Experiment 9 Identification of Unknowns April 456 2017
























- Slides: 37

Experiment 9: Identification of Unknowns April 4/5/6, 2017

Course Outline April 4/5/6 Experiment 9: Identification of Unknowns. Solubility, Functional Groups, Reports. Infra Red Spectroscopy (IR, FTIR). Reading assignment: Experiment 10. April 11/12/13 Experiment 9: Identification of Unknowns (continued). Experiment 10 (multistep synthesis): Synthesis of Benzoin. Experiments 7 and 8 are due. April 18/19/20 Experiment 10 (multistep synthesis, continued). April 25/26/27 Experiment 10 (multistep synthesis, continued). May 2/3/4 Laboratory Checkout

Identification of Unknowns • Each of you will receive two vials: one containing a liquid unknown (ca 5 m. L) and one - a solid unknown (ca. 5 g). Record the number! You will perform a number of experimental procedures on these compounds to gather data. You will determine the actual structure of your unknowns. • You should submit a Preliminary Unknown Report at the end of the first lab session.

Laboratory April 4/5/6 • Take a melting point of your solid unknown and a boiling point of your liquid unknown by distillation (recover the liquid!) • Take an IR of both unknowns • Perform solubility / miscibility tests with both unknowns • Fill a preliminary report and hand it over to the TAs.

How to proceed • • • Measure MP or BP. Your mp and bp must be accurate in order to narrow the possible choices within that class. Consider all possibilities within at least 5 °C of your mp, 10 °C for bp up to 175 °C; and a wider range for mp and bp above 175 °C. Take an IR Do solubility/miscibility tests. Submit your preliminary report: At this stage, you should have determined at least the class of your compound (e. g. , aldehyde, ketone, alcohol, etc. ) Once the preliminary report has been approved, proceed to the next step. If the report is not approved – follow the recommendations. Perform specific functionality (classification) test(s) Try to determine the actual structure of your unknown. Possible compounds and their derivatives are listed in the manual. Do the final structure elucidation and prepare your final report Submit formal report, detailed laboratory report and all remaining unknowns and derivatives thereof.

Solubility Tests • • • use a few mg of your compound in 1 m. L of solution to give you a dilute solution. If your compound is not completely soluble consider it insoluble. After obtaining data at room temperature, heat your solution in the water bath at 70°C or higher and observe any further changes. The results you record herein may have more meaning later when you know more about your unknown compound. You will test the solubility/reactivity of your compound in four different solutions: water; 5% aq HCl; 5% aq Na. HCO 3; and 5% aq. Na. OH. Most organic compounds are not water soluble. Salts of acids or bases will often be soluble. If the compound is not soluble in water, solubility in acid suggests that it is basic (eg, amines), solubility in strong base suggests that it is at least weakly acidic (eg, phenols) and solubility in weak base (bicarbonate) suggests that it is a stronger acid (eg, carboxylic acids). Whatever determination you make from this test, you will corroborate that suggestion with other data including class tests and analysis of the compound's infrared spectrum. Compounds, which are insoluble in all of the above solutions can be tested for solubility and color change in concentrated sulfuric acid. Compounds giving a color change or showing solubility include neutral compounds such as alkenes, alkynes, alcohols, ketones, aldehydes, esters, ethers, amides and nitro compounds. Compounds that appear inert include alkanes, alkyl halides, and simple aromatic hydrocarbons. Dispose of the sulfuric acid solution carefully by pouring it into a beaker of cold water, carefully rinsing the test tube with water, neutralizing the acid with sodium bicarbonate until bubbling stops and washing both down the sink.

