CHEM 1020 4242017 Monday FUNCTIONAL GROUPS NOMENCLATURE FUNCTIONAL



- Slides: 53

CHEM 1020 4/24/2017 Monday FUNCTIONAL GROUPS & NOMENCLATURE

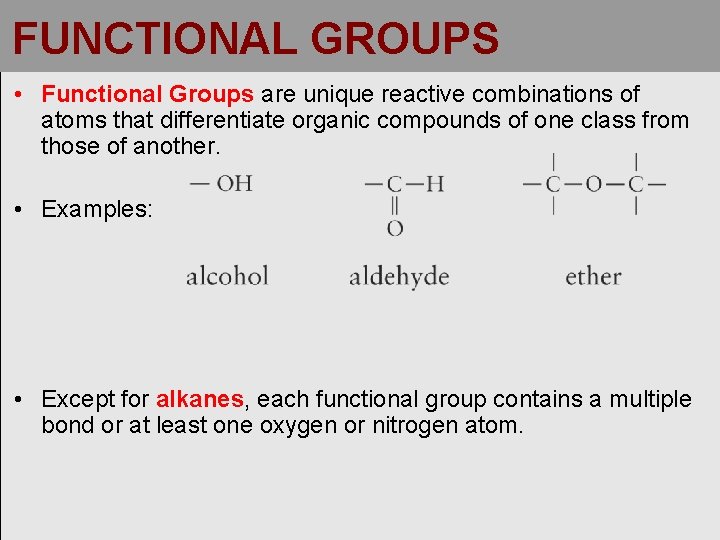
FUNCTIONAL GROUPS • Functional Groups are unique reactive combinations of atoms that differentiate organic compounds of one class from those of another. • Examples: • Except for alkanes, each functional group contains a multiple bond or at least one oxygen or nitrogen atom.

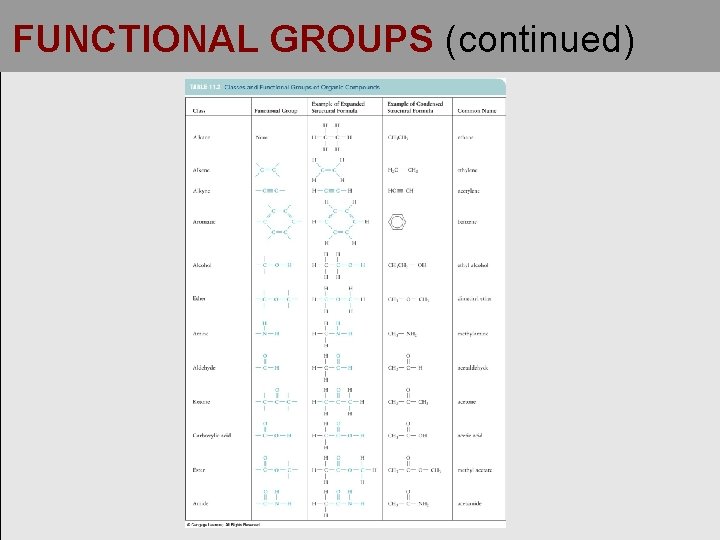
FUNCTIONAL GROUPS (continued)

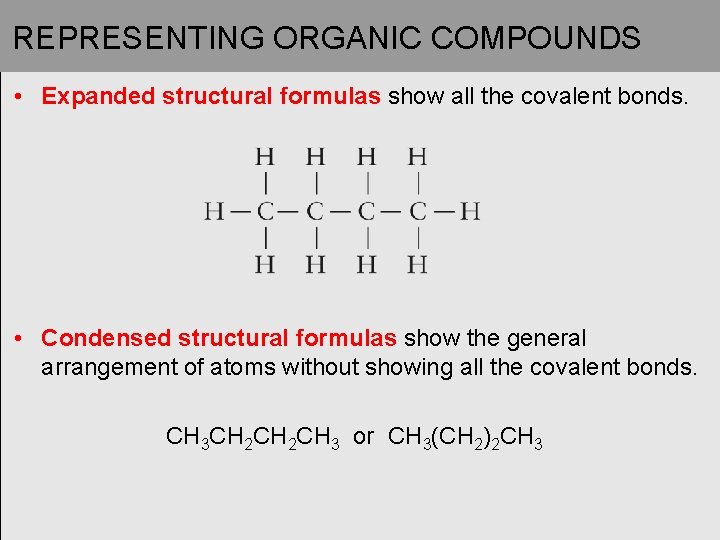
REPRESENTING ORGANIC COMPOUNDS • Expanded structural formulas show all the covalent bonds. • Condensed structural formulas show the general arrangement of atoms without showing all the covalent bonds. CH 3 CH 2 CH 3 or CH 3(CH 2)2 CH 3

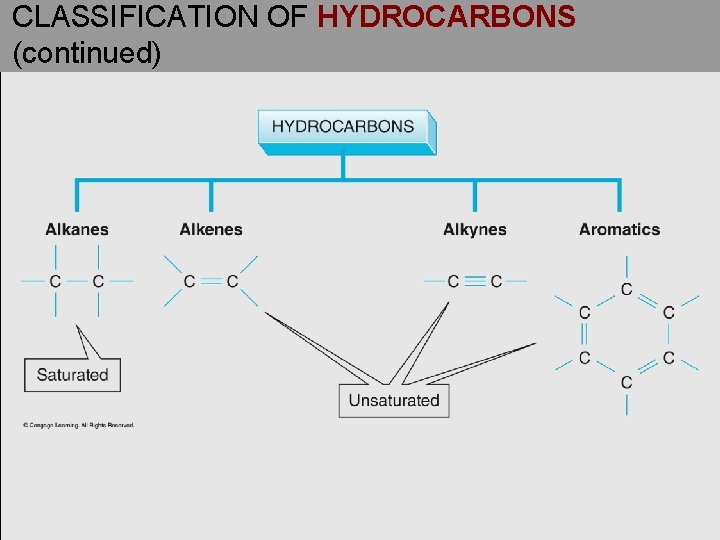
CLASSIFICATION OF HYDROCARBONS (continued)

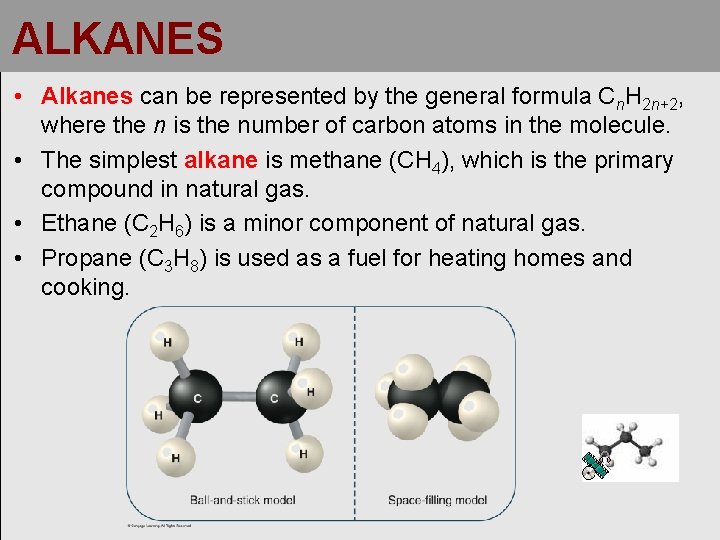
ALKANES • Alkanes can be represented by the general formula Cn. H 2 n+2, where the n is the number of carbon atoms in the molecule. • The simplest alkane is methane (CH 4), which is the primary compound in natural gas. • Ethane (C 2 H 6) is a minor component of natural gas. • Propane (C 3 H 8) is used as a fuel for heating homes and cooking.

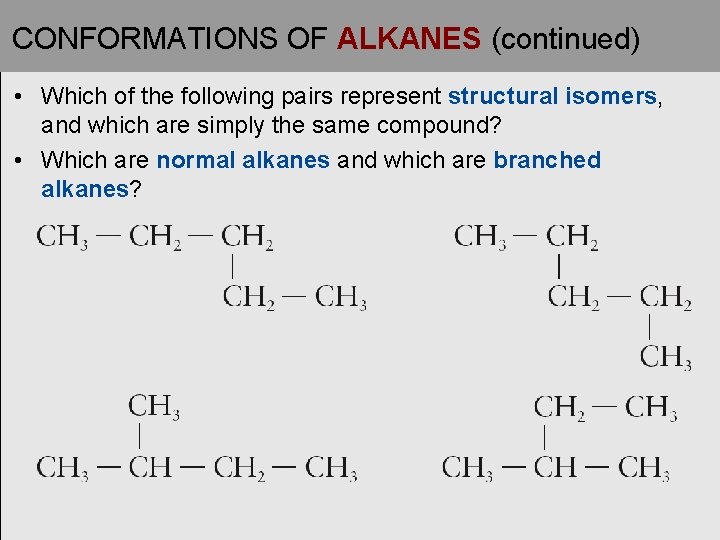
CONFORMATIONS OF ALKANES (continued) • Which of the following pairs represent structural isomers, and which are simply the same compound? • Which are normal alkanes and which are branched alkanes?

