OCR 2016 A Level Chemistry A Topic Exploration











- Slides: 24

© OCR 2016

A Level Chemistry A Topic Exploration Pack – Organic Synthesis Two-step synthesis © OCR 2016

To begin… • The easiest way to begin thinking about synthesis is to start with one-step reactions. • Identify the functional groups in the reactants and products. • Find them on the synthesis map. • Find the easiest route between them. © OCR 2016

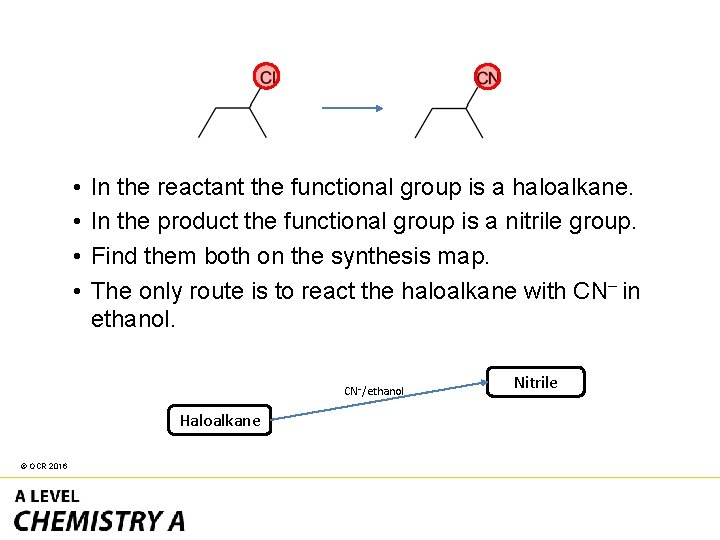
• • In the reactant the functional group is a haloalkane. In the product the functional group is a nitrile group. Find them both on the synthesis map. The only route is to react the haloalkane with CN– in ethanol. CN–/ethanol Haloalkane © OCR 2016 Nitrile

• • In the reactant the functional group is an acyl chloride. In the product the functional group is a 2 o amide. Find them both on the synthesis map. The only route is to react the acyl chloride with a primary amine, but now determine which! Acyl chloride primary amine © OCR 2016 2 o Amide

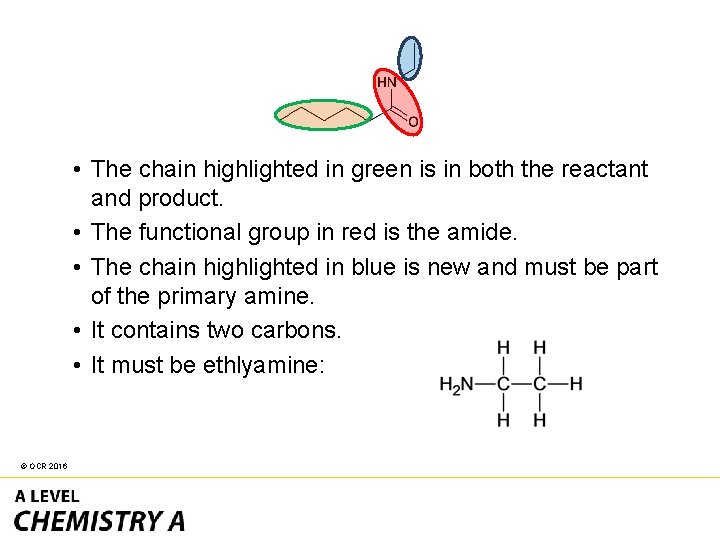
• The chain highlighted in green is in both the reactant and product. • The functional group in red is the amide. • The chain highlighted in blue is new and must be part of the primary amine. • It contains two carbons. • It must be ethlyamine: © OCR 2016