Rules for solubility • Only small polar molecules are soluble in water • Acids are better soluble in bases • Bases are better soluble in acids • Phenols are weak acids • Everything is soluble in concentrated sulfuric acid except for very inert compounds (alkanes, alkyl halides, aromatics)


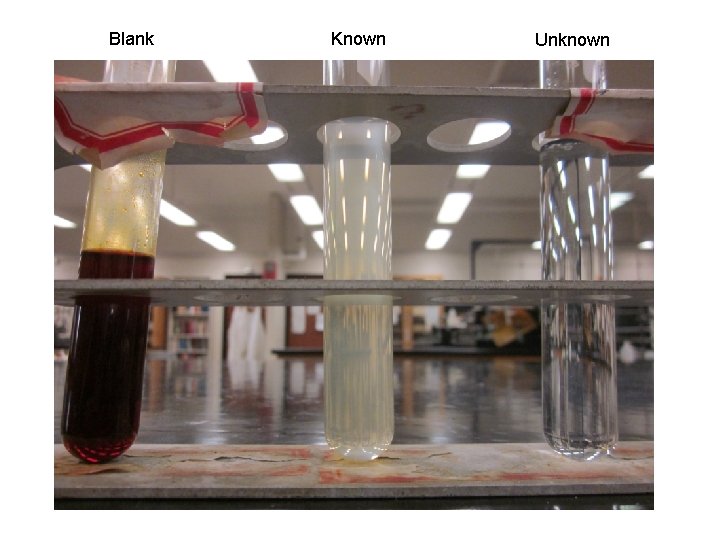
Preliminary Report Department of Chemistry and Biochemistry University of Missouri-St. Louis Solid (or Liquid) Unknown Chemistry 2633 Preliminary Report Form Name: ________ Date: ____ Unknown Number ______ Physical Properties a) appearance: b) melting point: c) boiling point: d) other properties you may have measured: Solubility Tests (use +/- to indicate positive, negative tests) H 2 O: _____; 5% Na. OH, _____; 5% Na. HCO 3, _____; 5%HCl, _____ ; H 2 SO 4, _____. p. H(aqueous solution): _____.

Experiment 9 • Seek feedback regarding your melting and boiling points. • Solubility experiments with Na. HCO 3 can be inconclusive. • Perform several classification tests to corroborate your functional group (one test is typically not enough).

IR spectra Take IR spectra of all compounds you are synthesizing and of both unknowns. Assign all major peaks above 1500 cm-1 to functional groups. Describe how your spectrum matches the isolated / obtained compound

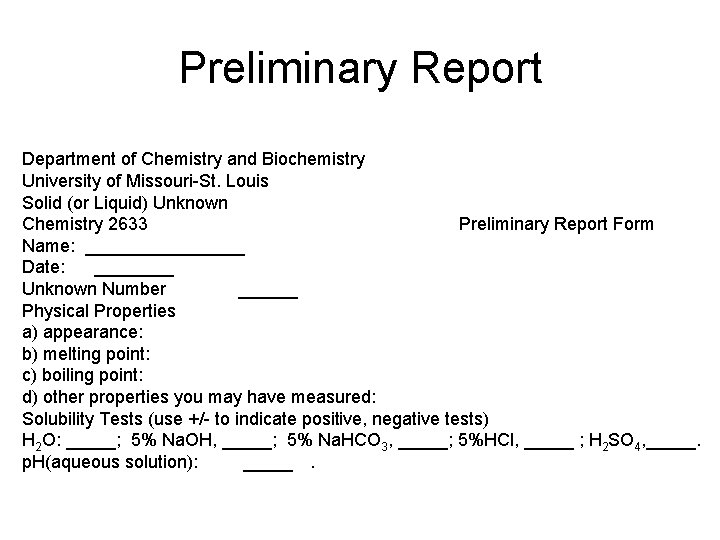
Classification Tests Halogens, Aromatics • Beilstein test for halogenated compounds. (copper wire/green flame) For knowns use bromobenzene or chlorbenzene for the liquid and a halogenated benzoic acid for the solid. This test should be done in a hood. • Ignition Test for Aromaticity. (spatula/yellow, sooty flame) Use naphthalene for the solid known and toluene or xylene for the liquid known. This test should be done in a hood.