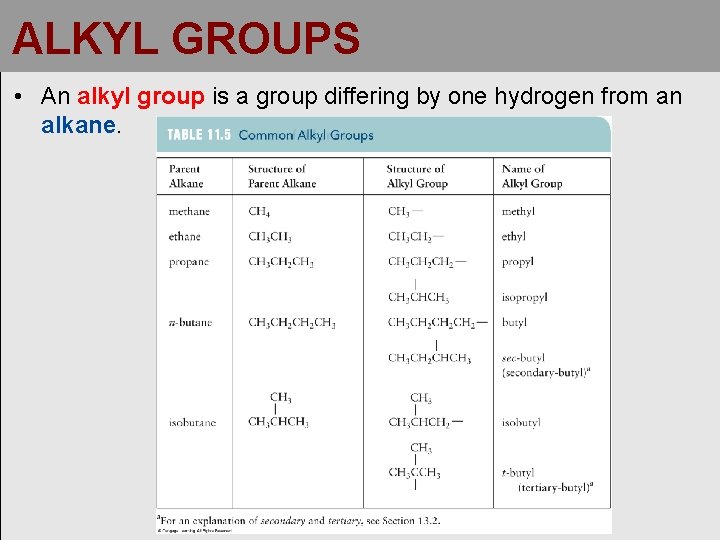
ALKYL GROUPS • An alkyl group is a group differing by one hydrogen from an alkane.

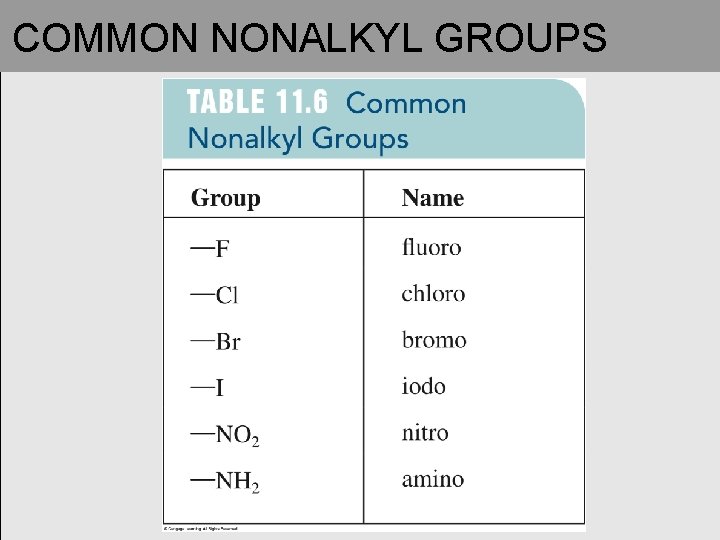
COMMON NONALKYL GROUPS

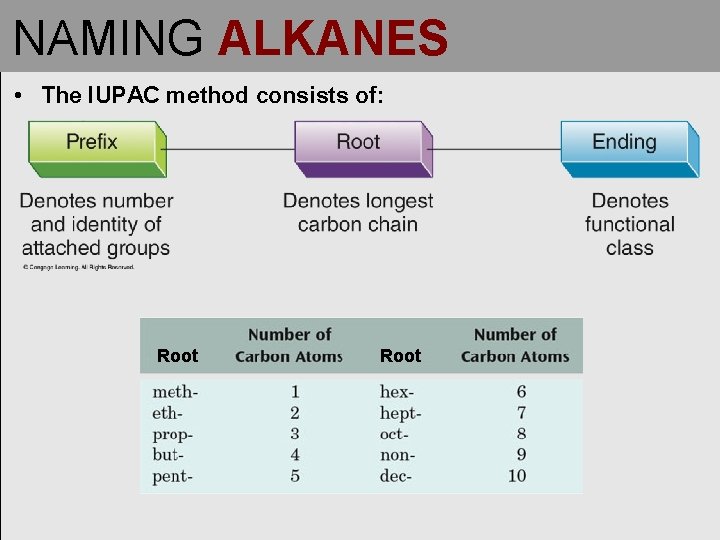
NAMING ALKANES • The IUPAC method consists of: Root

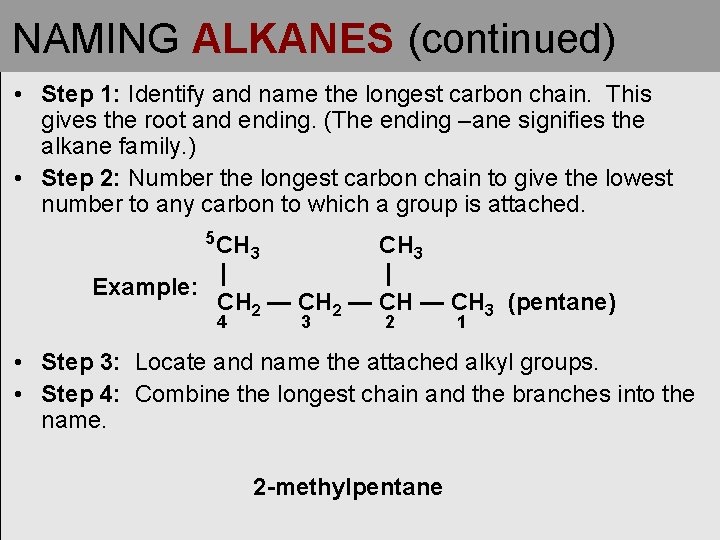
NAMING ALKANES (continued) • Step 1: Identify and name the longest carbon chain. This gives the root and ending. (The ending –ane signifies the alkane family. ) • Step 2: Number the longest carbon chain to give the lowest number to any carbon to which a group is attached. 5 CH CH 3 | | Example: CH 2 — CH 3 (pentane) 4 3 3 2 1 • Step 3: Locate and name the attached alkyl groups. • Step 4: Combine the longest chain and the branches into the name. 2 -methylpentane

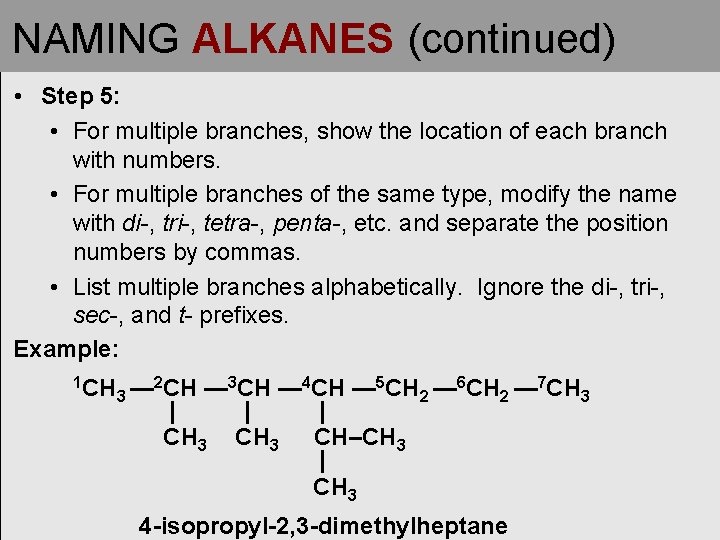
NAMING ALKANES (continued) • Step 5: • For multiple branches, show the location of each branch with numbers. • For multiple branches of the same type, modify the name with di-, tri-, tetra-, penta-, etc. and separate the position numbers by commas. • List multiple branches alphabetically. Ignore the di-, tri-, sec-, and t- prefixes. Example: 1 CH 3— 2 CH — 3 CH — 4 CH — 5 CH 2 — 6 CH 2 — 7 CH 3 | | | CH 3 CH–CH 3 | CH 3 4 -isopropyl-2, 3 -dimethylheptane

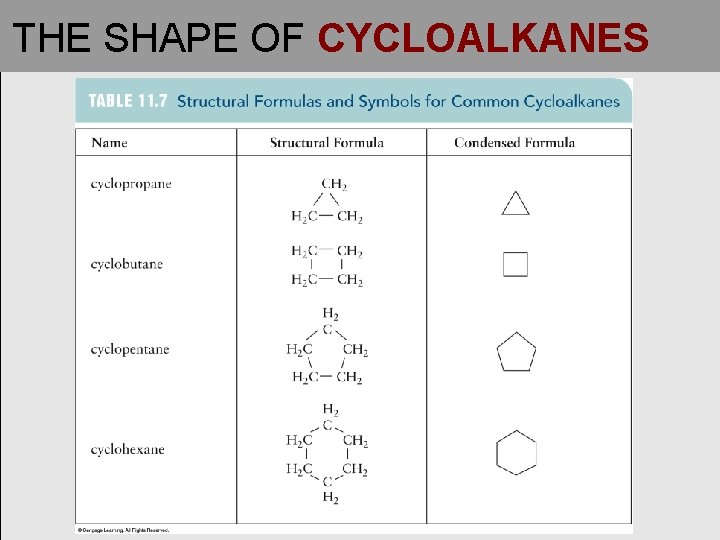
THE SHAPE OF CYCLOALKANES

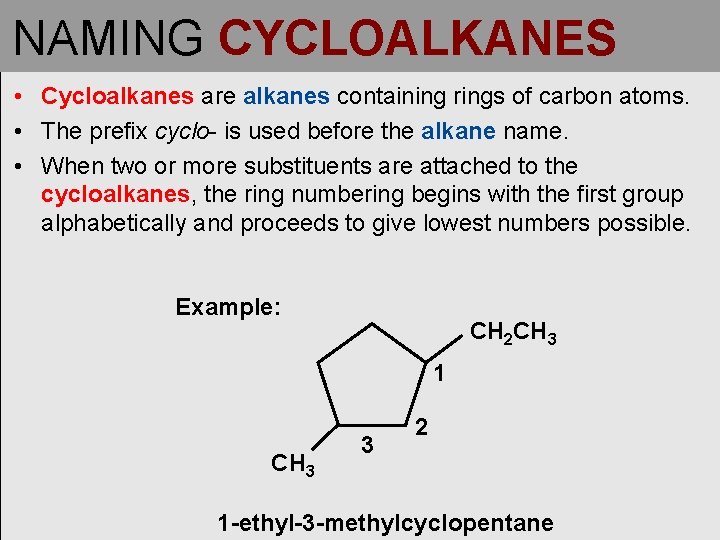
NAMING CYCLOALKANES • Cycloalkanes are alkanes containing rings of carbon atoms. • The prefix cyclo- is used before the alkane name. • When two or more substituents are attached to the cycloalkanes, the ring numbering begins with the first group alphabetically and proceeds to give lowest numbers possible. Example: CH 2 CH 3 1 CH 3 3 2 1 -ethyl-3 -methylcyclopentane