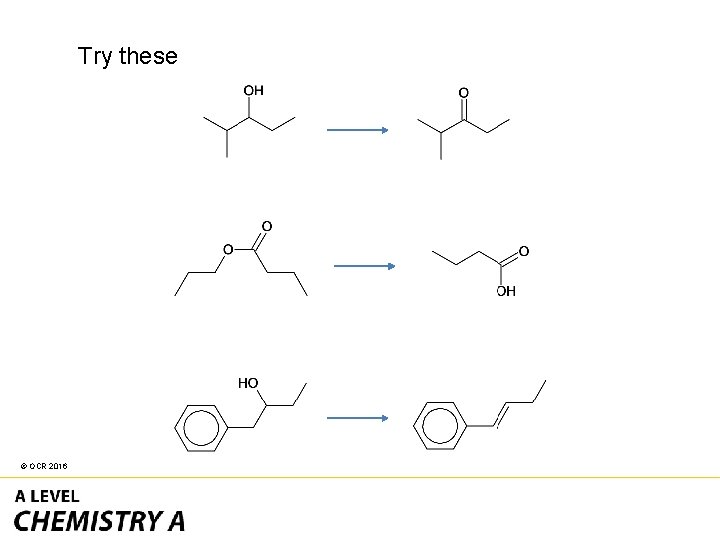
Try these © OCR 2016

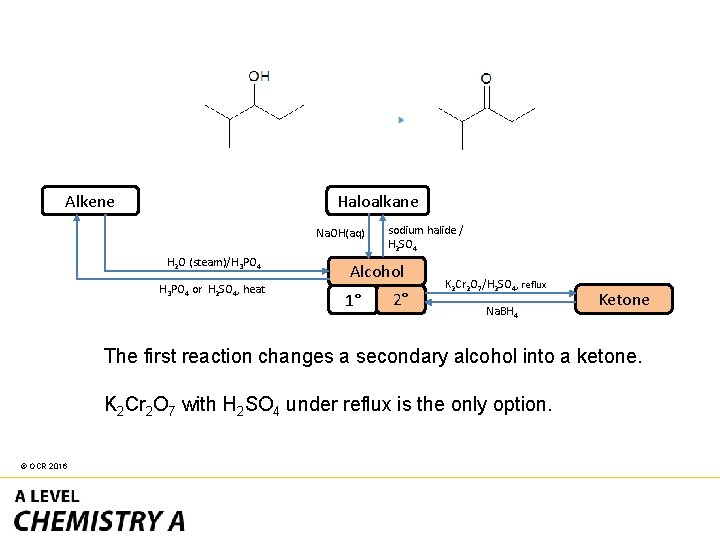
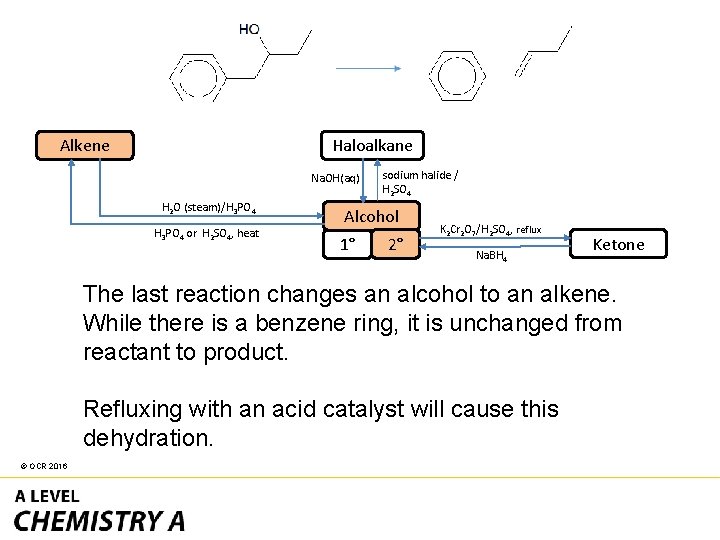
Haloalkane Alkene Na. OH(aq) H 2 O (steam)/H 3 PO 4 or H 2 SO 4, heat sodium halide / H 2 SO 4 Alcohol 1° 2° K 2 Cr 2 O 7/H 2 SO 4, reflux Na. BH 4 Ketone The first reaction changes a secondary alcohol into a ketone. K 2 Cr 2 O 7 with H 2 SO 4 under reflux is the only option. © OCR 2016

Alcohol The second reaction changes an ester into a carboxylic acid. Refluxing with dilute acid is the only option. The alcohol propanol will also be formed. carboxylic acid/ conc. H 2 SO 4 OR acid anhydride Ester OH– heat Carboxylate © OCR 2016 alcohol/conc. H 2 SO 4 dilute acid, heat Carboxylic acid alcohol Acyl chloride

Haloalkane Alkene Na. OH(aq) H 2 O (steam)/H 3 PO 4 or H 2 SO 4, heat sodium halide / H 2 SO 4 Alcohol 1° 2° K 2 Cr 2 O 7/H 2 SO 4, reflux Na. BH 4 Ketone The last reaction changes an alcohol to an alkene. While there is a benzene ring, it is unchanged from reactant to product. Refluxing with an acid catalyst will cause this dehydration. © OCR 2016