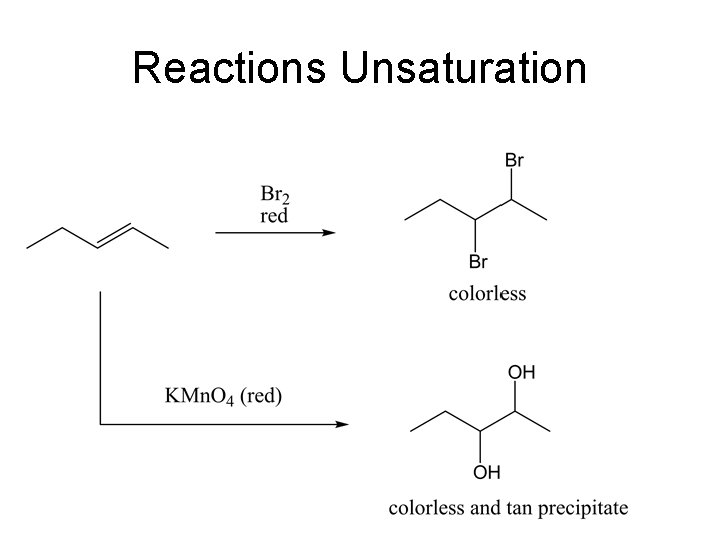
Classification Tests • All classification tests should include a known, unknown, and blank. • The known compound gives you a positive test for reference and tells you if the reagents or procedure are faulty. Knowns are provided in the hood. • The blank gives you the other extreme, a negative test. • The results you obtain from your unknown can then be put into perspective. • The triplicate tests should be done simultaneously thereby taking little extra time. Use clean test tubes. For certain tests, the presence of even trace amounts of acetone can give false positives. • Do only those tests that you think will be useful based upon solubility results and your analysis of the infrared spectrum of the compound. If that analysis has not been done and you wish to do some class tests, do them in the order shown below.

Reactions for Beilstein and Flame Test

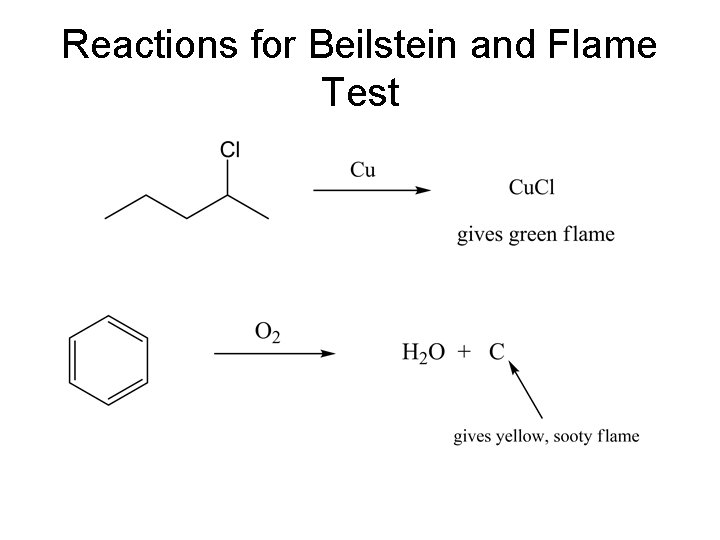
Classification Tests Unsaturation • Bromine Test for Unsaturation: (multiple bond/bromine color disappearance) Use cyclohexene, octene, or another simple alkene as the known. Warning: the reagent deteriorates with the formation of HBr so compare the results with a blank. • Permanganate Test for Unsaturation (Baeyer Test, multiple bonds/brown precipitate). Other compounds including alcohols, aldehydes, phenols, and aromatic amines also react slowly with the reagent so interpret your results carefully and look for corroboration from the other tests. Use cyclohexene, octene or another simple alkene as the known.