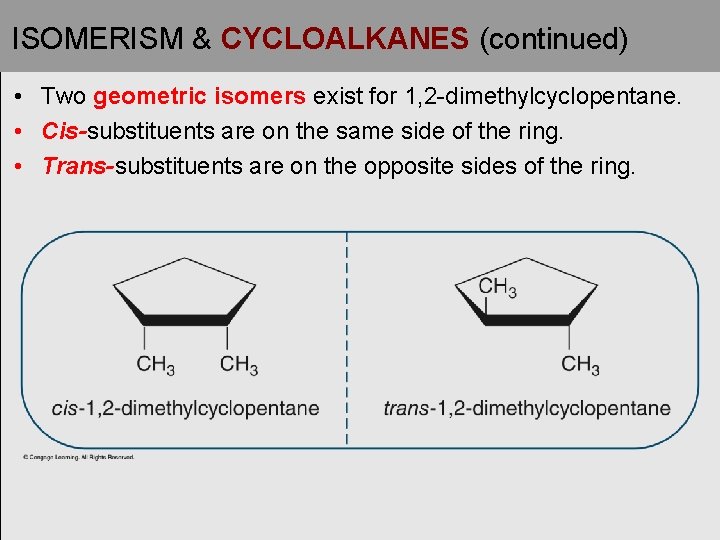
ISOMERISM & CYCLOALKANES (continued) • Two geometric isomers exist for 1, 2 -dimethylcyclopentane. • Cis-substituents are on the same side of the ring. • Trans-substituents are on the opposite sides of the ring.

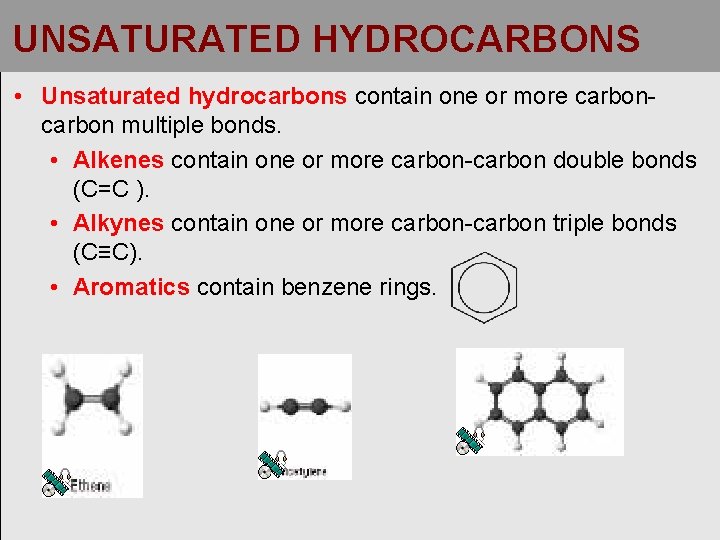
UNSATURATED HYDROCARBONS • Unsaturated hydrocarbons contain one or more carbon multiple bonds. • Alkenes contain one or more carbon-carbon double bonds (C=C ). • Alkynes contain one or more carbon-carbon triple bonds (C≡C). • Aromatics contain benzene rings.

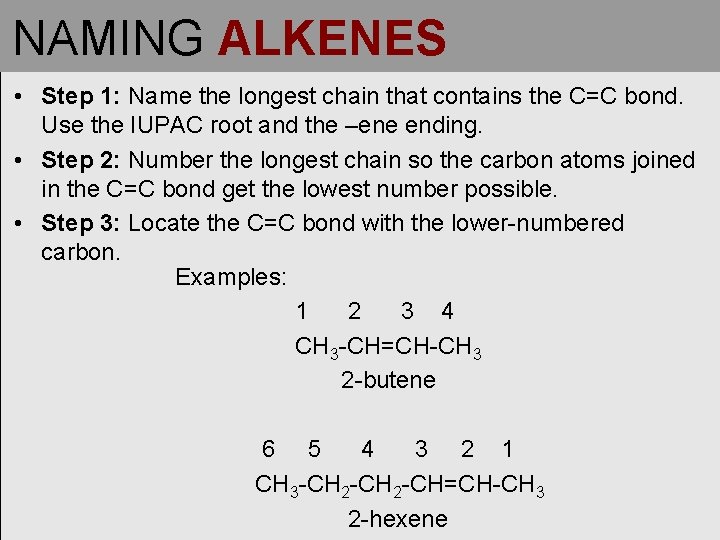
NAMING ALKENES • Step 1: Name the longest chain that contains the C=C bond. Use the IUPAC root and the –ene ending. • Step 2: Number the longest chain so the carbon atoms joined in the C=C bond get the lowest number possible. • Step 3: Locate the C=C bond with the lower-numbered carbon. Examples: 1 2 3 4 CH 3 -CH=CH-CH 3 2 -butene 6 5 4 3 2 1 CH 3 -CH 2 -CH=CH-CH 3 2 -hexene

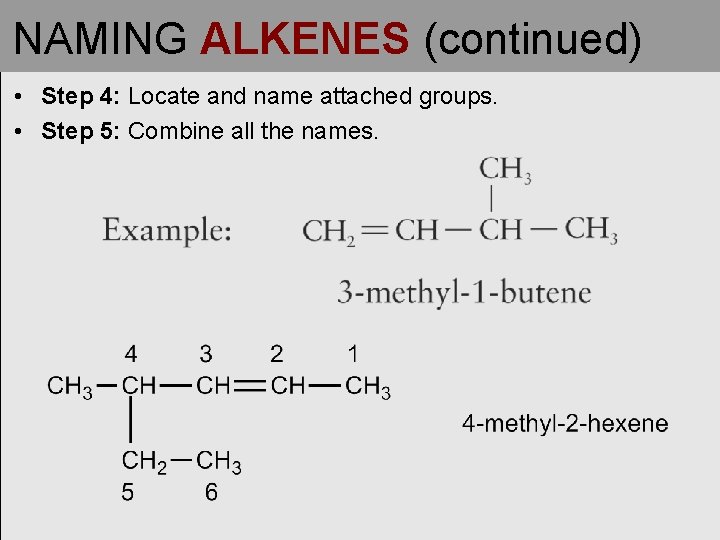
NAMING ALKENES (continued) • Step 4: Locate and name attached groups. • Step 5: Combine all the names.

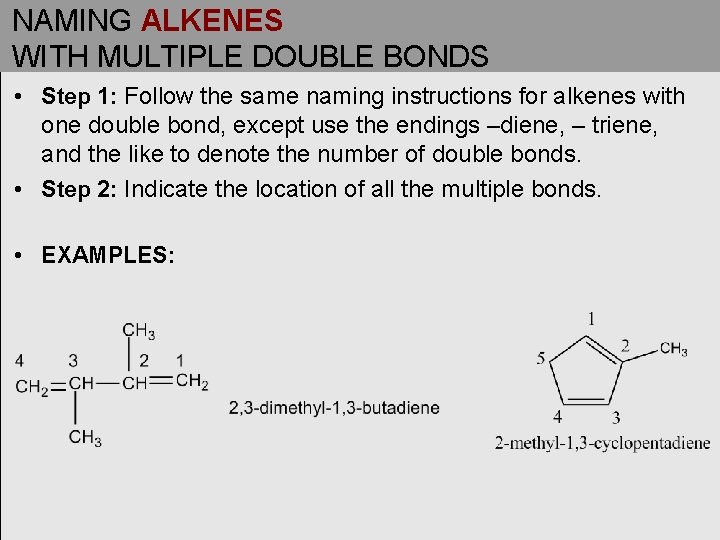
NAMING ALKENES WITH MULTIPLE DOUBLE BONDS • Step 1: Follow the same naming instructions for alkenes with one double bond, except use the endings –diene, – triene, and the like to denote the number of double bonds. • Step 2: Indicate the location of all the multiple bonds. • EXAMPLES:

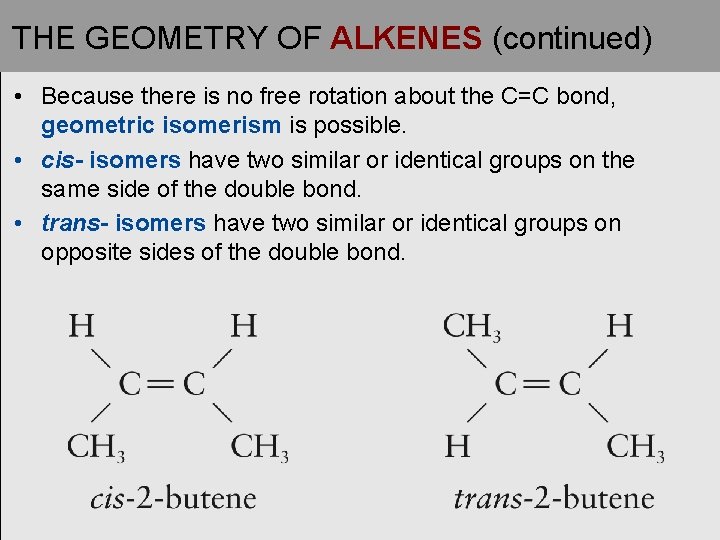
THE GEOMETRY OF ALKENES (continued) • Because there is no free rotation about the C=C bond, geometric isomerism is possible. • cis- isomers have two similar or identical groups on the same side of the double bond. • trans- isomers have two similar or identical groups on opposite sides of the double bond.