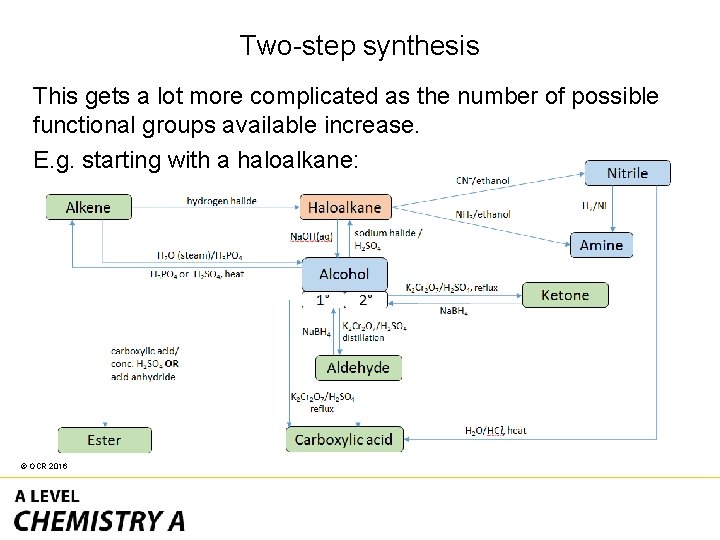
Two-step synthesis This gets a lot more complicated as the number of possible functional groups available increase. E. g. starting with a haloalkane: © OCR 2016

Two-step synthesis • In one step a haloalkane can change to three other functional groups. • In two steps this increases to eight. © OCR 2016

Two-step synthesis To help reduce the number of options you can remove certain possibilities quickly with the following questions. • Are there any benzene rings or phenol groups which change? - See the aromatic / phenol reaction pathways map. • Is there the same number of carbon atoms in reactant and product? - This suggests addition of a nitrile or formation of an ester/amide. • Are there other elements in the main chain which change? - Look for esters/amides. © OCR 2016

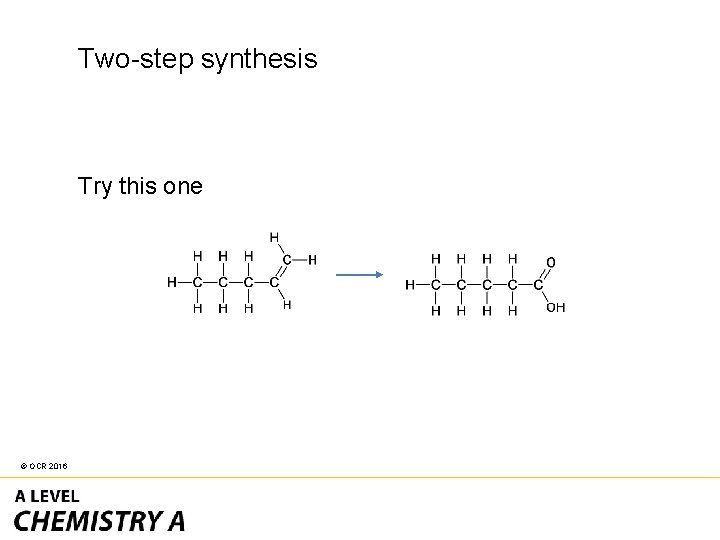
Two-step synthesis Try this one © OCR 2016