Reactions Unsaturation

Blank Known Unknown

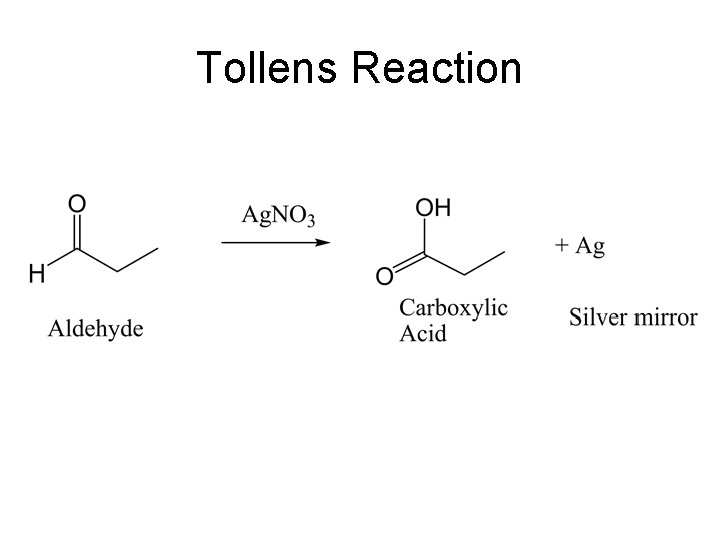
Classification Tests Aldehyde/Ketones • Aldehydes and Ketones with 2, 4 -dinitrophenylhydrazine (DNP, colored precipitate). For knowns, use cyclohexanone and benzaldehyde. Warning: even the trace of acetone used to clean your glassware will give a positive test. • Tollens Test for Aldehydes and other easily oxidized functional groups. (silver mirror) Do not use the known or the blank for this test. Warning: Wash any minor amounts of residual Tollens reagent into a sink and flush with water. The reagent forms silver fulminate which is very explosive.

DNP-Reactions with Ketones/Aldehydes Colored precipitate

Tollens Reaction

Classification Tests Aldehydes / Alcohols • Chromic Acid Test for Aldehydes and Alcohols. (color change from orange to green) Tertiary alcohols are not oxidized. Aliphatic aldehydes are oxidized in less than a minute, aromatic aldehydes take a bit longer. It is recommended to carefully run the blank to be certain that the acetone itself is not giving a false positive. Use benzaldehyde and an aliphatic aldehyde for aldehyde knowns and 1 - and 2 -butanol for alcohol knowns. Warning: Cr(VI) compounds are considered suspect carcinogens and should be handled carefully.

Alcohols

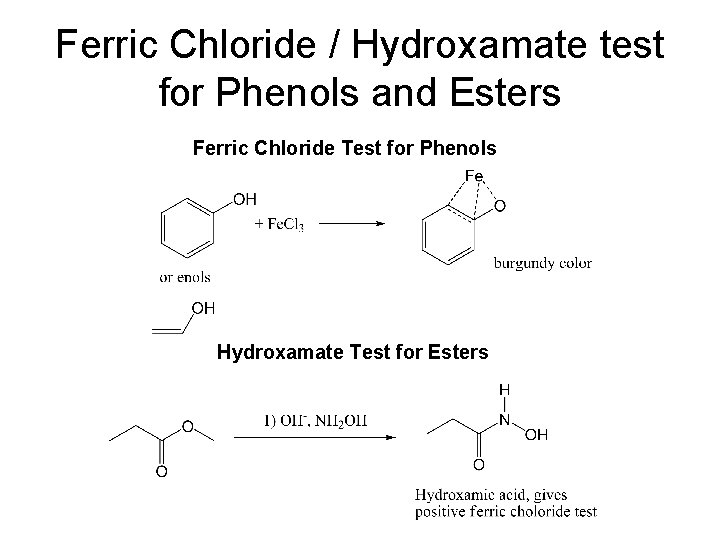
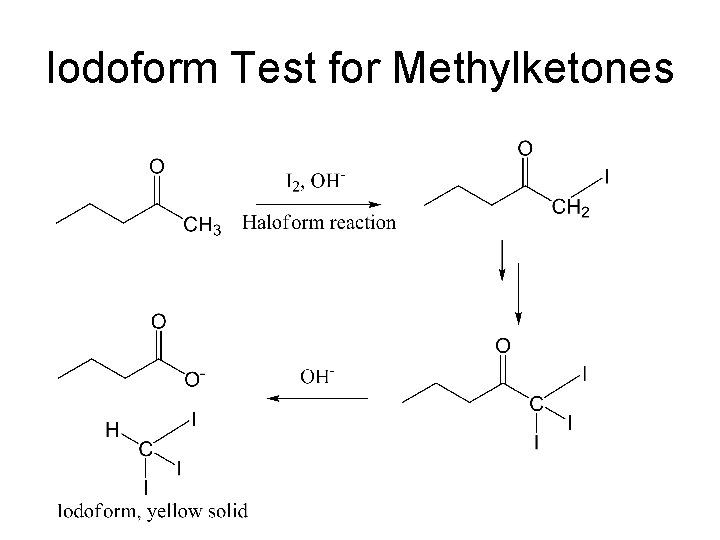
Classification Tests • Ferric Hydroxamate Test for Esters. (deep burgundy color). Use banana oil or methyl benzoate as knowns. • Ferric Chloride test for Phenols. (An intense color is a positive test) Use phenol as a known. Not all phenols will give a positive test! • Iodoform test for methyl ketones. (yellow solid) Acetone can be used for the known.