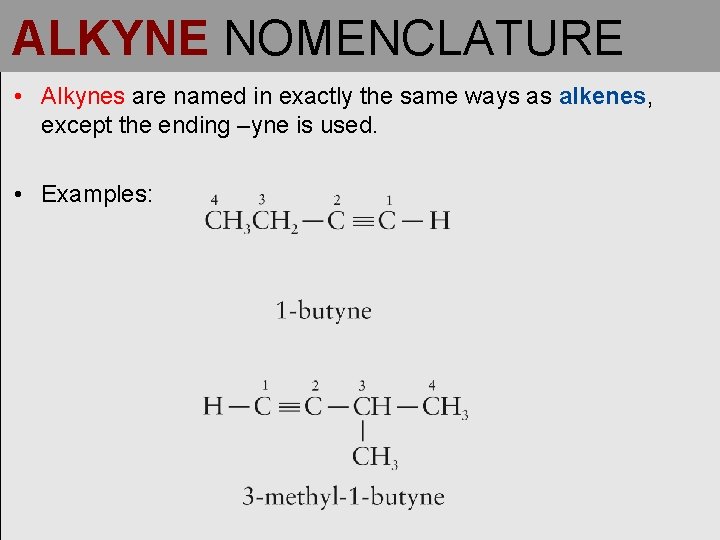
ALKYNE NOMENCLATURE • Alkynes are named in exactly the same ways as alkenes, except the ending –yne is used. • Examples:

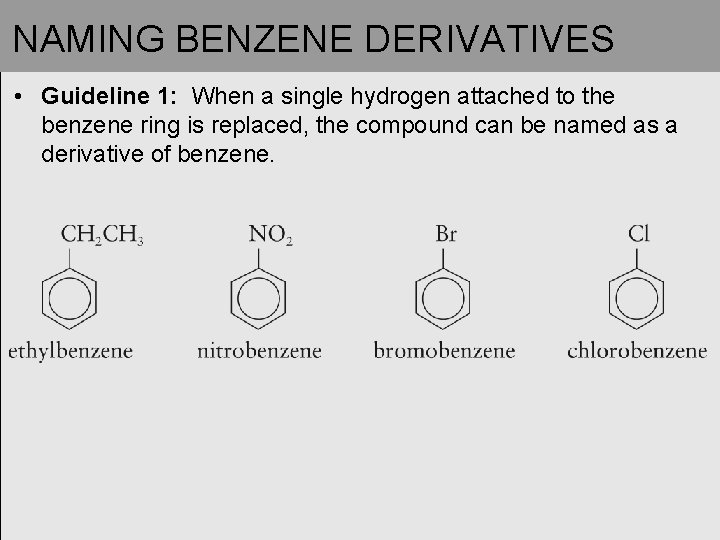
NAMING BENZENE DERIVATIVES • Guideline 1: When a single hydrogen attached to the benzene ring is replaced, the compound can be named as a derivative of benzene.

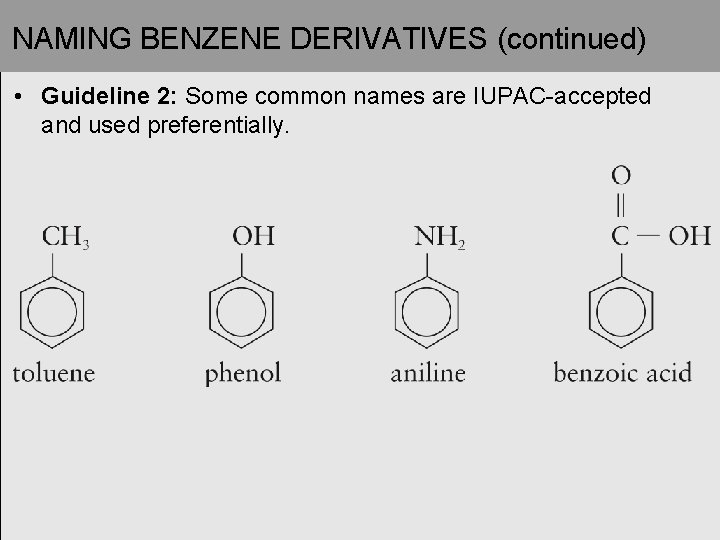
NAMING BENZENE DERIVATIVES (continued) • Guideline 2: Some common names are IUPAC-accepted and used preferentially.

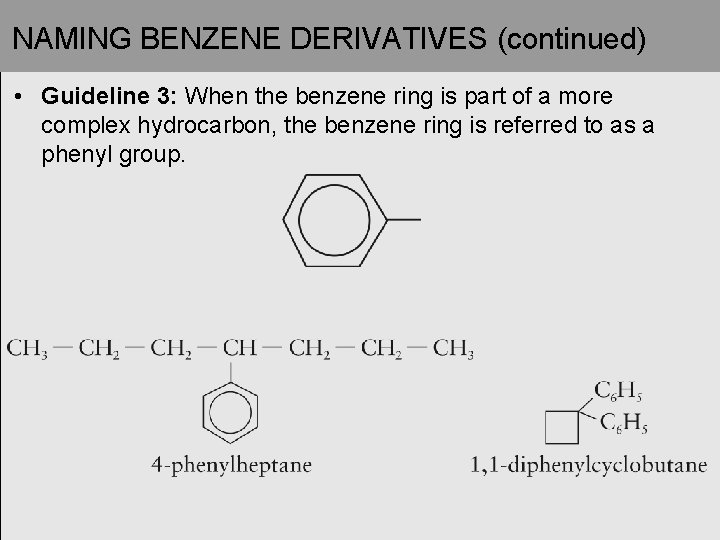
NAMING BENZENE DERIVATIVES (continued) • Guideline 3: When the benzene ring is part of a more complex hydrocarbon, the benzene ring is referred to as a phenyl group.

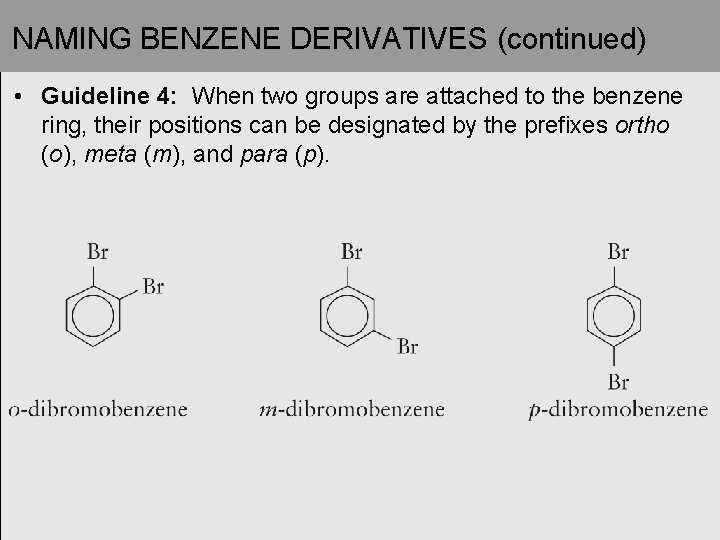
NAMING BENZENE DERIVATIVES (continued) • Guideline 4: When two groups are attached to the benzene ring, their positions can be designated by the prefixes ortho (o), meta (m), and para (p).

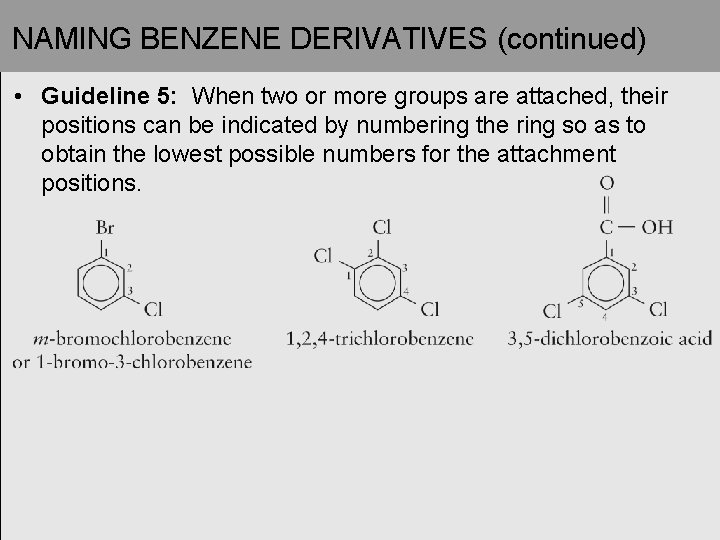
NAMING BENZENE DERIVATIVES (continued) • Guideline 5: When two or more groups are attached, their positions can be indicated by numbering the ring so as to obtain the lowest possible numbers for the attachment positions.

