PYROLYSIS OF ETHYL ESTERS IN A MICROREACTOR Paper


- Slides: 17

PYROLYSIS OF ETHYL ESTERS IN A MICRO-REACTOR Paper 3978 CORY ROGERS, Department of Mechanical Engineering, University of Colorado Boulder, CO JESSIE P PORTERFIELD, Radio and Geoastronomy Division, Harvard-Smithsonian Center for Astrophysics, Cambridge, MA, JOHN W DAILY, Department of Mechanical Engineering, University of Colorado Boulder, CO BARNEY ELLISON, Department of Chemistry, University of Colorado, Boulder, CO NICOLE LABBE, Department of Mechanical Engineering, University of Colorado, Boulder, CO

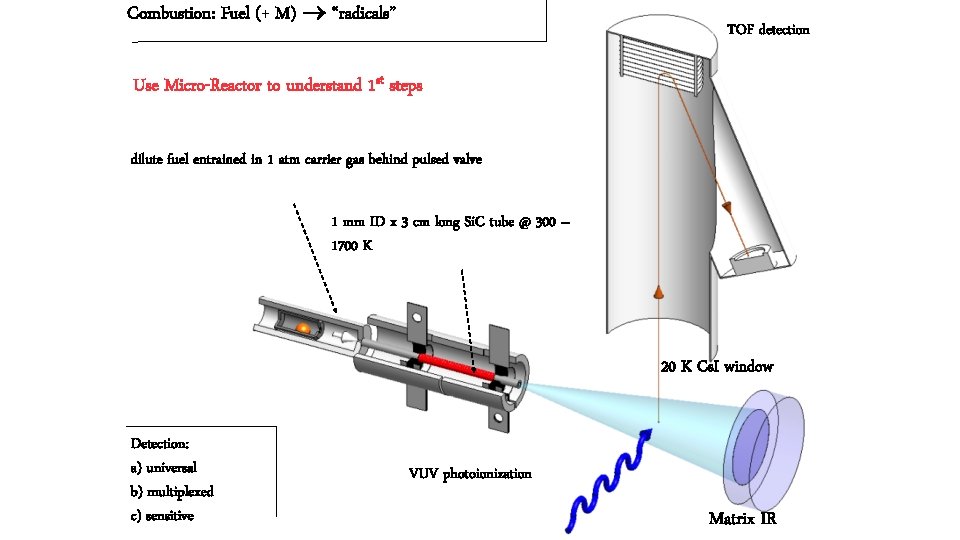