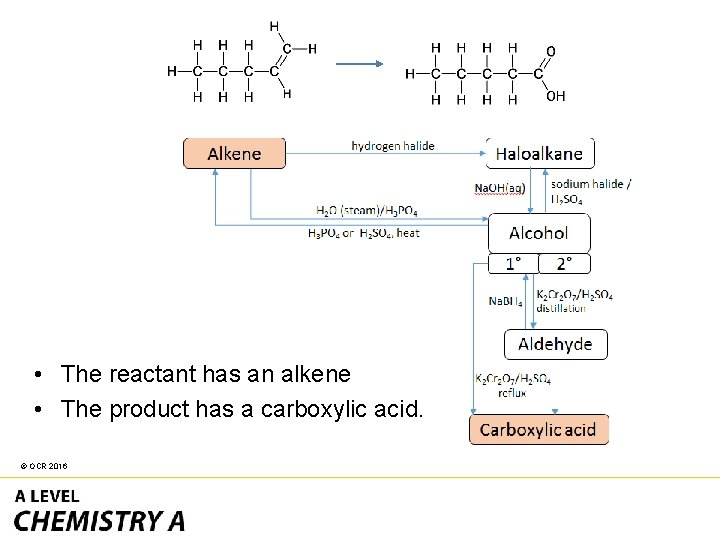
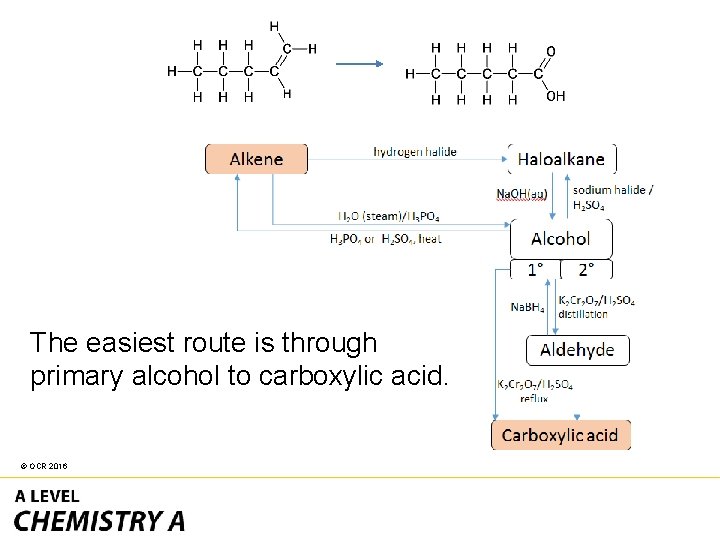
• The reactant has an alkene • The product has a carboxylic acid. © OCR 2016

The easiest route is through primary alcohol to carboxylic acid. © OCR 2016

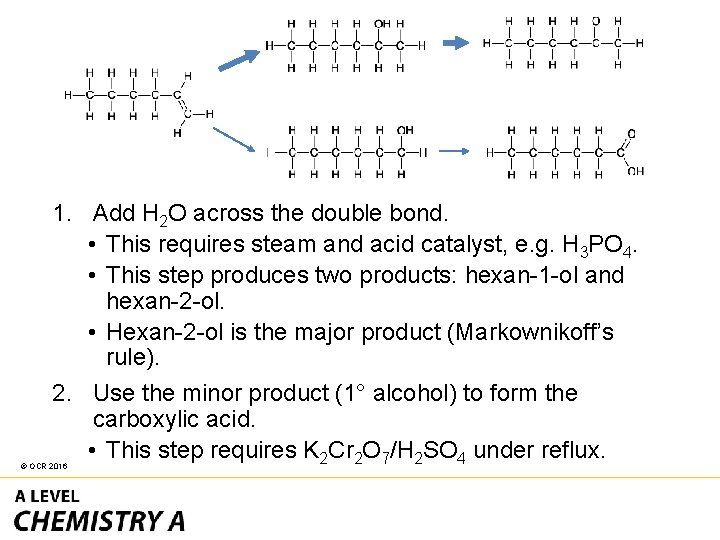
1. Add H 2 O across the double bond. • This requires steam and acid catalyst, e. g. H 3 PO 4. • This step produces two products: hexan-1 -ol and hexan-2 -ol. • Hexan-2 -ol is the major product (Markownikoff’s rule). 2. Use the minor product (1° alcohol) to form the carboxylic acid. • This step requires K 2 Cr 2 O 7/H 2 SO 4 under reflux. © OCR 2016

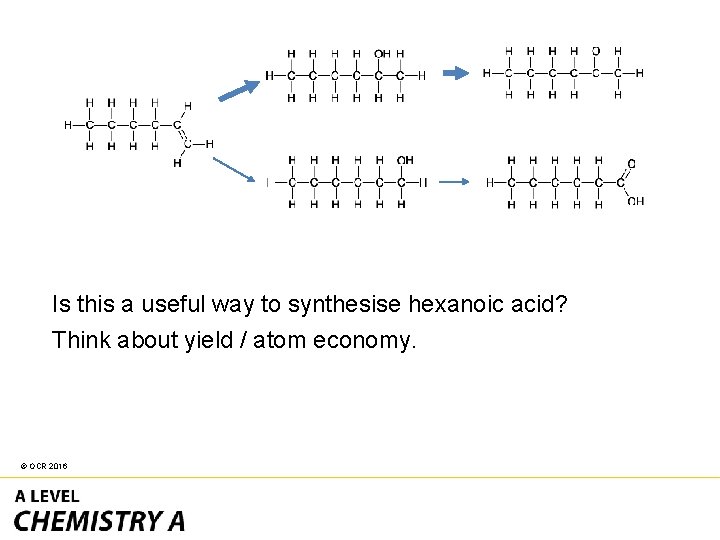
Is this a useful way to synthesise hexanoic acid? Think about yield / atom economy. © OCR 2016