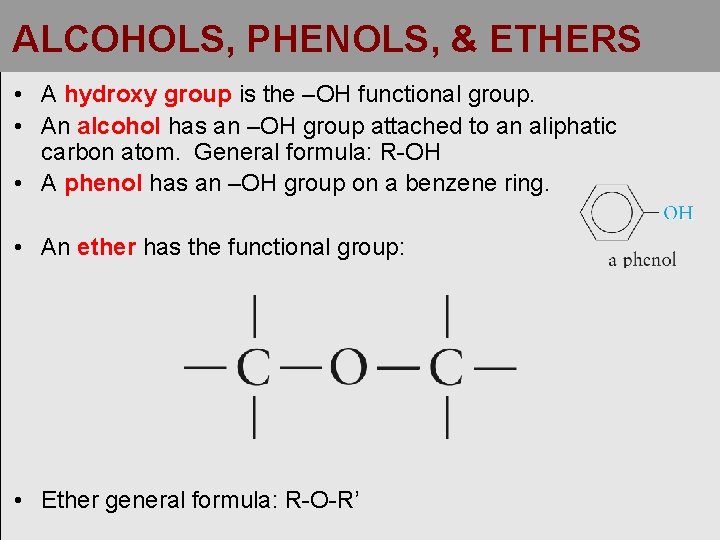
ALCOHOLS, PHENOLS, & ETHERS • A hydroxy group is the –OH functional group. • An alcohol has an –OH group attached to an aliphatic carbon atom. General formula: R-OH • A phenol has an –OH group on a benzene ring. • An ether has the functional group: • Ether general formula: R-O-R’

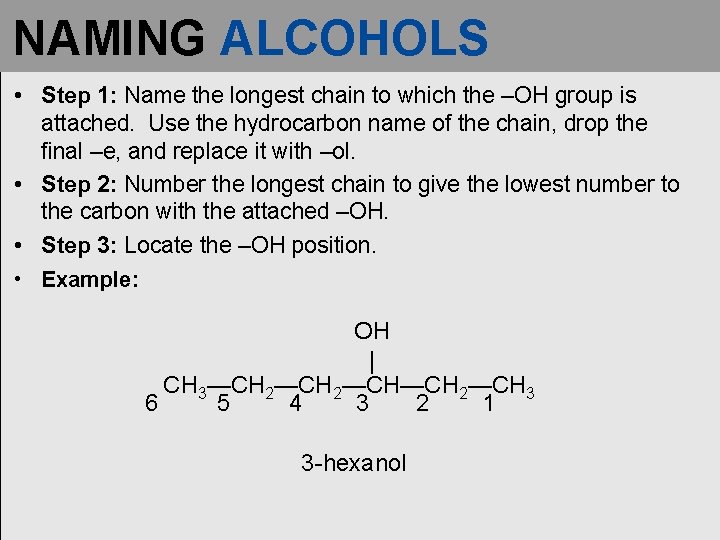
NAMING ALCOHOLS • Step 1: Name the longest chain to which the –OH group is attached. Use the hydrocarbon name of the chain, drop the final –e, and replace it with –ol. • Step 2: Number the longest chain to give the lowest number to the carbon with the attached –OH. • Step 3: Locate the –OH position. • Example: OH | CH 3—CH 2—CH—CH 2—CH 3 6 5 4 3 2 1 3 -hexanol

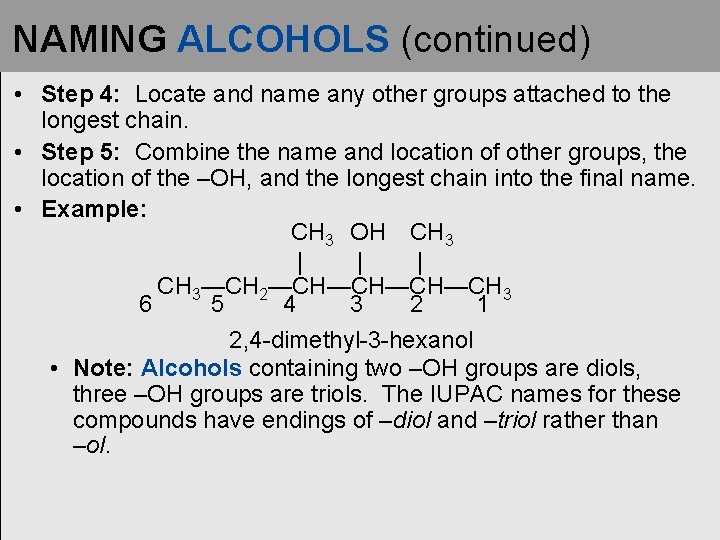
NAMING ALCOHOLS (continued) • Step 4: Locate and name any other groups attached to the longest chain. • Step 5: Combine the name and location of other groups, the location of the –OH, and the longest chain into the final name. • Example: CH 3 OH CH 3 | | | CH 3—CH 2—CH—CH 3 6 5 4 3 2 1 2, 4 -dimethyl-3 -hexanol • Note: Alcohols containing two –OH groups are diols, three –OH groups are triols. The IUPAC names for these compounds have endings of –diol and –triol rather than –ol.

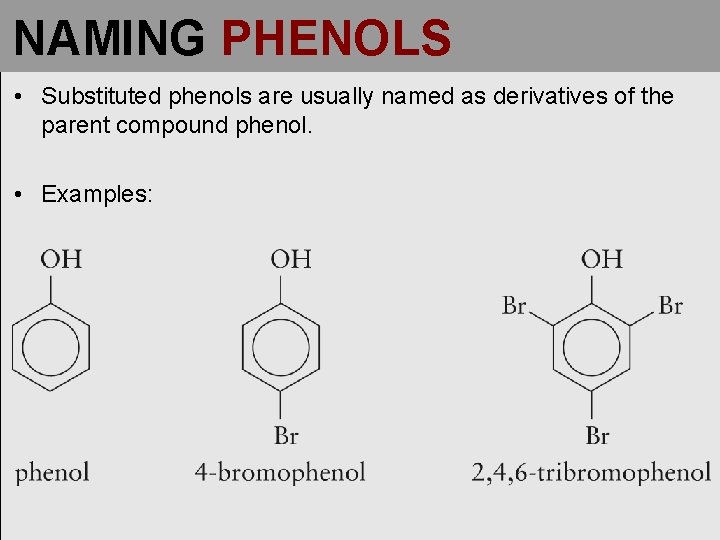
NAMING PHENOLS • Substituted phenols are usually named as derivatives of the parent compound phenol. • Examples:

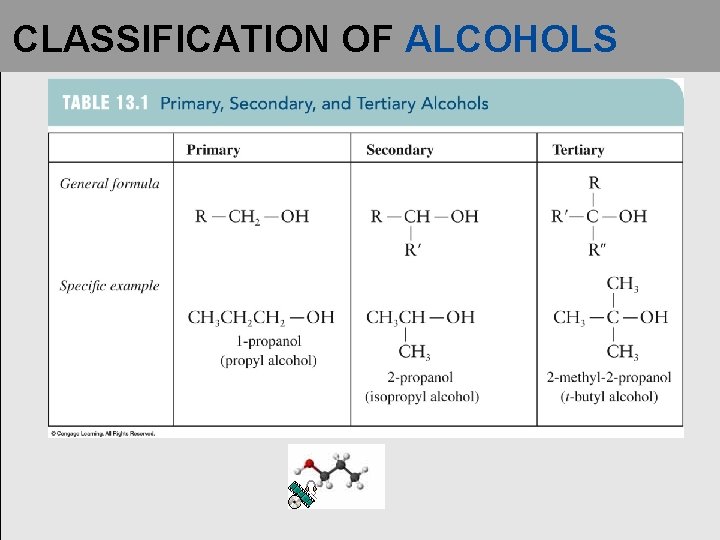
CLASSIFICATION OF ALCOHOLS

NAMING ETHERS • IUPAC name: Name the smaller of the two R groups as an alkoxy group attached to the parent chain by replacing the –yl ending of the R group with –oxy. • Common name: Name the groups attached to the oxygen alphabetically and add the word ether. common: sec-butyl ether

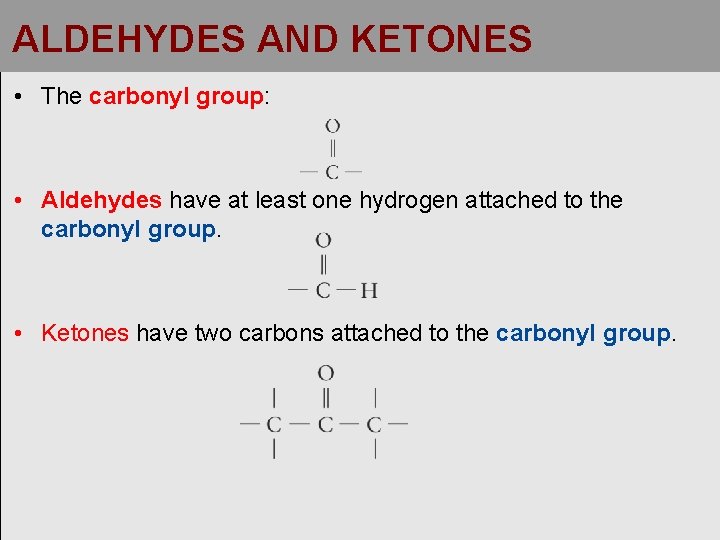
ALDEHYDES AND KETONES • The carbonyl group: • Aldehydes have at least one hydrogen attached to the carbonyl group. • Ketones have two carbons attached to the carbonyl group.