Combustion: Fuel (+ M) “radicals” TOF detection Use Micro-Reactor to understand 1 st steps dilute fuel entrained in 1 atm carrier gas behind pulsed valve 1 mm ID x 3 cm long Si. C tube @ 300 – 1700 K 20 K Cs. I window Detection: a) universal b) multiplexed c) sensitive VUV photoionization Matrix IR

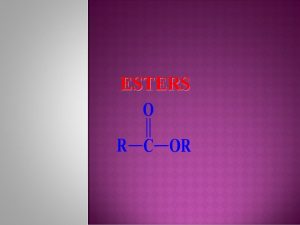
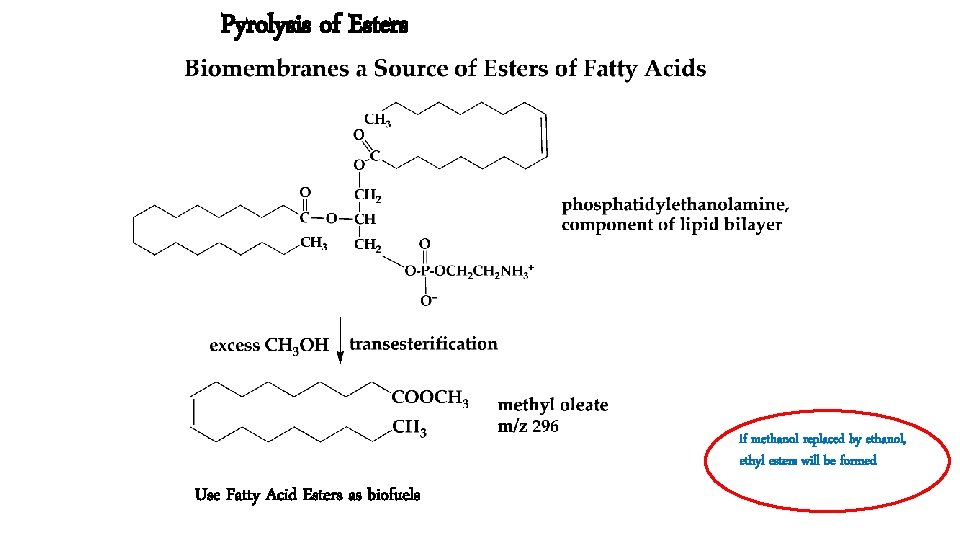
Pyrolysis of Esters if methanol replaced by ethanol, ethyl esters will be formed Use Fatty Acid Esters as biofuels

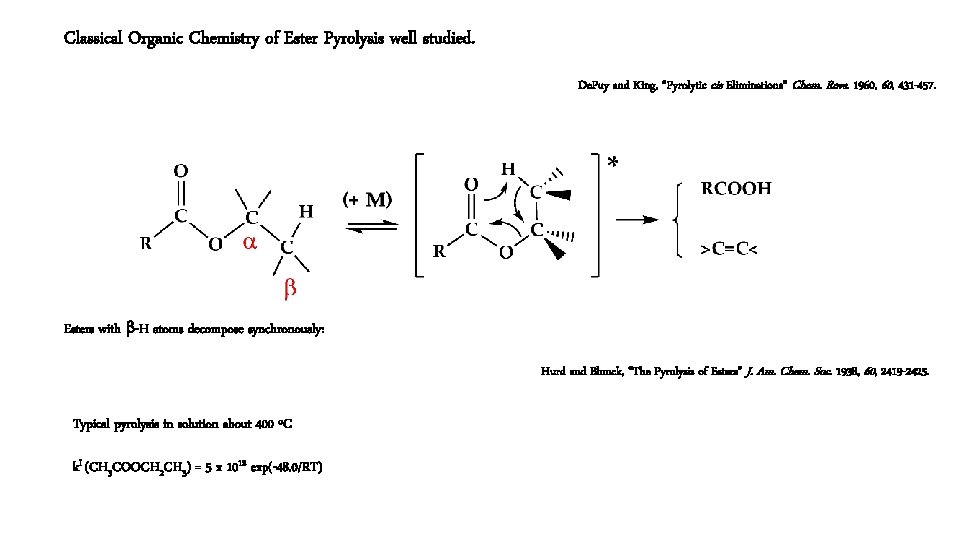
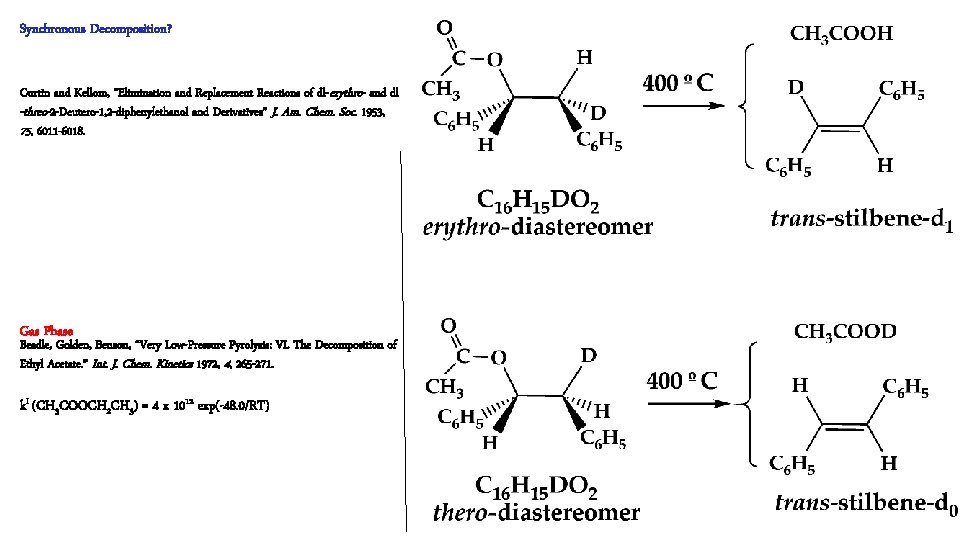
Classical Organic Chemistry of Ester Pyrolysis well studied. De. Puy and King, “Pyrolytic cis Eliminations” Chem. Revs. 1960, 431 -457. Esters with b-H atoms decompose synchronously: Hurd and Blunck, “The Pyrolysis of Esters” J. Am. Chem. Soc. 1938, 60, 2419 -2425. Typical pyrolysis in solution about 400 ºC k. I (CH 3 COOCH 2 CH 3) = 5 x 1012 exp(-48. 0/RT)

Synchronous Decomposition? Curtin and Kellom, “Elimination and Replacement Reactions of dl- erythro- and dl -threo-2 -Deutero-1, 2 -diphenylethanol and Derivatives” J. Am. Chem. Soc. 1953, 75, 6011 -6018. Gas Phase Beadle, Golden, Benson, “Very Low-Pressure Pyrolysis: VI. The Decomposition of Ethyl Acetate. ” Int. J. Chem. Kinetics 1972, 4, 265 -271. k. I (CH 3 COOCH 2 CH 3) = 4 x 1012 exp(-48. 0/RT)

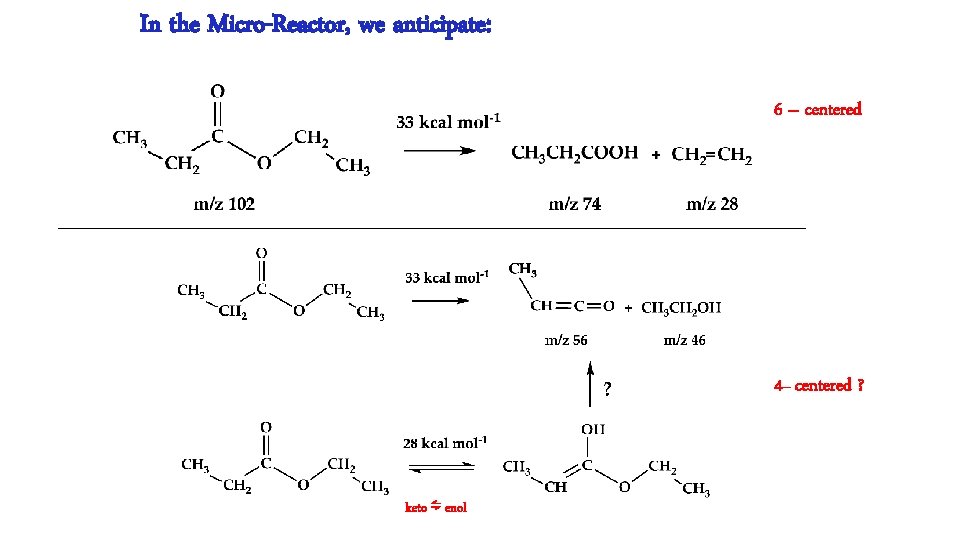
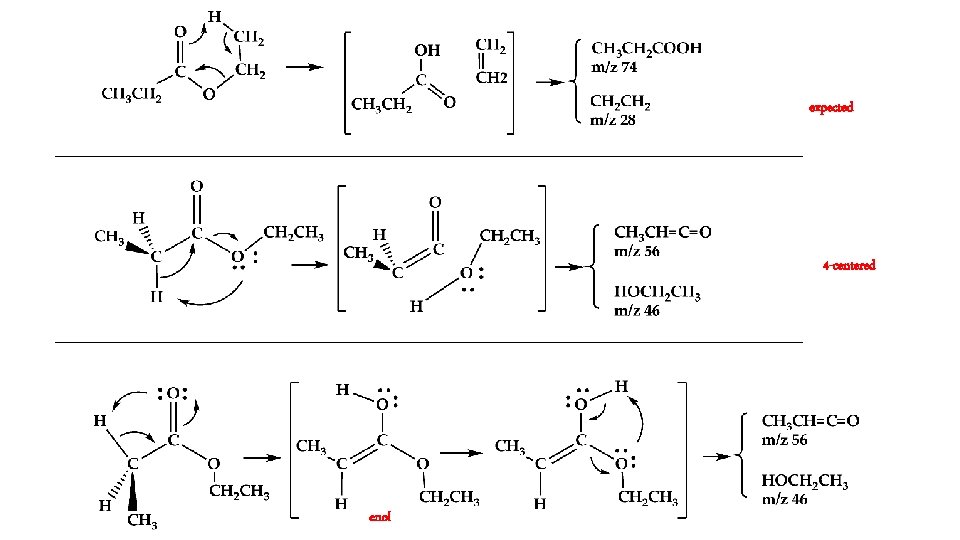
In the Micro-Reactor, we anticipate: 6 – centered 4– centered ? keto ⇋ enol

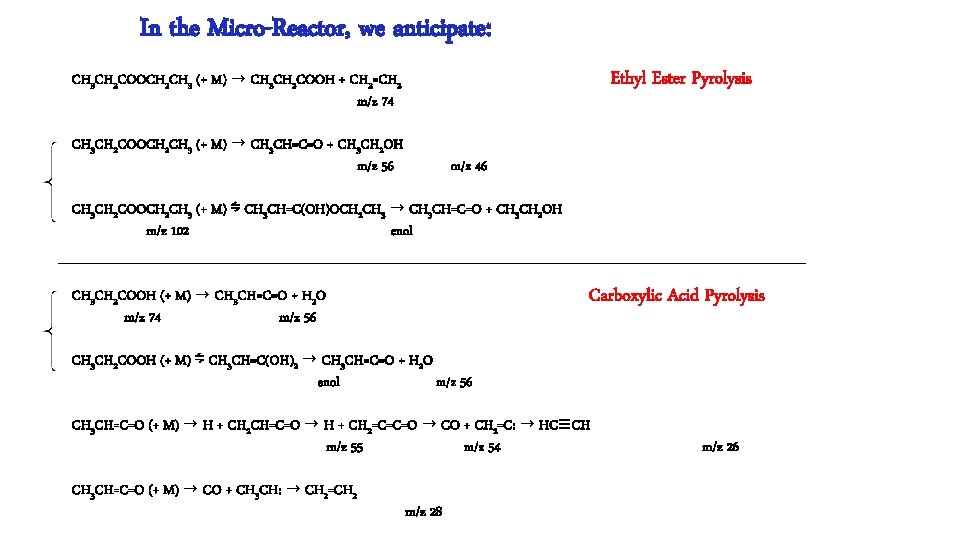
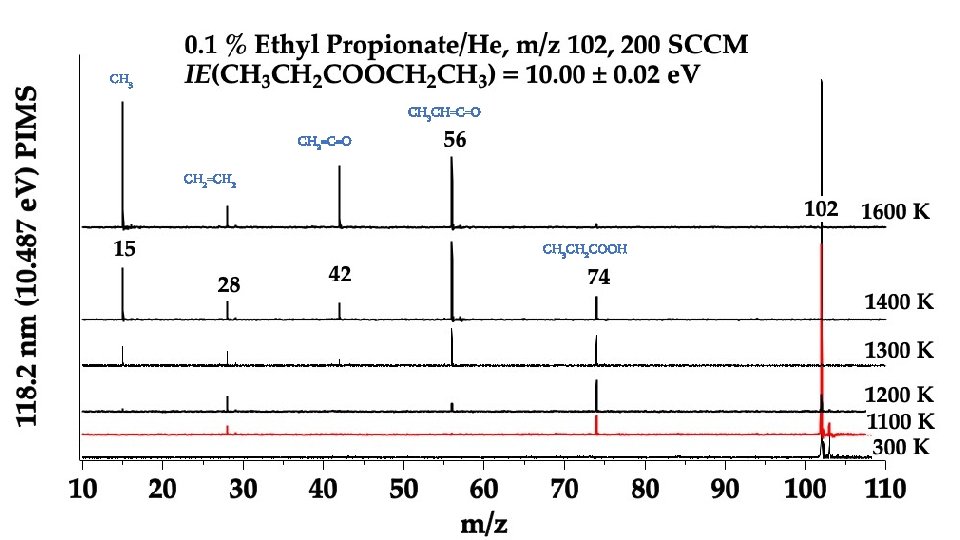
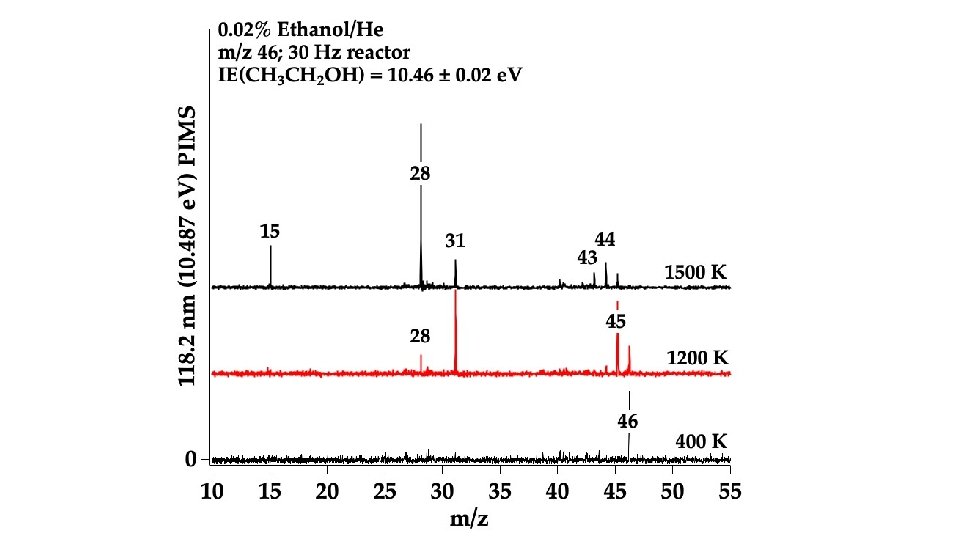
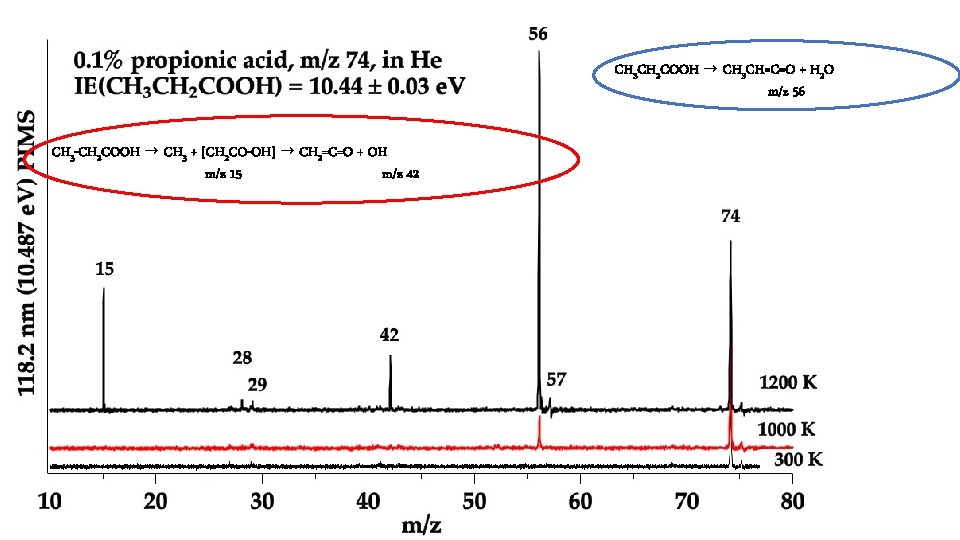
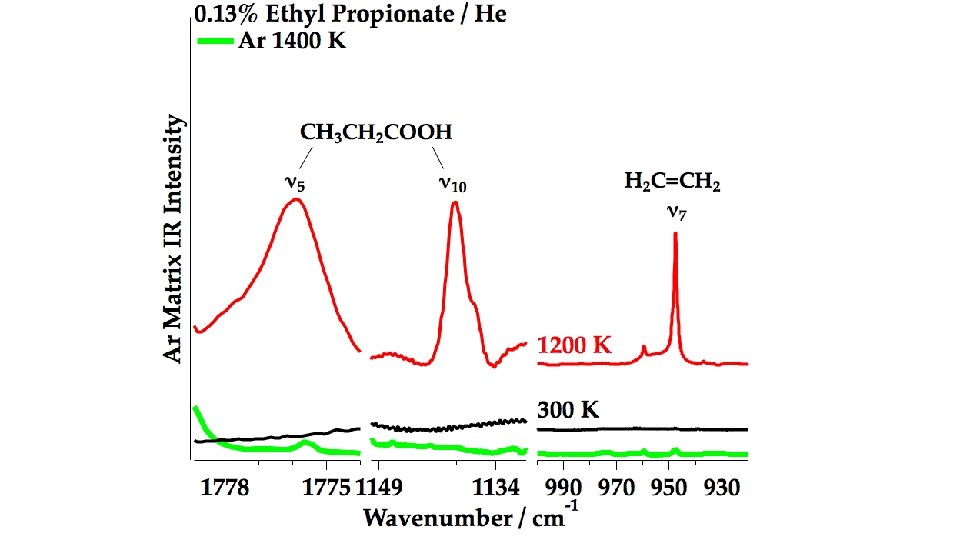
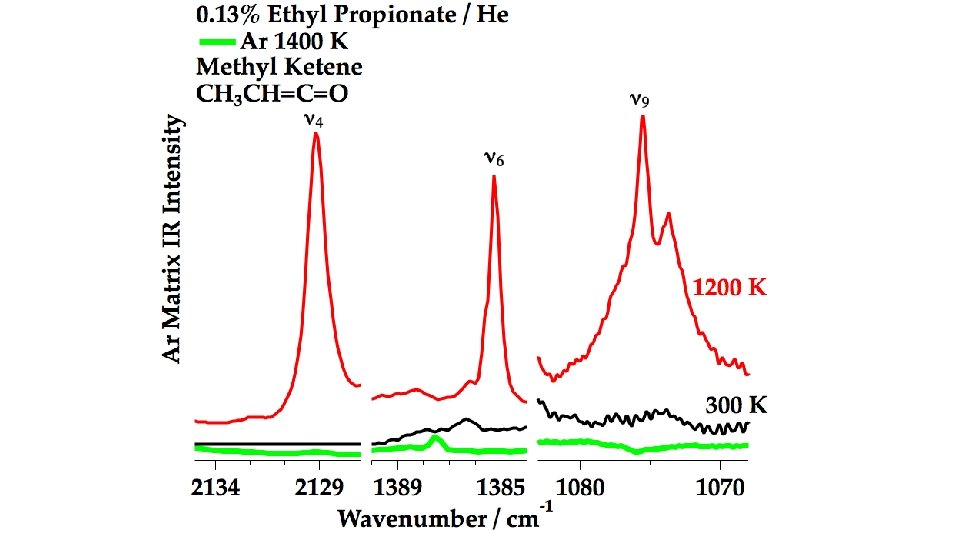
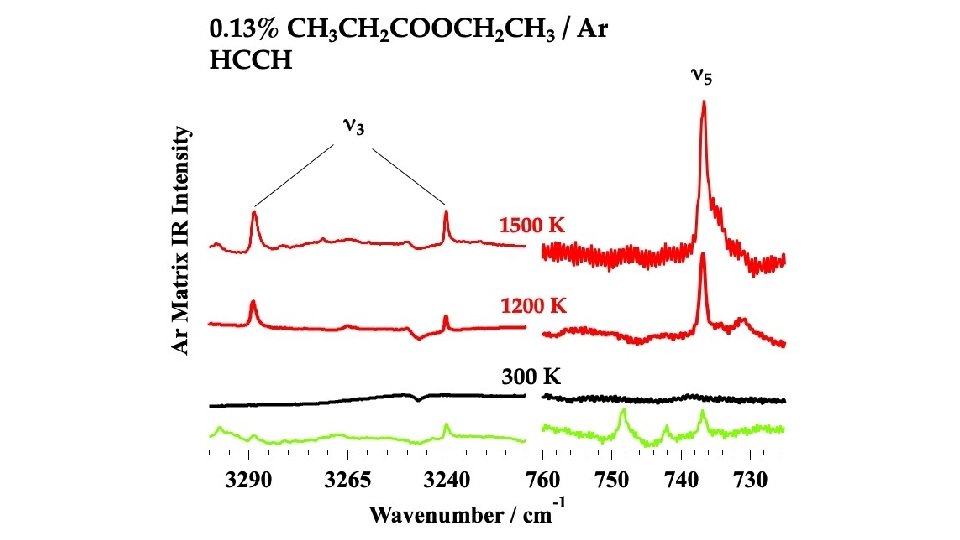
In the Micro-Reactor, we anticipate: CH 3 CH 2 COOCH 2 CH 3 (+ M) → CH 3 CH 2 COOH + CH 2=CH 2 m/z 74 Ethyl Ester Pyrolysis CH 3 CH 2 COOCH 2 CH 3 (+ M) → CH 3 CH=C=O + CH 3 CH 2 OH m/z 56 m/z 46 CH 3 CH 2 COOCH 2 CH 3 (+ M) ⇋ CH 3 CH=C(OH)OCH 2 CH 3 → CH 3 CH=C=O + CH 3 CH 2 OH m/z 102 enol CH 3 CH 2 COOH (+ M) → CH 3 CH=C=O + H 2 O m/z 74 m/z 56 Carboxylic Acid Pyrolysis CH 3 CH 2 COOH (+ M) ⇋ CH 3 CH=C(OH)2 → CH 3 CH=C=O + H 2 O enol m/z 56 CH 3 CH=C=O (+ M) → H + CH 2 CH=C=O → H + CH 2=C=C=O → CO + CH 2=C: → HC≡CH m/z 55 m/z 54 CH 3 CH=C=O (+ M) → CO + CH 3 CH: → CH 2=CH 2 m/z 28 m/z 26


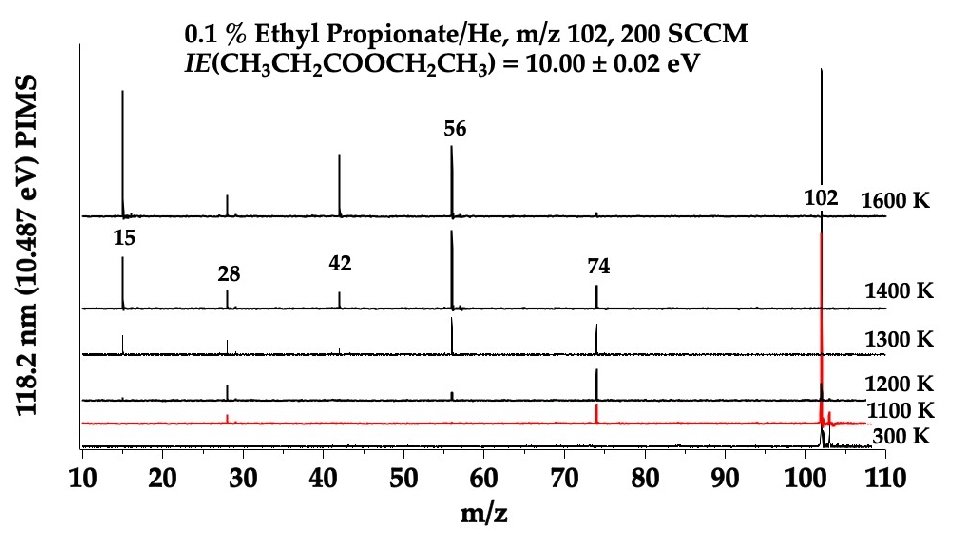
CH 3 CH=C=O CH 2=CH 2 CH 3 CH 2 COOH

expected 4 -centered enol


CH 3 CH 2 COOH → CH 3 CH=C=O + H 2 O m/z 56 CH 3 -CH 2 COOH → CH 3 + [CH 2 CO-OH] → CH 2=C=O + OH m/z 15 m/z 42