Ferric Chloride / Hydroxamate test for Phenols and Esters Ferric Chloride Test for Phenols Hydroxamate Test for Esters

Iodoform Test for Methylketones

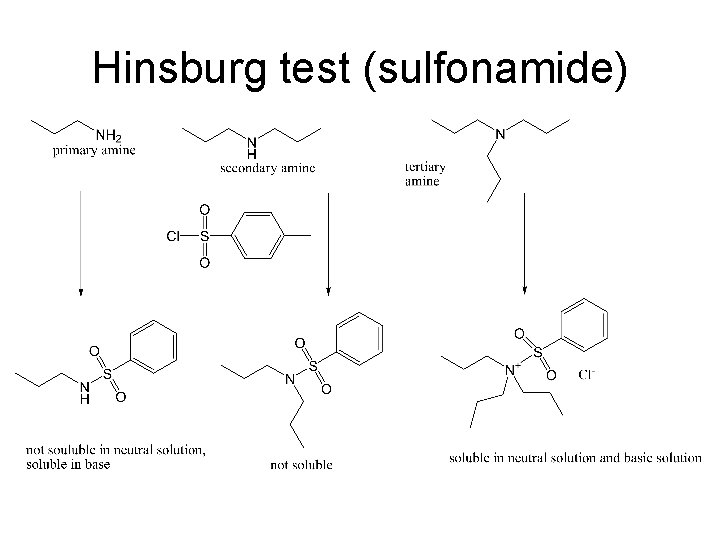
Hinsburg Test for Amines • You will react the amine with a sulfonyl chloride forming an insoluble sulfonamide of a primary or secondary amine or the soluble salt of a tertiary amine. The insoluble sulfonamide of a primary amine will be made soluble in base (via removal of the slightly acidic proton on N) but that of a secondary amine will not (no proton on N to remove). • Use aniline, N-methylaniline and N, Ndimethylaniline for knowns.

Hinsburg test (sulfonamide)

Titration of an acid • Determination of Equivalent Weight (or Neutralization Equivalent) • Molecular Weight Determination • Generally, any acid (or base) can be titrated using standard solutions of base (or acid). The neutralization equivalent obtained is usually a simple fraction of the molecular weight (1, 1/2, 1/3, etc). In the titration of an acid with standard base, the endpoint is reached when all the acid is neutralized and a drop of excess base is added. If phenolphthalein is used as the indicator, it will turn red at this instant. For a dicarboxylic acid such as malonic acid, the endpoint is reached when the last of the acid is converted to the carboxylate anion. The neutralization equivalent will be one-half its molecular weight. CHx(CO 2 H)n + n Na. OH → CHx(CO 2 Na)n + n H 2 O n = 1, 2, 3

Titration Acids • • Into a 125 ml Erlenmeyer flask place approx. 150 mg of the unknown, accurately weighed to the nearest 1 mg. Add approx. 5 -10 ml of 95% ethanol to dissolve the unknown and add an equal amount of water. Add a drop or two of phenolphthalein solution, and a magnetic stirring bar if you do not want to swirl. Titrate with standardized aqueous base to the first permanent pink color that lasts about 60 seconds. The equivalent weight (neutralization equivalent) can be calculated as follows: V (ml) x M (mmol/ml)= wt (mg)/equivalent weight (mg/equiv); V = volume of standard base used measured accurately to at least 3 significant figures; M = molarity of standard base as long as the base is monobasic, probably listed as 0. 100 M Na. OH or KOH (note three significant figures); Wt = weight of unknown used. An equivalent weight of 120 means that the molecular weight is some whole number multiple of 120; for example, 120 (if monoacid) or 240 (if diacid) or 360 (if triacid), etc.

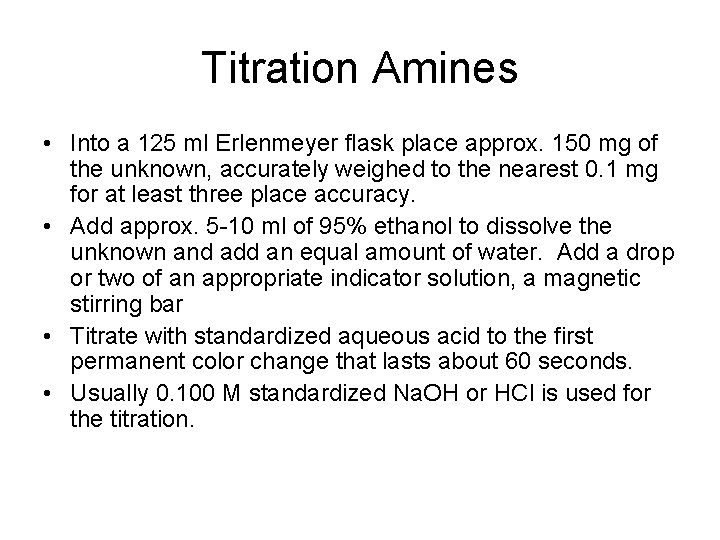
Titration Amines • Into a 125 ml Erlenmeyer flask place approx. 150 mg of the unknown, accurately weighed to the nearest 0. 1 mg for at least three place accuracy. • Add approx. 5 -10 ml of 95% ethanol to dissolve the unknown and add an equal amount of water. Add a drop or two of an appropriate indicator solution, a magnetic stirring bar • Titrate with standardized aqueous acid to the first permanent color change that lasts about 60 seconds. • Usually 0. 100 M standardized Na. OH or HCl is used for the titration.

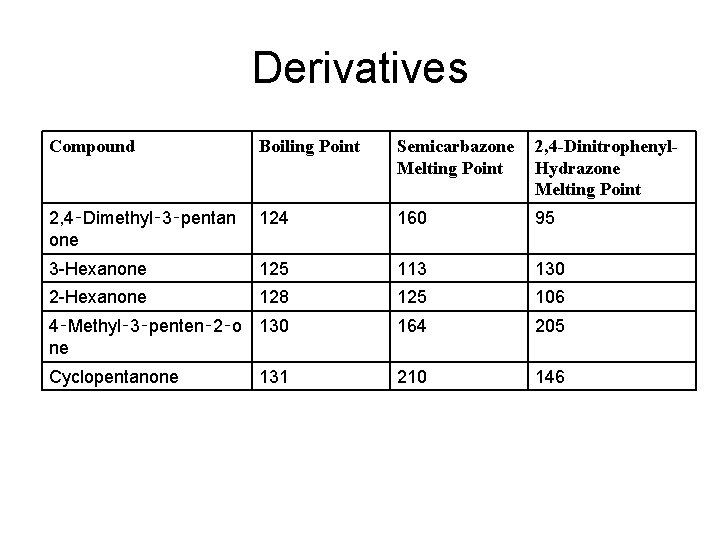
Derivatives Compound Boiling Point Semicarbazone Melting Point 2, 4 -Dinitrophenyl. Hydrazone Melting Point 2, 4‑Dimethyl‑ 3‑pentan one 124 160 95 3 -Hexanone 125 113 130 2 -Hexanone 128 125 106 4‑Methyl‑ 3‑penten‑ 2‑o 130 ne 164 205 Cyclopentanone 210 146 131