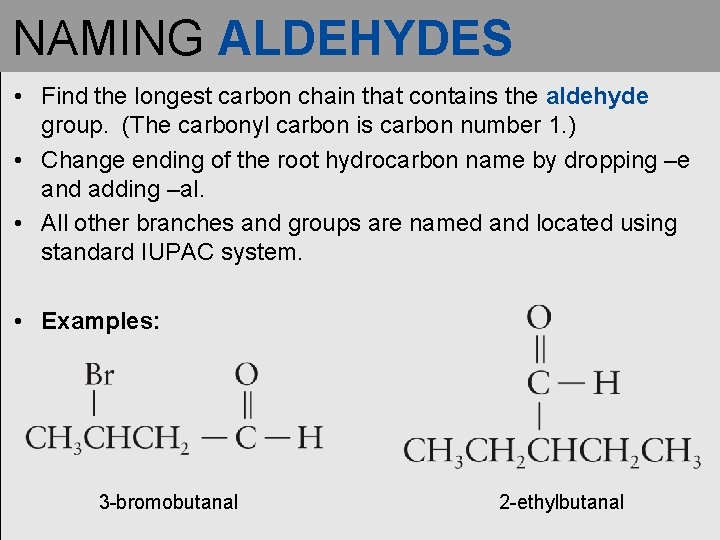
NAMING ALDEHYDES • Find the longest carbon chain that contains the aldehyde group. (The carbonyl carbon is carbon number 1. ) • Change ending of the root hydrocarbon name by dropping –e and adding –al. • All other branches and groups are named and located using standard IUPAC system. • Examples: 3 -bromobutanal 2 -ethylbutanal

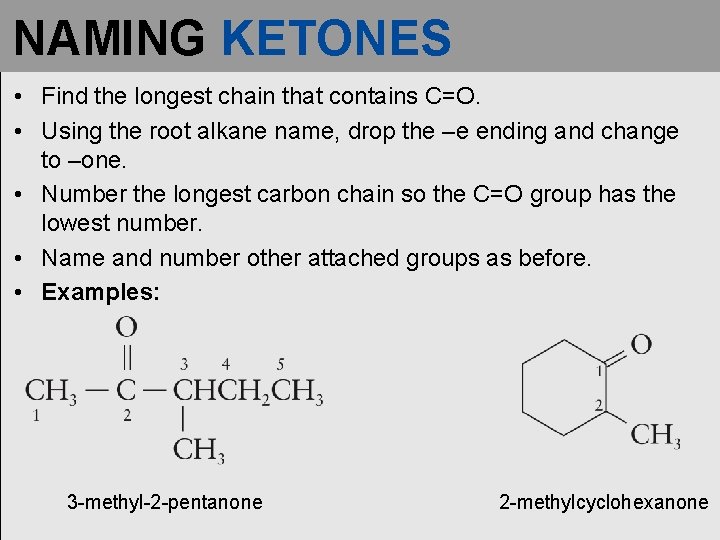
NAMING KETONES • Find the longest chain that contains C=O. • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other attached groups as before. • Examples: 3 -methyl-2 -pentanone 2 -methylcyclohexanone

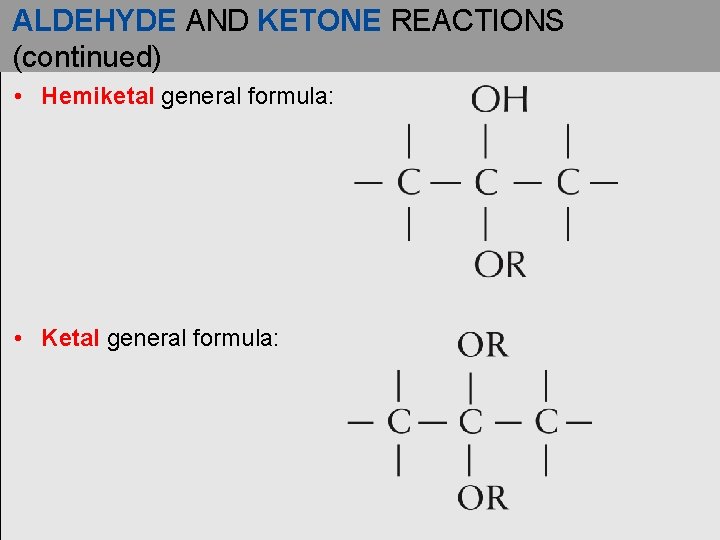
ALDEHYDE AND KETONE REACTIONS (continued) • Hemiketal general formula: • Ketal general formula:

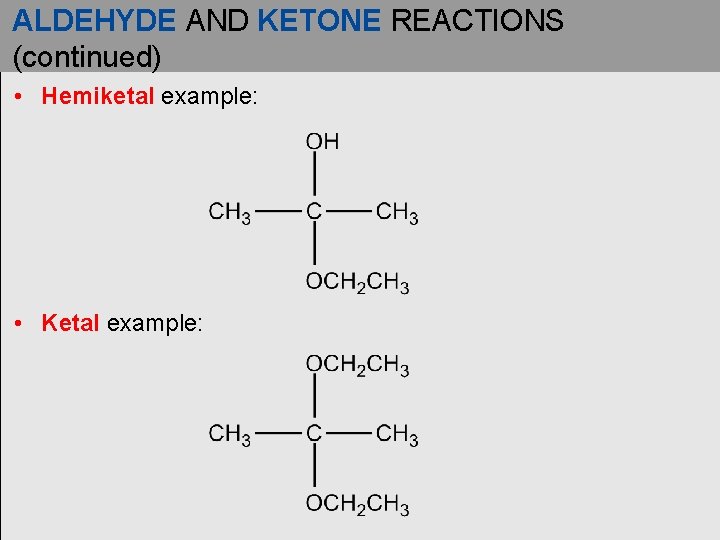
ALDEHYDE AND KETONE REACTIONS (continued) • Hemiketal example: • Ketal example:

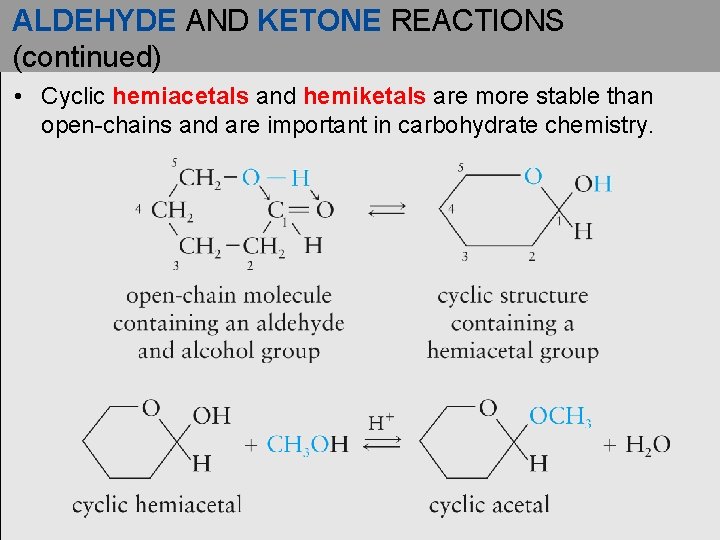
ALDEHYDE AND KETONE REACTIONS (continued) • Cyclic hemiacetals and hemiketals are more stable than open-chains and are important in carbohydrate chemistry.

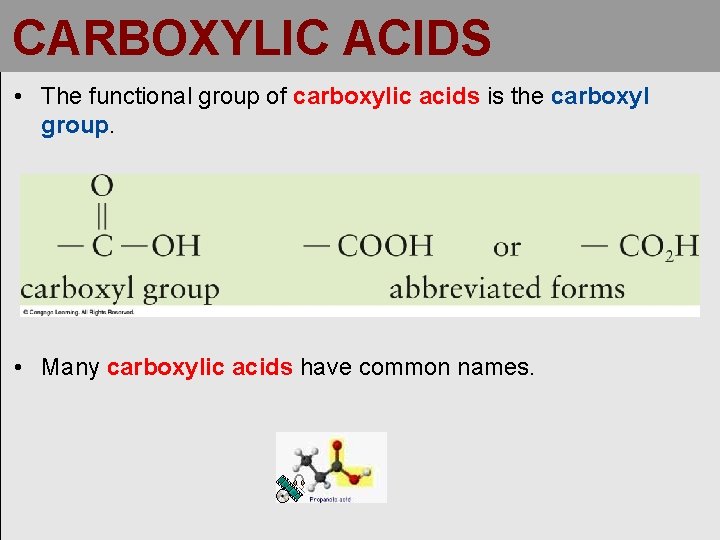
CARBOXYLIC ACIDS • The functional group of carboxylic acids is the carboxyl group. • Many carboxylic acids have common names.

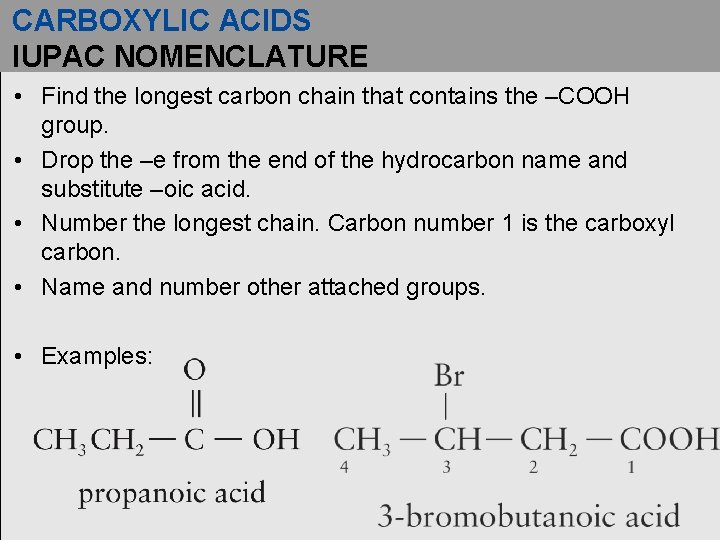
CARBOXYLIC ACIDS IUPAC NOMENCLATURE • Find the longest carbon chain that contains the –COOH group. • Drop the –e from the end of the hydrocarbon name and substitute –oic acid. • Number the longest chain. Carbon number 1 is the carboxyl carbon. • Name and number other attached groups. • Examples:

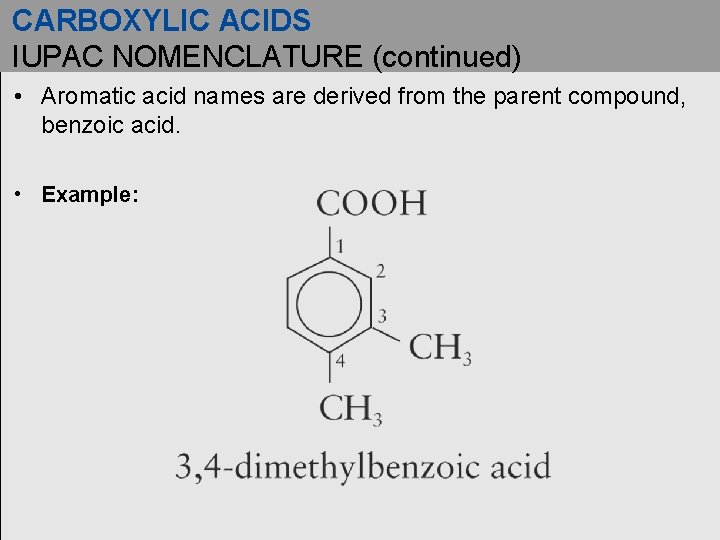
CARBOXYLIC ACIDS IUPAC NOMENCLATURE (continued) • Aromatic acid names are derived from the parent compound, benzoic acid. • Example:

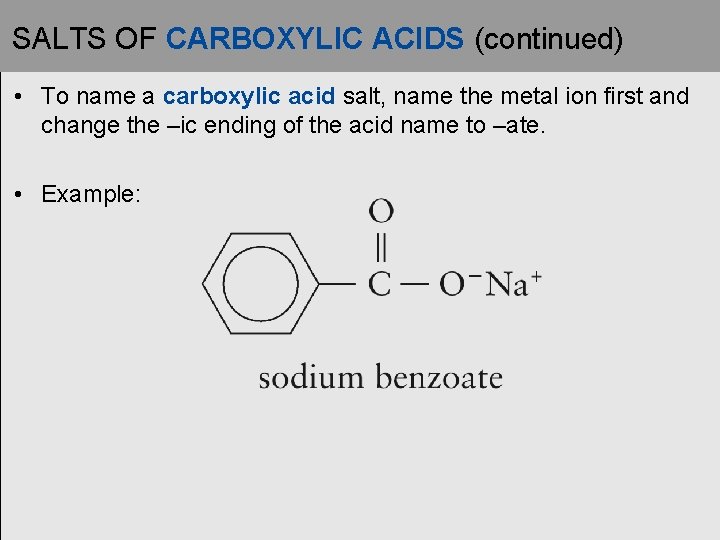
SALTS OF CARBOXYLIC ACIDS (continued) • To name a carboxylic acid salt, name the metal ion first and change the –ic ending of the acid name to –ate. • Example:

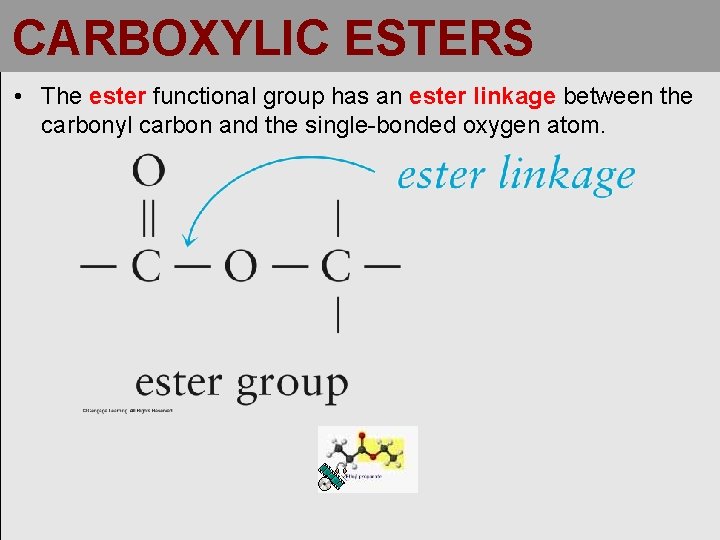
CARBOXYLIC ESTERS • The ester functional group has an ester linkage between the carbonyl carbon and the single-bonded oxygen atom.

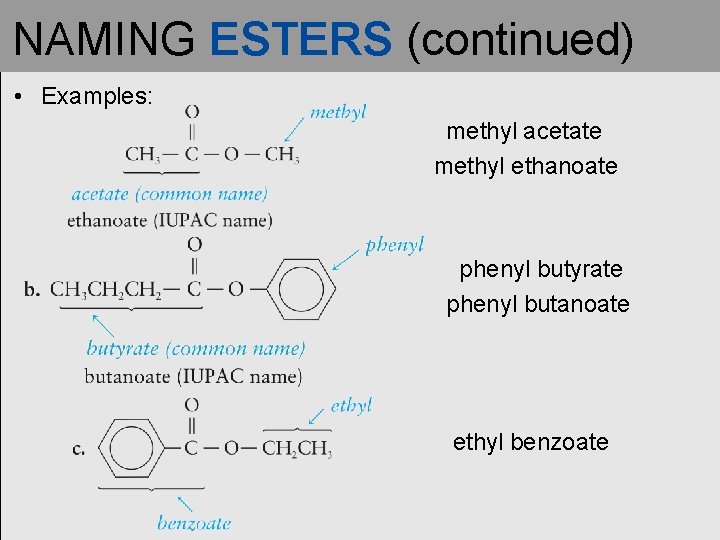
NAMING ESTERS • The first word of the name of an ester is the name of the alkyl or aromatic group (R') contributed by the alcohol. • The second word is the carboxylic acid name, with the –ic acid ending changed to –ate.

NAMING ESTERS (continued) • Examples: methyl acetate methyl ethanoate phenyl butyrate phenyl butanoate ethyl benzoate

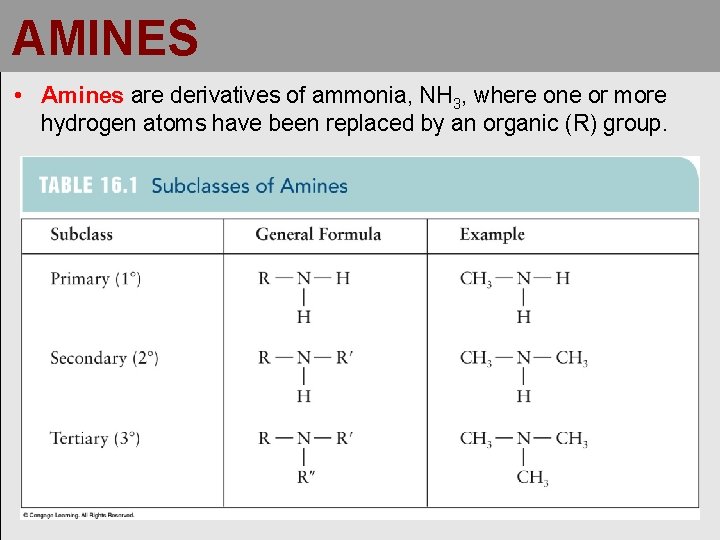
AMINES • Amines are derivatives of ammonia, NH 3, where one or more hydrogen atoms have been replaced by an organic (R) group.

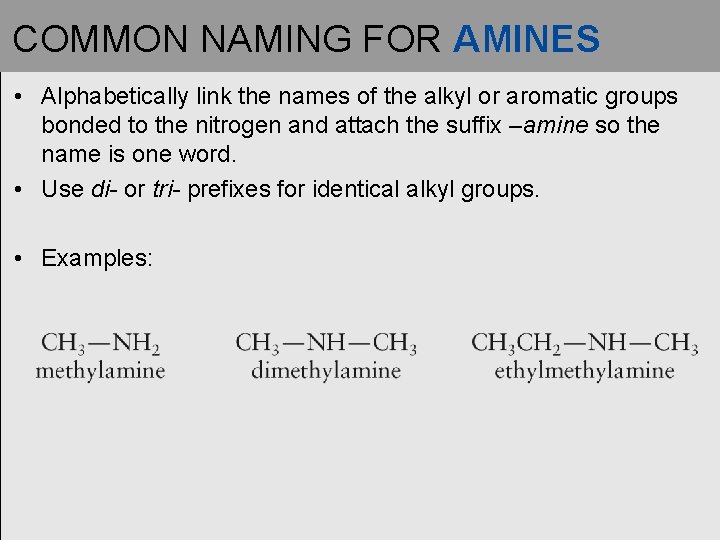
COMMON NAMING FOR AMINES • Alphabetically link the names of the alkyl or aromatic groups bonded to the nitrogen and attach the suffix –amine so the name is one word. • Use di- or tri- prefixes for identical alkyl groups. • Examples:

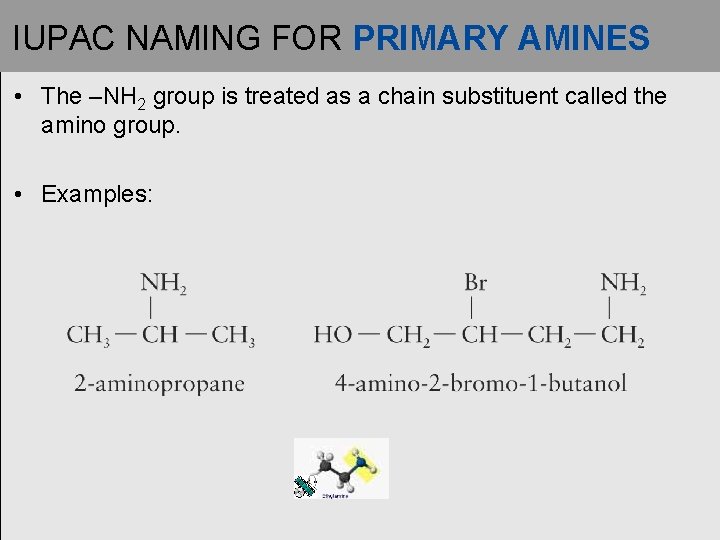
IUPAC NAMING FOR PRIMARY AMINES • The –NH 2 group is treated as a chain substituent called the amino group. • Examples:

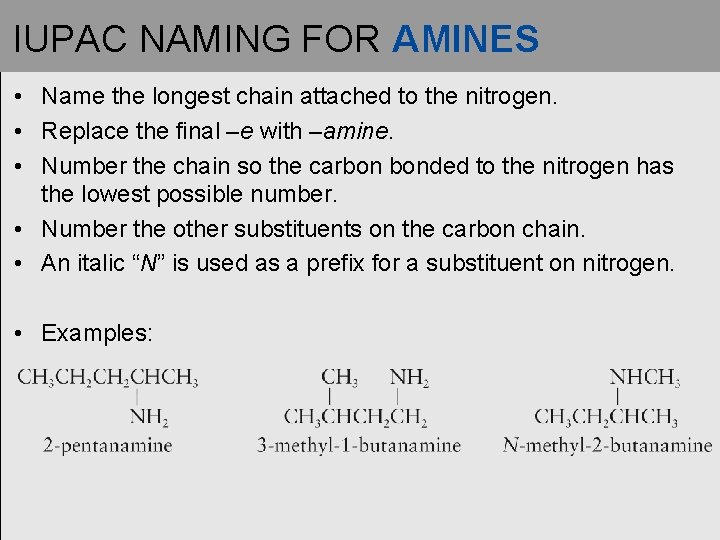
IUPAC NAMING FOR AMINES • Name the longest chain attached to the nitrogen. • Replace the final –e with –amine. • Number the chain so the carbon bonded to the nitrogen has the lowest possible number. • Number the other substituents on the carbon chain. • An italic “N” is used as a prefix for a substituent on nitrogen. • Examples:

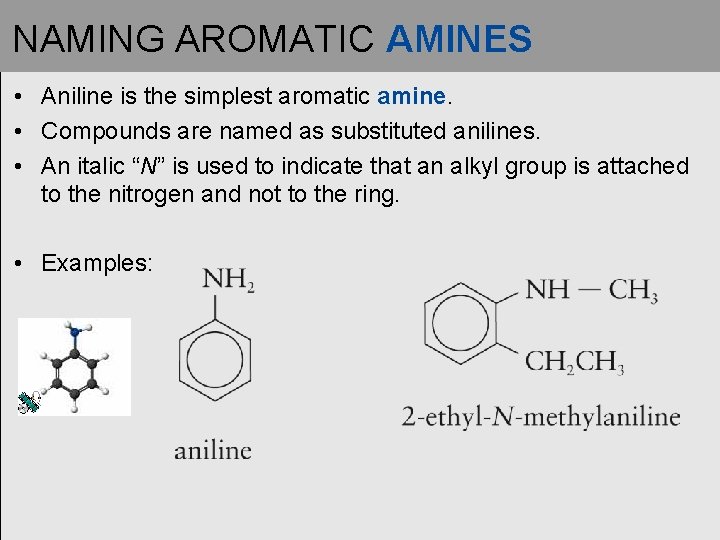
NAMING AROMATIC AMINES • Aniline is the simplest aromatic amine. • Compounds are named as substituted anilines. • An italic “N” is used to indicate that an alkyl group is attached to the nitrogen and not to the ring. • Examples:

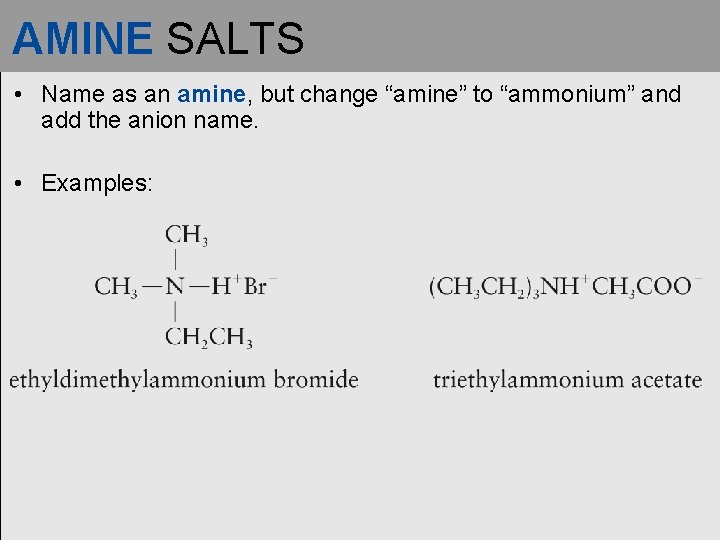
AMINE SALTS • Name as an amine, but change “amine” to “ammonium” and add the anion name. • Examples:

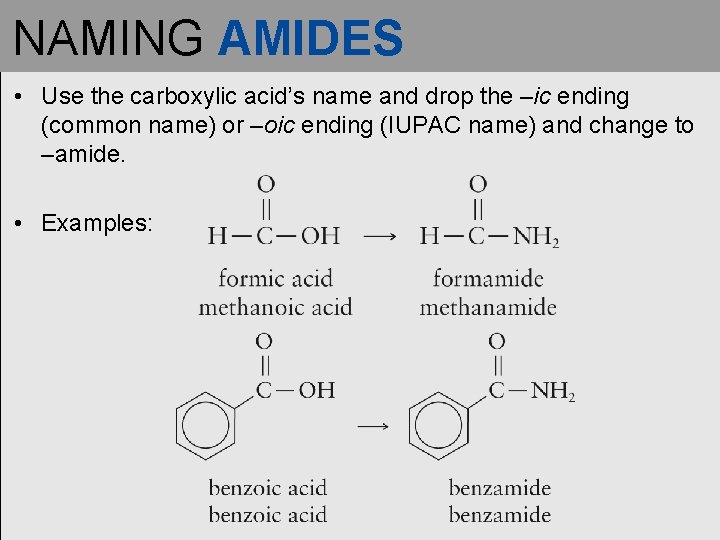
NAMING AMIDES • Use the carboxylic acid’s name and drop the –ic ending (common name) or –oic ending (IUPAC name) and change to –amide. • Examples:

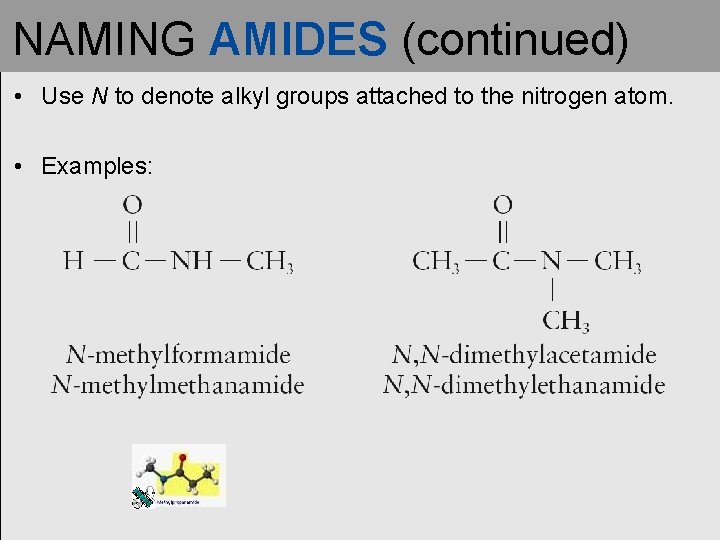
NAMING AMIDES (continued) • Use N to denote alkyl groups attached to the nitrogen atom. • Examples: