Boiling Points Distillations Ethylene glycol HOCH 2 OH

Boiling Points - Distillations Ethylene glycol, HOCH 2 OH, boils at 198 o. C and melts at -13 o C. What happens to the melting point of water if you add antifreeze? Melting point goes down.

Add Antifreeze What happens to the boiling point of water if you add antifreeze? The boiling point goes up.

Mixtures Solids Usually melt low Liquids Usually boil between the two components

Vapor pressure of water vs. temperature Solution boils when the vapor pressure = applied pressure

Add Salt If you add salt, Na. Cl, to water what happens to the melting point? Impurities depress the melting point so it goes down.

Salt Boiling Point If you add salt, Na. Cl, to water what happens to the boiling point? The boiling point goes up.
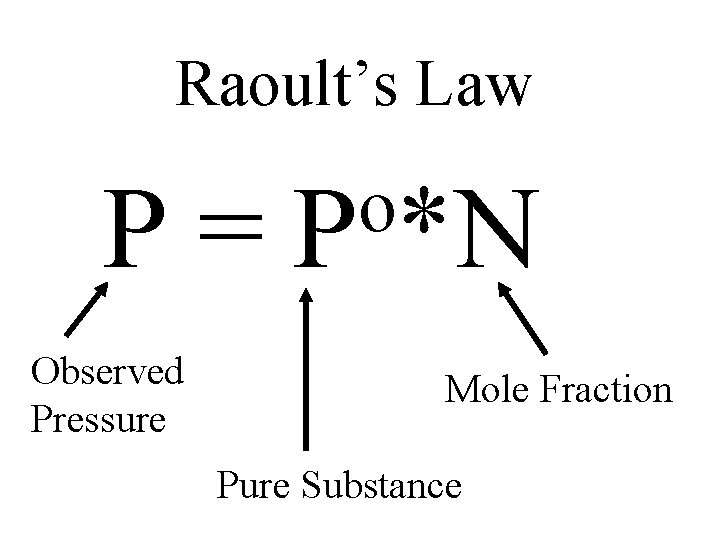
Raoult’s Law P= Observed Pressure o P *N Mole Fraction Pure Substance

Two Volatile Liquids

Two Volatile Liquids P a = P oa * N a Pb = o P b * Nb Mixture boils when Pa + Pb = Papplied
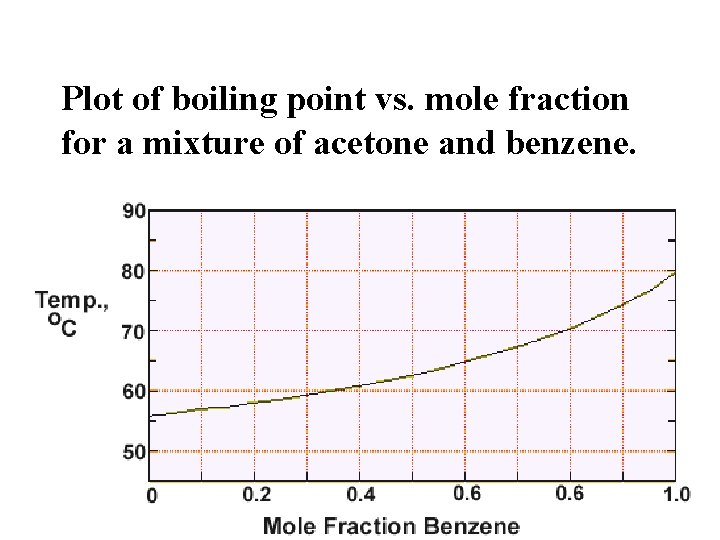
Boiling point vs. mole fraction Plot of boiling point vs. mole fraction for a mixture of acetone and benzene.
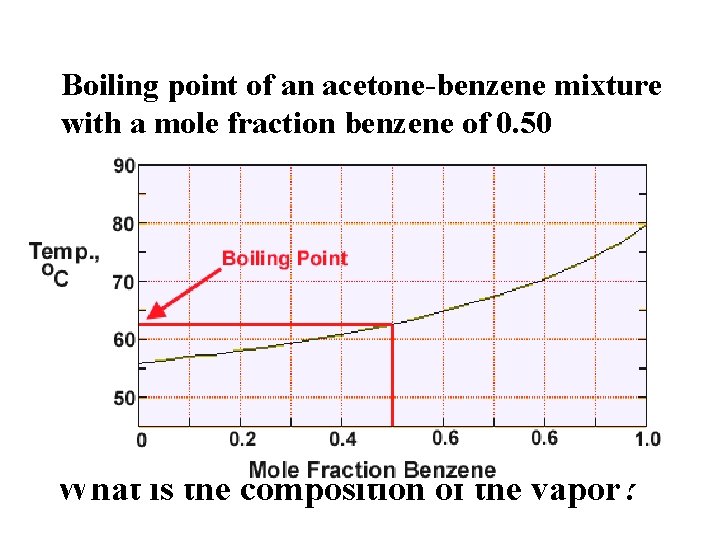
Boiling point vs. mole fraction Boiling point of an acetone-benzene mixture with a mole fraction benzene of 0. 50 What is the composition of the vapor?

Vapor richer in the lower boiling component

Fractional Distillation
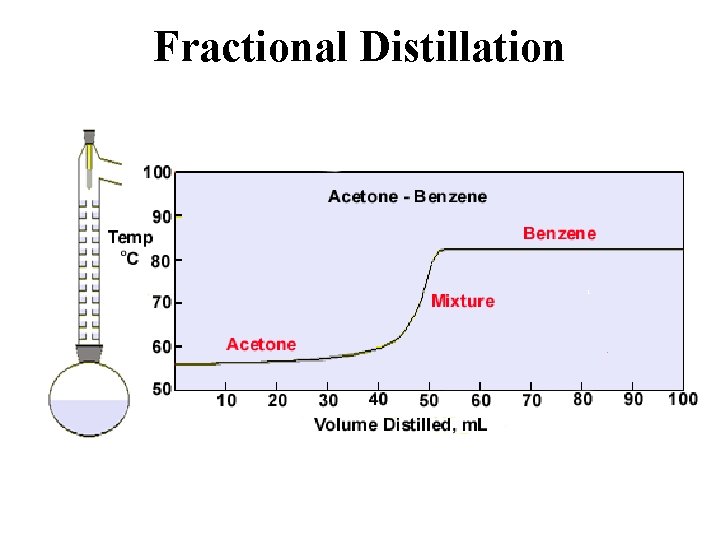
Fractional Distillation

Packed Column HETP = 1. 5 cm

Vigreux Column HETP = 10 cm

Which column has the greater holdup? Packed because it has the larger surface area.

Separations Which column would you use to separate a mixture of pentane, b. p. 36 o and octane, b. p. 99 o? Vigreux

Separations Which column would you use to separate a mixture of methyl alcohol, b. p. 65 o and water, b. p. 100 o ? Packed

Experiment Separate mixture of methanol and water. Plot volume distilled vs. temperature.

Setup

CH 3 OH Methyl alcohol is toxic!

Standard Taper Joints 14/20 14 mm 20 mm

Grease joints to prevent sticking.

Thermowell

Plug thermowell into the variable transformer

Put boiling chips in bottom of flask Boiling Chips

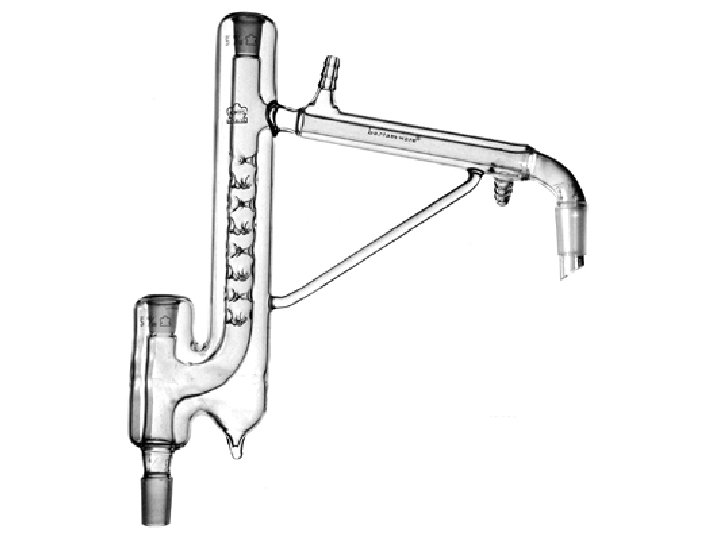
Distilling Column

Thermometer Adapter

Digital Thermometer

Probe for digital thermometer
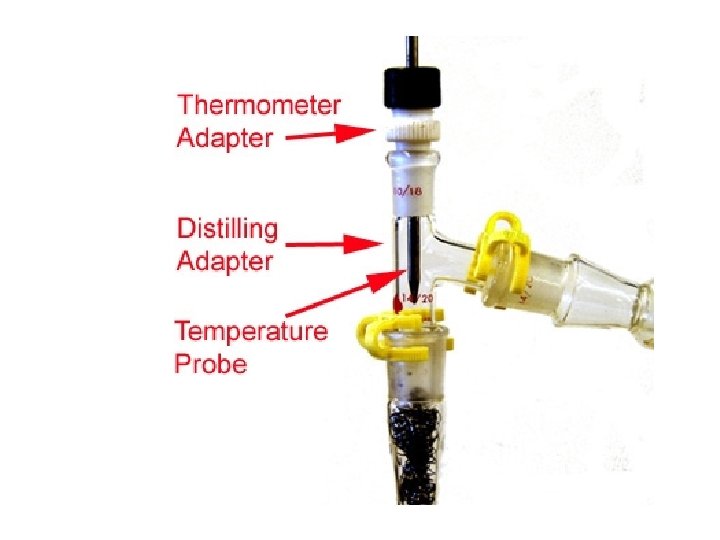
Digital Thermometer
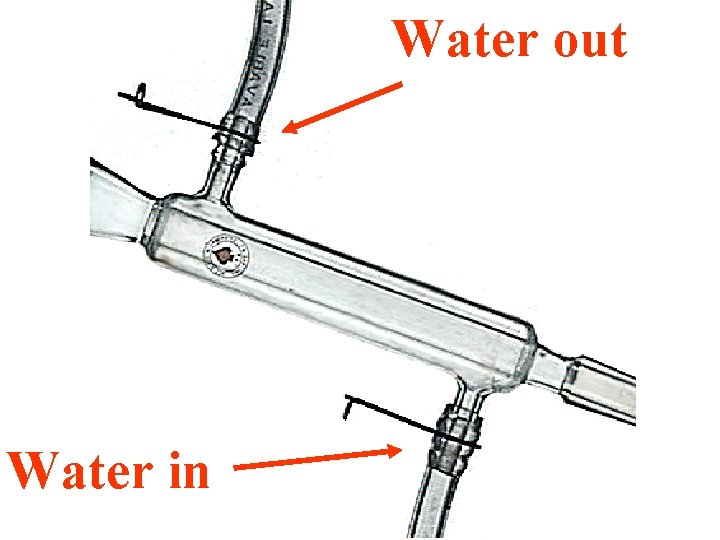
Water out Water in