Two-step synthesis Try this one. © OCR 2016

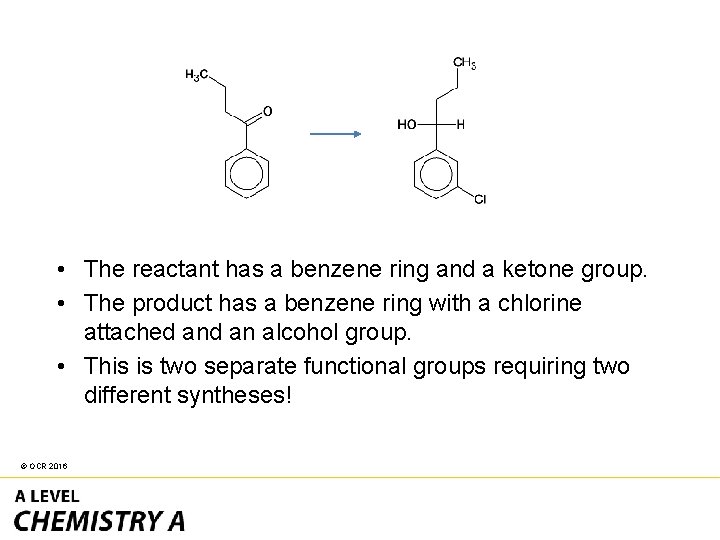
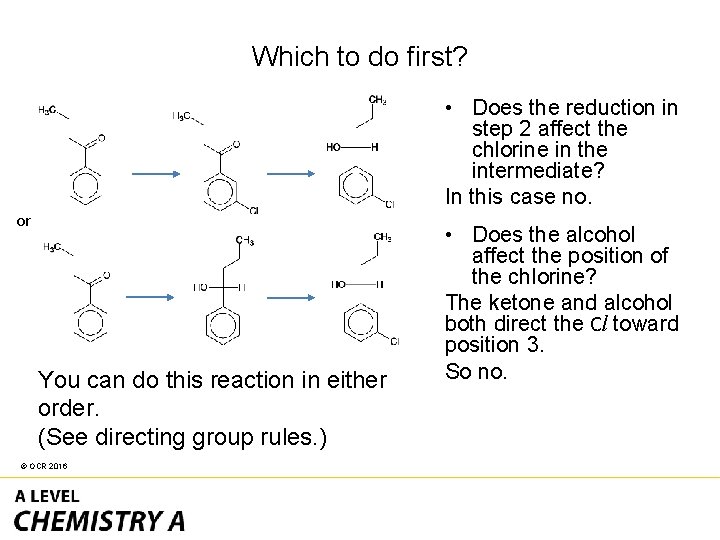
• The reactant has a benzene ring and a ketone group. • The product has a benzene ring with a chlorine attached an alcohol group. • This is two separate functional groups requiring two different syntheses! © OCR 2016

• The two reactions are straightforward: – Na. BH 4 to reduce the ketone – Cl 2 and a halogen carrier to add chlorine to the benzene ring. • In a question like this it is important to think about which reaction to do first. © OCR 2016

Which to do first? • Does the reduction in step 2 affect the chlorine in the intermediate? In this case no. or You can do this reaction in either order. (See directing group rules. ) © OCR 2016 • Does the alcohol affect the position of the chlorine? The ketone and alcohol both direct the Cl toward position 3. So no.

Next Steps • The best way improve is to practise. • The process becomes more difficult when you do not have the synthesis map in front of you. Like in the exam. • As you improve try to do more without using the map, or use the blank map which does not show the reagents needed. • Have a go at the two-step synthesis sheet. • Then, start thinking about multi-step syntheses. © OCR 2016

Thank you for using this OCR resource Other OCR resources are available at www. ocr. org. uk To give us feedback on, or ideas about the OCR resources you have used, email resourcesfeedback@ocr. org. uk OCR Resources: the small print OCR’s resources are provided to support the teaching of OCR specifications, but in no way constitute an endorsed teaching method that is required by the Board, and the decision to use them lies with the individual teacher. Whilst every effort is made to ensure the accuracy of the content, OCR cannot be held responsible for any errors or omissions within these resources. © OCR 2016 - This resource may be freely copied and distributed, as long as the OCR logo and this message remain intact and OCR is acknowledged as the originator of this work. © OCR 2016