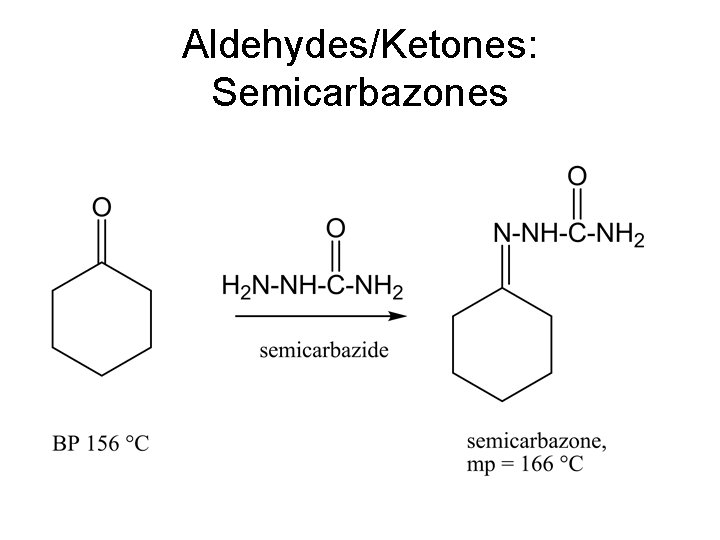
Aldehydes/Ketones: Semicarbazones

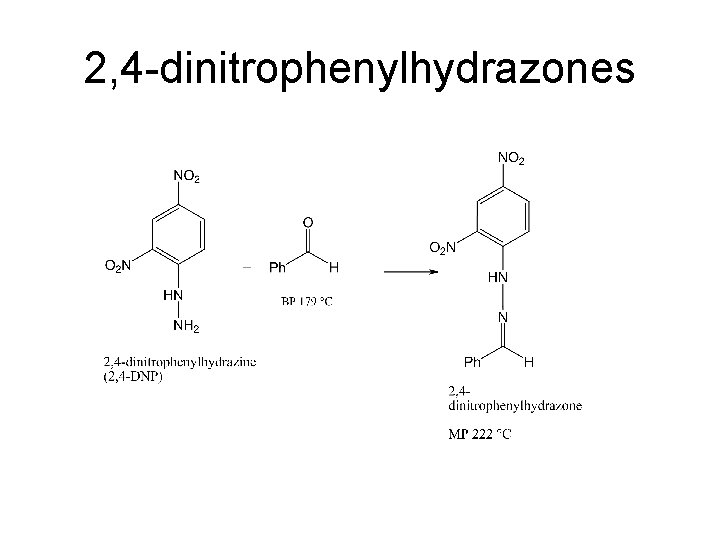
2, 4 -dinitrophenylhydrazones

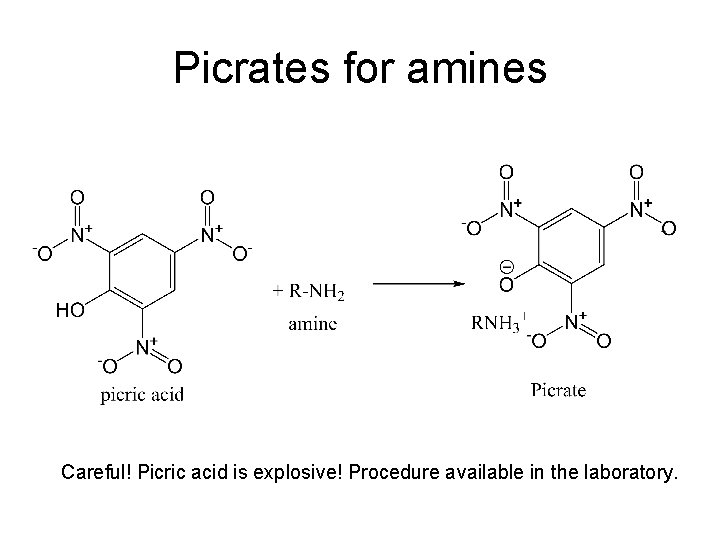
Picrates for amines Careful! Picric acid is explosive! Procedure available in the laboratory.

Derivatives. Aldehydes and Ketones 2, 4 -Dinitrophenylhydrazones: • Place 10 m. L of a solution of 2, 4 -dinitrophenylhydrazine [2, 4 -DNP] (in methanol/hydrochloric acid) into a test tube • Add 150 -250 mg of the unknown compound (or about 1 mmol). If the unknown is a solid, dissolve the compound in a minimum amount of 95% ethanol or 1, 2 -dimethoxyethane first. • Crystallization will probably not occur immediately (the reaction in ethanol/sulfuric acid is faster), so gently warm the solution for a minute on a hot plate and then set it aside to crystallize. It may take several hours. • As a backup, you can repeat the procedure using the 2, 4 -DNP solution prepared in ethanol/sulfuric acid but the methanolic solution will probably give you better crystals (higher mp).

Semicarbazones • The stock solution is prepared by dissolving 1. 11 g of semicarbazide hydrochloride (MW 111. 5) in 5 m. L of water. Place 0. 5 m. L of this solution in a small test tube. • Add an estimated 1 mmol (approx 150 -250 mg) of the unknown. If the unknown does not dissolve in the aqueous solution, or if it becomes cloudy, add some methanol dropwise to dissolve the solid or produce a clear solution. • Add 10 drops of pyridine and heat the mixture gently on a steam bath or a hot plate for 5 minutes. The product should begin to crystallize. • The product can be recrystallized from ethanol if necessary. Alternative Method: • Dissolve 1 g of semicarbazide hydrochloride and 1. 5 g of sodium acetate in 5 m. L of water. • Dissolve 1. 0 g of the unkown in 10 m. L of ethanol. Mix the two solutions together in a 50 m. L Erlenmeyer flask and heat to boiling for about 5 minutes. • Place the flask in a beaker of ice and scratch the sides of the flask to induce crystallization.

Final report for the unknowns: • Number, your name, appropriate properties, the results of the solubility and the class tests, the neutralization equivalent if taken (must be reported for all acids and bases), other possible compounds within that class, a thorough analysis of your infrared spectrum that includes as many features of your compound as you can determine (major functional group, other functional groups, aromatic units, double or triple bonds). Submit the derivative(s) in a properly labeled vial which includes your name, name of product, mp of product, lit mp of product, date prepared. Also return unused unknown compound. • When you submit the formal final report you should attach your properly labeled infrared spectrum (your name, the compound name and/or unknown number and the major peaks >1600 cm-1 labeled) and the detailed lab report. • Having the correct structure is insufficient! In your lab report you must list other possibilities and explain how you differentiated between the “correct” compound and those other possibilities. Write a detective/forensic story if your wish.
 Experiment 456
Experiment 456 Equation with two unknowns
Equation with two unknowns Known unknowns matrix
Known unknowns matrix Solve 2 equations 2 unknowns maple
Solve 2 equations 2 unknowns maple Central pocket vs plain whorl
Central pocket vs plain whorl Slab design
Slab design 123 12345678
123 12345678 Ccx provider portal
Ccx provider portal 800 456 3355
800 456 3355 Slump value as per is 456
Slump value as per is 456 Ralphs annual income is about $32 000
Ralphs annual income is about $32 000 Penilaian gcs
Penilaian gcs 123 + 456 + 789
123 + 456 + 789 Write a number in expanded form. 456
Write a number in expanded form. 456 123456 xxxx
123456 xxxx Slump value as per is 456
Slump value as per is 456 Non stationary process
Non stationary process Gcs 456
Gcs 456 123456+789
123456+789 Subtitle123
Subtitle123 Infix to postfix conversion rules
Infix to postfix conversion rules Bbm456
Bbm456 Cmsc 456 3 cryptology
Cmsc 456 3 cryptology What gangs are in oklahoma city
What gangs are in oklahoma city 456 name
456 name Cmsc 456
Cmsc 456 Pcb alignment pin
Pcb alignment pin Tornado outbreak of april 20, 2004
Tornado outbreak of april 20, 2004 April 23, 1564
April 23, 1564 Vitaj april
Vitaj april Iris light april greiman
Iris light april greiman Dreißig tage hat september
Dreißig tage hat september Why is april a busy month for birds
Why is april a busy month for birds April 2006 calendar
April 2006 calendar April art project
April art project Reformdjp/quiz/kuis-mini-april
Reformdjp/quiz/kuis-mini-april April fleming
April fleming April 26 1564
April 26 1564