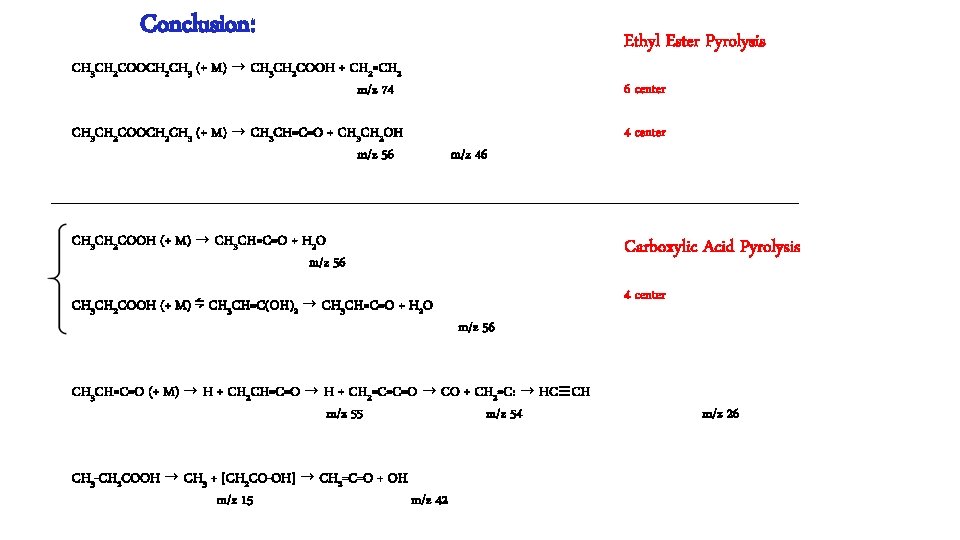
Conclusion: Ethyl Ester Pyrolysis CH 3 CH 2 COOCH 2 CH 3 (+ M) → CH 3 CH 2 COOH + CH 2=CH 2 m/z 74 CH 3 CH 2 COOCH 2 CH 3 (+ M) → CH 3 CH=C=O + CH 3 CH 2 OH m/z 56 6 center m/z 46 4 center CH 3 CH 2 COOH (+ M) → CH 3 CH=C=O + H 2 O m/z 56 Carboxylic Acid Pyrolysis CH 3 CH 2 COOH (+ M) ⇋ CH 3 CH=C(OH)2 → CH 3 CH=C=O + H 2 O 4 center m/z 56 CH 3 CH=C=O (+ M) → H + CH 2 CH=C=O → H + CH 2=C=C=O → CO + CH 2=C: → HC≡CH m/z 55 m/z 54 CH 3 -CH 2 COOH → CH 3 + [CH 2 CO-OH] → CH 2=C=O + OH m/z 15 m/z 42 m/z 26

Thermal decomposition of methyl esters is a mess. CH 3 COOCH 3 → products CH 3 CH 2 COOCH 3 → products Porterfield, Bross, Ruscic, Thorpe, Nguyen, Baraban, Stanton, Daily, Ellison, “Thermal Decomposition of Potential Ester Biofuels Part I: Methyl Acetate and Methyl Butanoate. ” J. Phys. Chem. A 2017, 121, 4658 -4677.
 Oorja systems & consultants
Oorja systems & consultants The scale structure covering the exterior of the hair
The scale structure covering the exterior of the hair Naamgeving esters oefenen
Naamgeving esters oefenen Naming phenols
Naming phenols Carboxylic acid to an ester
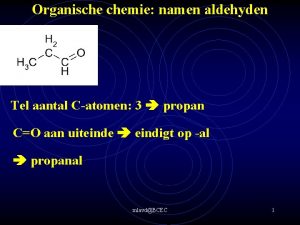
Carboxylic acid to an ester Structuurformule propaanzuur
Structuurformule propaanzuur Ch3 ch3 ch2
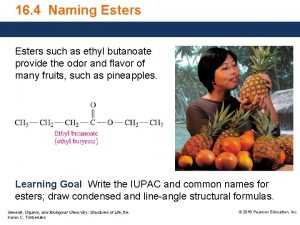
Ch3 ch3 ch2 Ethyl butanoate structure
Ethyl butanoate structure Esters naming
Esters naming Chemical reactions of esters
Chemical reactions of esters San esters
San esters Hydration of ethene
Hydration of ethene Esterification of propanoic acid
Esterification of propanoic acid Carboxylic acid derivatives reactions
Carboxylic acid derivatives reactions Formule générale des esters
Formule générale des esters Identification test for esters
Identification test for esters N-ethyl-n-propylbutylamin
N-ethyl-n-propylbutylamin Boiling point of hoch2oh
Boiling point of hoch2oh