Clamps
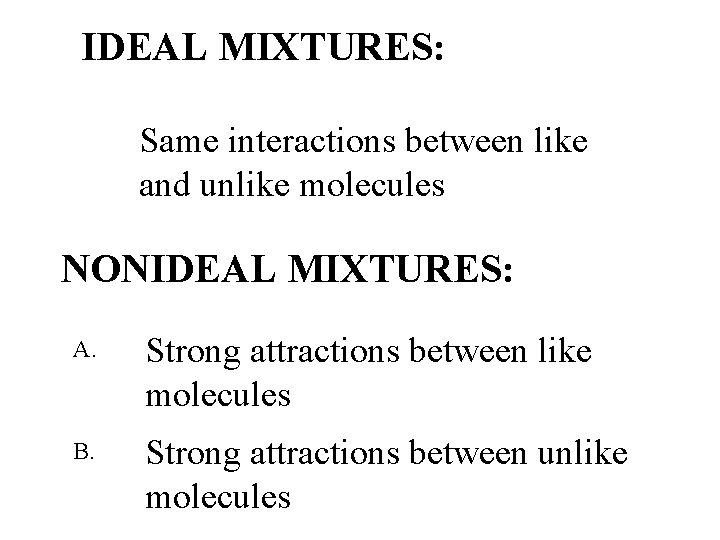
IDEAL MIXTURES: Same interactions between like and unlike molecules NONIDEAL MIXTURES: A. Strong attractions between like molecules B. Strong attractions between unlike molecules
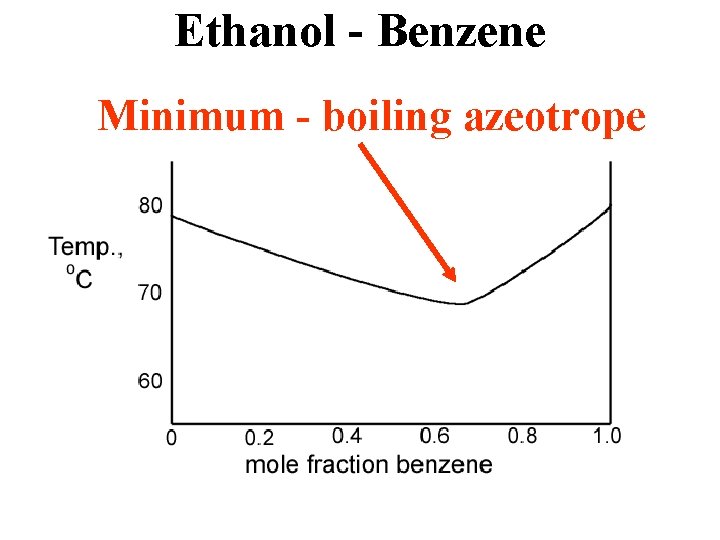
Ethanol - Benzene Minimum - boiling azeotrope
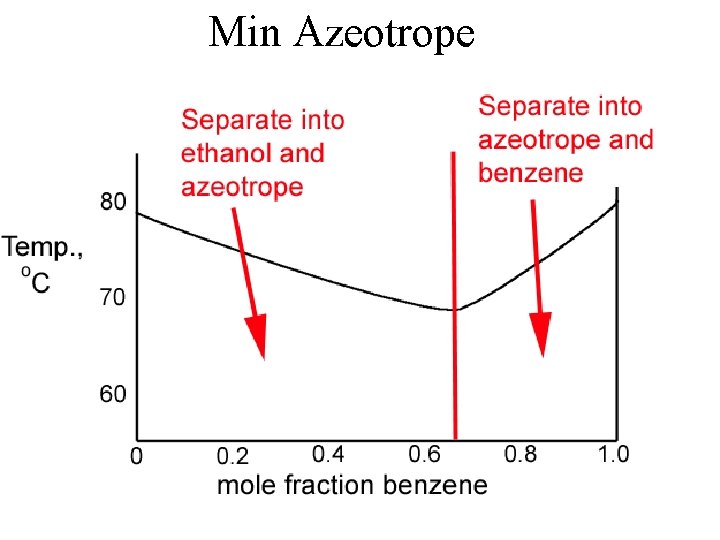
Min Azeotrope

Acetone - Chloroform Maximum-boiling azeotrope
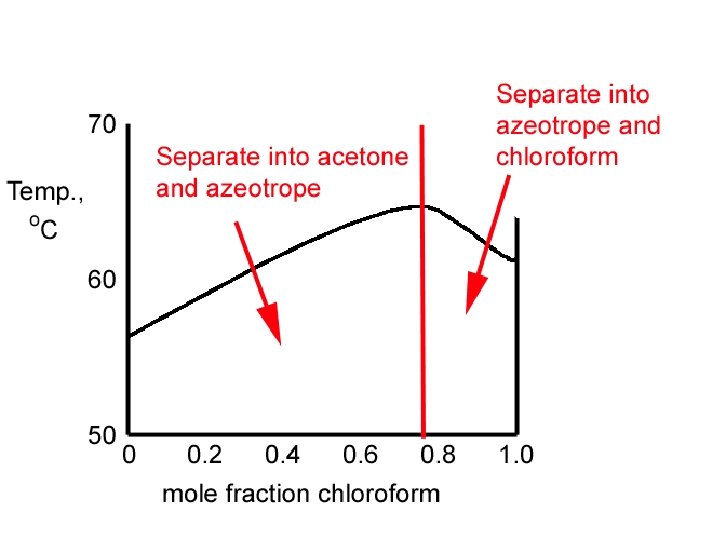
Max Azeotrope

Simple Distillation


- Slides: 43