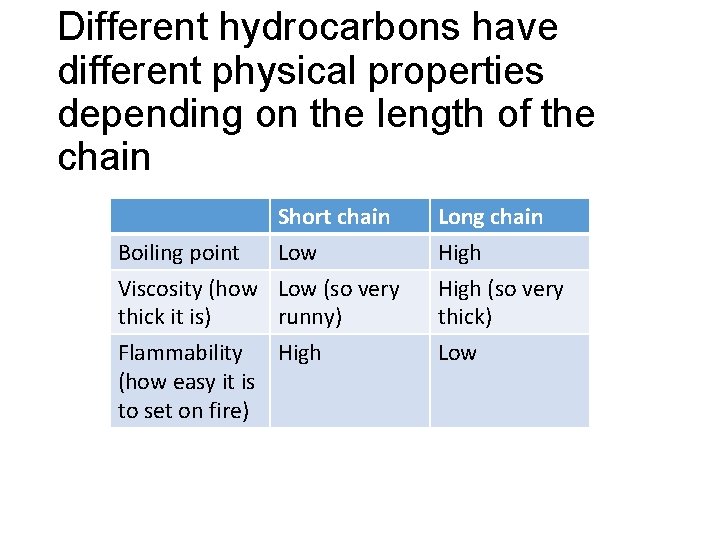
Different hydrocarbons have different physical properties depending on the length of the chain Boiling point Short chain Low Long chain High Viscosity (how Low (so very thick it is) runny) High (so very thick) Flammability High (how easy it is to set on fire) Low

Complete questions 5 -10

Boiling points and separation

Complete question 11 -26


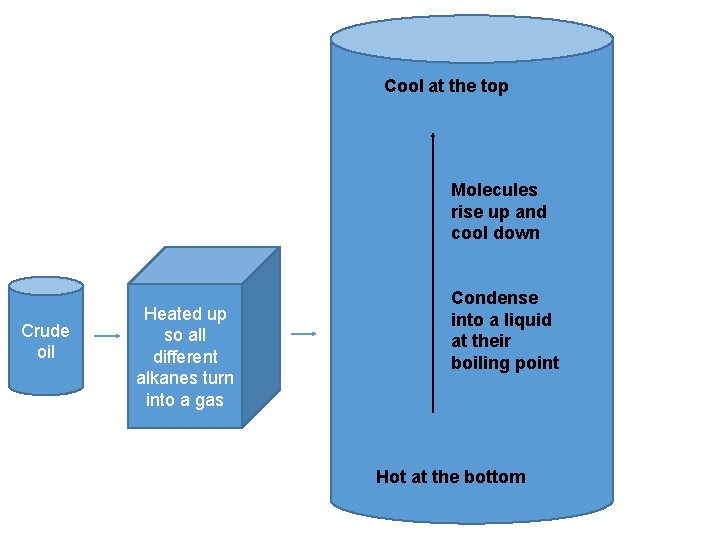
Fractional distillation

Cool at the top Molecules rise up and cool down Crude oil Heated up so all different alkanes turn into a gas Condense into a liquid at their boiling point Hot at the bottom

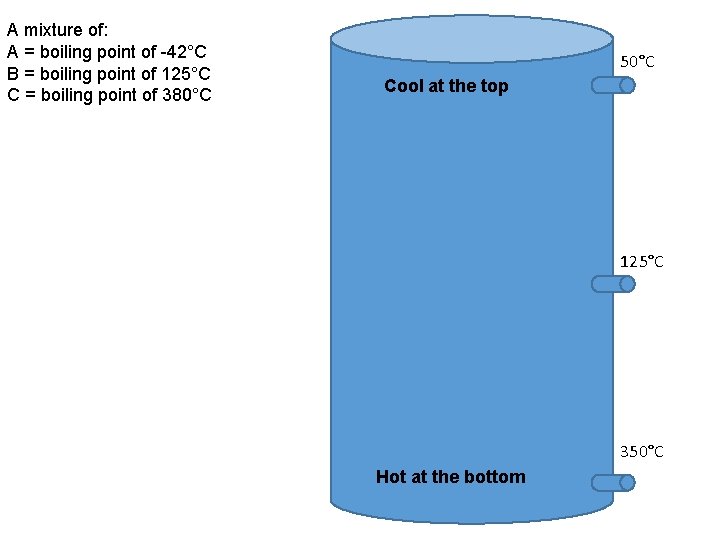
A mixture of: A = boiling point of -42°C B = boiling point of 125°C C = boiling point of 380°C 50°C Cool at the top 125°C 350°C Hot at the bottom


Combustion

• Combustion reactions are when a fuel reacts with oxygen • Complete combustion of alkanes always produces carbon dioxide and water • The reaction releases energy which can be used

E. g. complete combustion of methane • Word equation • Symbol equation • Note that carbon and hydrogen have been oxidised (had oxygen added to them) • Q 36

Incomplete combustion • Incomplete combustion occurs when there is not enough oxygen • Incomplete combustion produces carbon monoxide (which is a toxic gas) • Releases less energy • Can release soot (particles of carbon) • Methane example • Q 37+38

Cracking • There are long hydrocarbons and short ones • The shorter ones are more useful • Used as fuels and to help make polymers and other useful chemicals • Cracking turns the long ones into shorter ones • One way is to pass over a hot catalyst • Another way is to mix with steam and heat to a high temperature • Produce shorter alkanes and alkenes • Alkenes are useful substances that are more reactive than alkanes

Demo

Balancing equations showing cracking • C 10 H 22 C 5 H 12 + C 3 H 6 + C 2 H 4 • C 10 H 22 C 4 H 10 + C 3 H 6

• Test for unsaturation • Answer questions 37 -46

Further organic (triple only)

Alkenes Triple only • Double bond • Unsaturation • First four

• Questions 1 - 3 • Start reading page 158 -159

Reactions of alkenes • Combustion • Same as alkanes but more often incomplete combustion • Smoky flame • Less energy

• Questions 4 -6 • Read page 158 -159

Reactions • Additions with halogen • Room temperature • Halogen adds across the double bond • Addition with hydrogen • • 60°C, Ni catalyst Forms alkane Increases melting point Adding hydrogen to unsaturated fats makes spreadable oils • Addition with water (steam) • • Forms alcohol High temperature and pressure Catalyst Reversible

Alcohols • Structure • Functional group • Homologous series • Combustion • Q 27 -32 • Start reading page 162 -163

Alcohols with sodium • Sodium + ethanol sodium ethoxide + hydrogen • 2 Na + 2 C 2 H 5 OH 2 C 2 H 5 ONa + H 2 • Questions 15 -19

Alcohols in water • Smaller alcohols dissolve readily in water • Can be used as solvents

Alcohol formation • Alkene with steam • Fermentation of sugar

Alcohol with oxidising agent • Turn into carboxylic acids • Can occur naturally in the air • Can be done in the lab

Carboxylic acids • Formation from alcohols • Notation for oxidising agent • Reaction with carbonates • Q 20 -27

Esters • Formed from the reaction between an alcohol and a carboxylic acid • Sweet smelling, used in flavourings • Only need to know ethyl ethanoate • Q 28 -35

Polymers © Pearson Education Ltd 2016. Copying permitted for purchasing institutions only. This material is not copyright free.

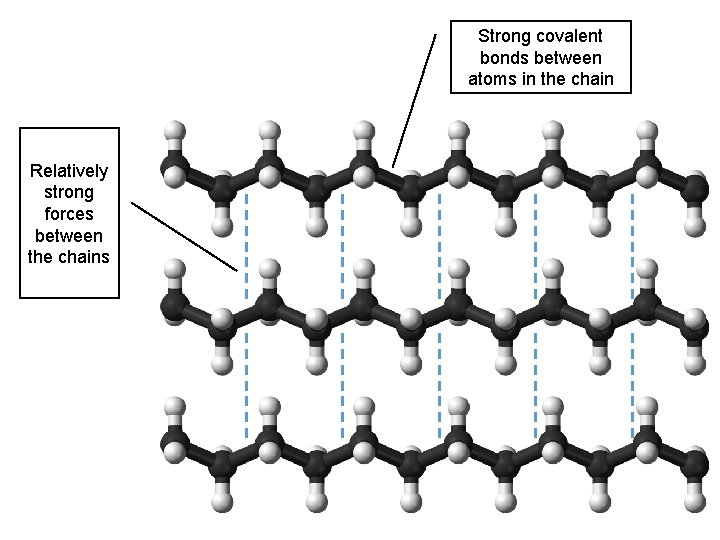
Strong covalent bonds between atoms in the chain Relatively strong forces between the chains

• Natural vs. synthetic polymers

• Addition polymerisation • Ethene poly(ethene) diagram • Note: • • • “n“ Double bond in ethene Single bonds in poly(ethene) Round brackets “Repeating unit” Naming convention

• Example 2 and 3 • Chloroethene • Butene • Questions 1 -8

Condensation Polymerisation

• Reaction between ethandiol and propanedioc acid • Questions 9 -12

Glucose, starch and cellulose

• Q 13 -18

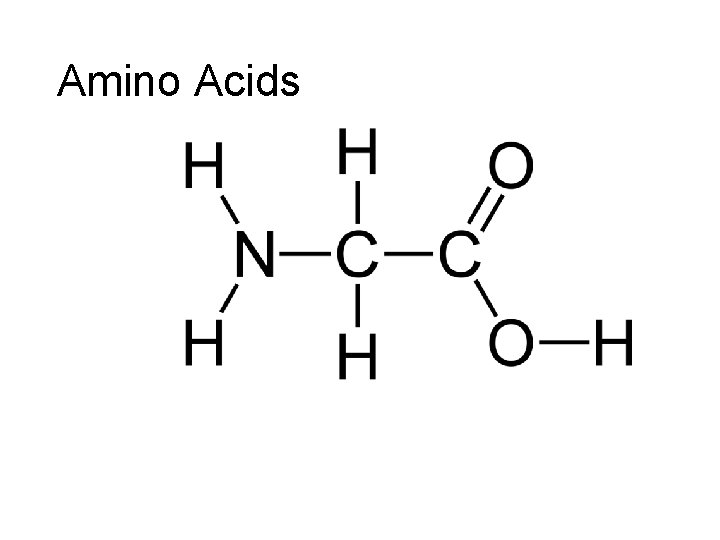
Amino Acids

• Q 19 -23

DNA

• Remaining questions • Page 177 all questions
 Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Mnemonic for crude oil fractions
Mnemonic for crude oil fractions Naphtha uses
Naphtha uses Canadian oil and gas trusts
Canadian oil and gas trusts Crude oil contains
Crude oil contains Crude oil
Crude oil Catalytic cracking diagram
Catalytic cracking diagram Is petroleum flammable
Is petroleum flammable Finite and non finite subordinate clauses
Finite and non finite subordinate clauses What is finite verb
What is finite verb Learning objectives of non finite verbs
Learning objectives of non finite verbs How to find finite and nonfinite verbs
How to find finite and nonfinite verbs Non finite forms of the verb qayda
Non finite forms of the verb qayda Titan oil recovery share price
Titan oil recovery share price Primary emulsion formula for fixed oil and mineral oil
Primary emulsion formula for fixed oil and mineral oil Founder of organic chemistry
Founder of organic chemistry Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Alkene table
Alkene table Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Leveling effect organic chemistry
Leveling effect organic chemistry Priority when naming organic compounds
Priority when naming organic compounds Organic chemistry lab report sample
Organic chemistry lab report sample Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Cyclo organic chemistry
Cyclo organic chemistry Importance of organic compounds
Importance of organic compounds Kiliani fischer synthesis
Kiliani fischer synthesis Math eth prop bute
Math eth prop bute How is cracking done
How is cracking done Organic chemistry vs biochemistry
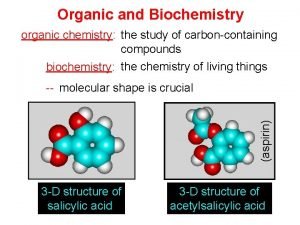
Organic chemistry vs biochemistry Organic chemistry myanmar
Organic chemistry myanmar Br2aq
Br2aq Gc organic chemistry
Gc organic chemistry Hono organic chemistry
Hono organic chemistry Father of organic chemistry
Father of organic chemistry Organic chemistry topic 11
Organic chemistry topic 11 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry nomenclature
Organic chemistry nomenclature What is organic chemistry like
What is organic chemistry like Ch4o organic or inorganic
Ch4o organic or inorganic Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis