Organic Chemistry Chapter 1 Introduction to organic chemistry





- Slides: 29

Organic Chemistry Chapter 1 Introduction to organic chemistry 1

Introduction to organic chemistry • History: • Earlier in the eighteenth century it was believed that in order to produce compounds by living cells, a “vital force” was needed. • These compounds were classified as organic compounds. • A german chemist, Friedrich Woehler in 1828 has shown this belief was incorrect 2

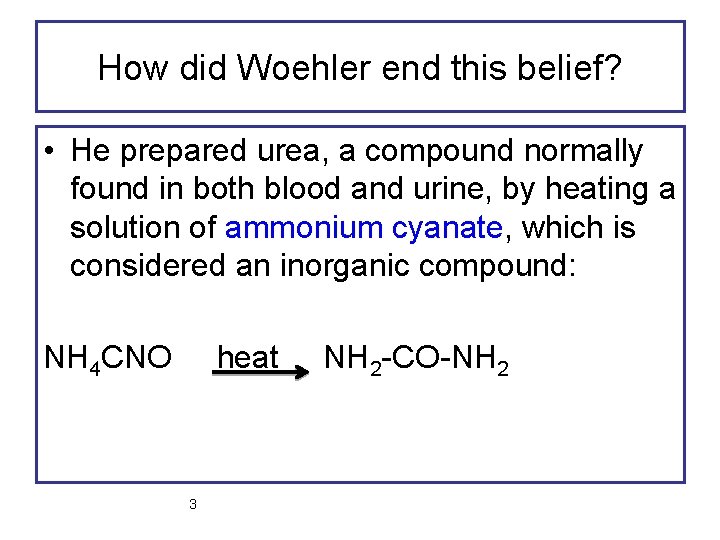
How did Woehler end this belief? • He prepared urea, a compound normally found in both blood and urine, by heating a solution of ammonium cyanate, which is considered an inorganic compound: NH 4 CNO heat 3 NH 2 -CO-NH 2

• Later on many other organic compounds were produced in the laboratory. • This has led to the subdivision of chemistry into 2 parts: • Organic • Inorganic 4

Organic Chemistry • Definition: With the simplest definition, organic chemistry is known as the chemistry of carbon compounds. 5

What makes Carbon so special to give it a whole branch of chemistry and place all other elements in the other branch? 1. Carbon is unique in that it forms covalent bonds to other carbon atoms as well as to other elements. 2. There are millions of organic compounds known today 6

Importance of organic chemistry • It’s importance comes from its association with all living matter in both plants and animals Examples: Ø Proteins, carbohydrates, lipids, hormones, vitamins, enzymes, and many other drugs are organic compounds. 7

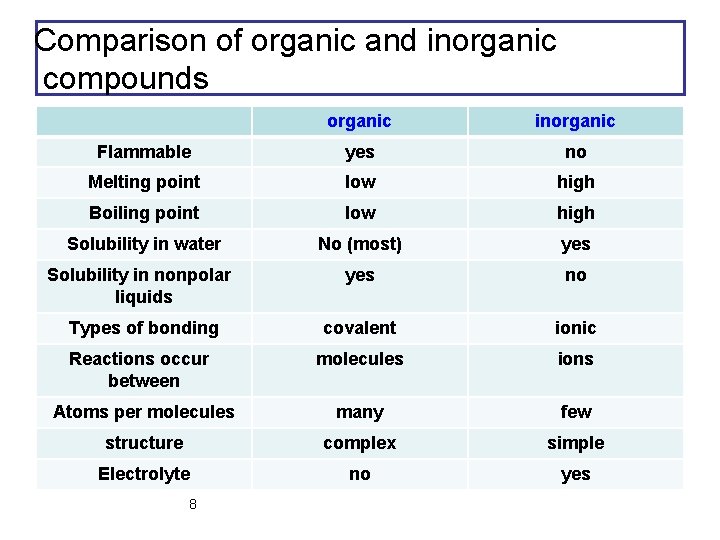
Comparison of organic and inorganic compounds organic inorganic Flammable yes no Melting point low high Boiling point low high Solubility in water No (most) yes Solubility in nonpolar liquids yes no Types of bonding covalent ionic Reactions occur between molecules ions Atoms per molecules many few structure complex simple Electrolyte no yes 8

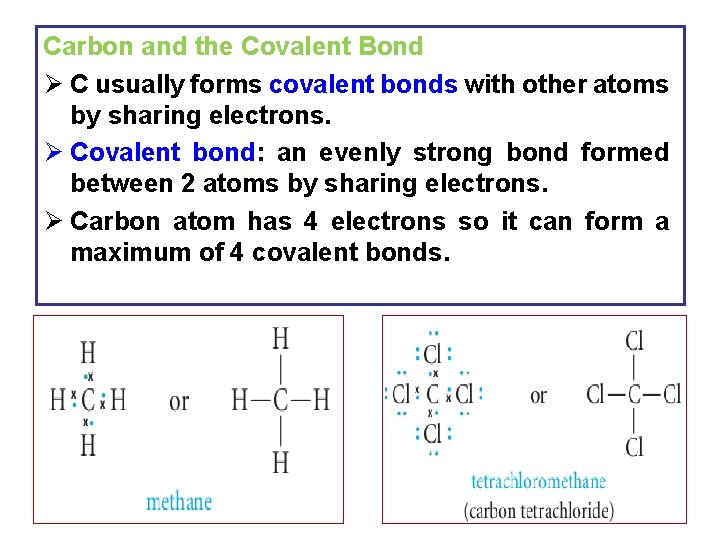
Carbon and the Covalent Bond Ø C usually forms covalent bonds with other atoms by sharing electrons. Ø Covalent bond: an evenly strong bond formed between 2 atoms by sharing electrons. Ø Carbon atom has 4 electrons so it can form a maximum of 4 covalent bonds. 9

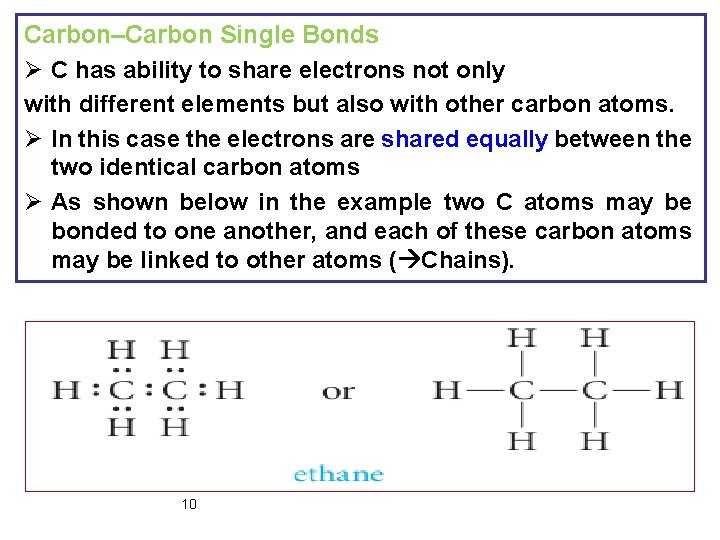
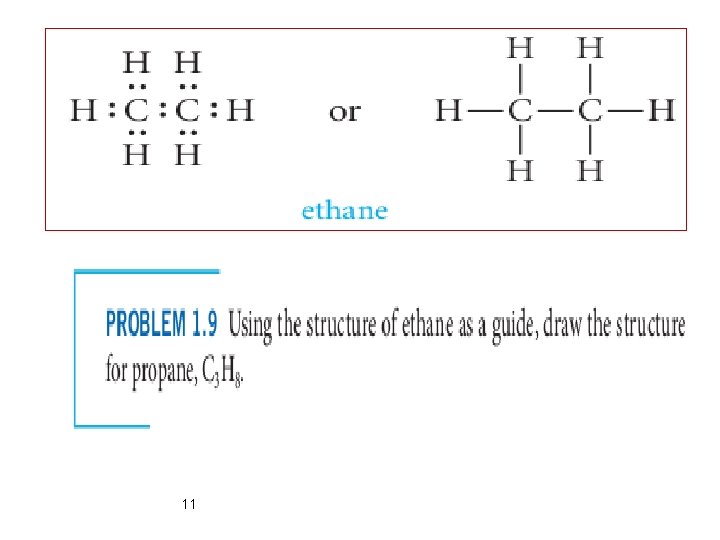
Carbon–Carbon Single Bonds Ø C has ability to share electrons not only with different elements but also with other carbon atoms. Ø In this case the electrons are shared equally between the two identical carbon atoms Ø As shown below in the example two C atoms may be bonded to one another, and each of these carbon atoms may be linked to other atoms ( Chains). 10

11

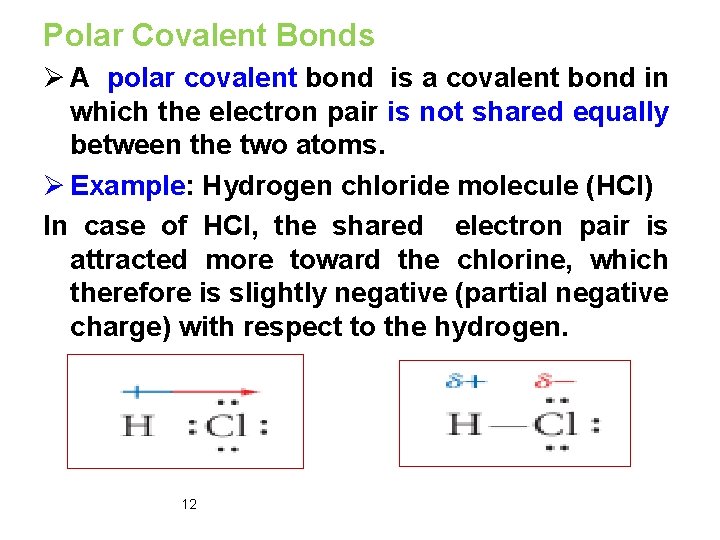
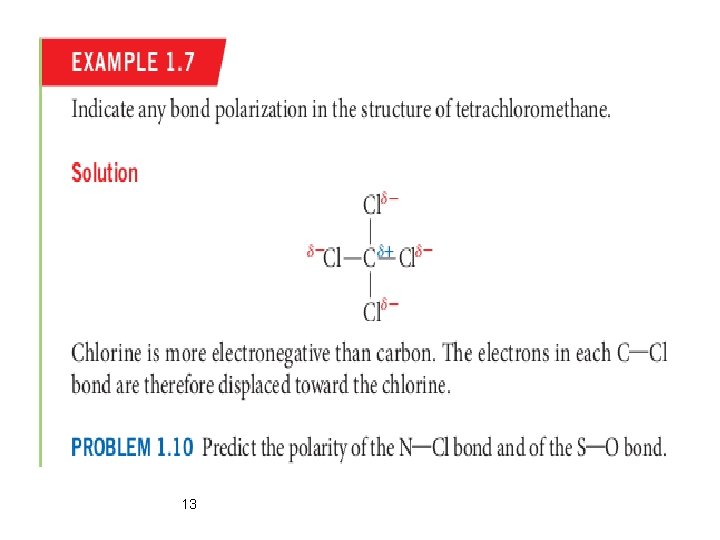
Polar Covalent Bonds Ø A polar covalent bond is a covalent bond in which the electron pair is not shared equally between the two atoms. Ø Example: Hydrogen chloride molecule (HCl) In case of HCl, the shared electron pair is attracted more toward the chlorine, which therefore is slightly negative (partial negative charge) with respect to the hydrogen. 12

13

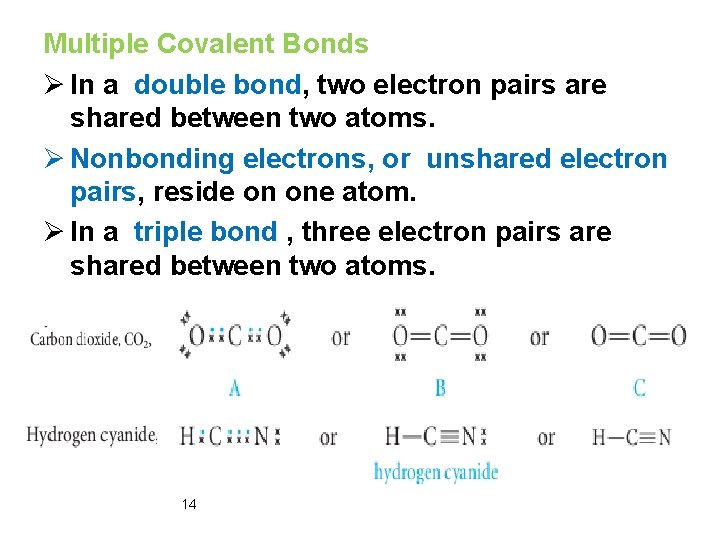
Multiple Covalent Bonds Ø In a double bond, two electron pairs are shared between two atoms. Ø Nonbonding electrons, or unshared electron pairs, reside on one atom. Ø In a triple bond , three electron pairs are shared between two atoms. 14

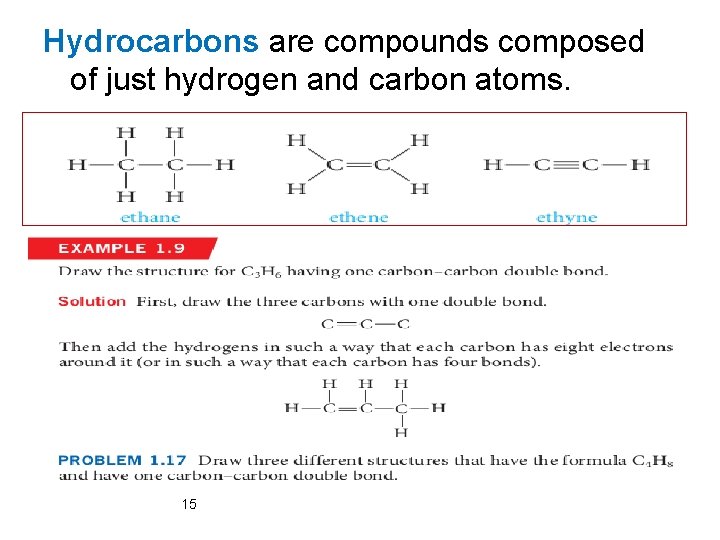
Hydrocarbons are compounds composed of just hydrogen and carbon atoms. 15

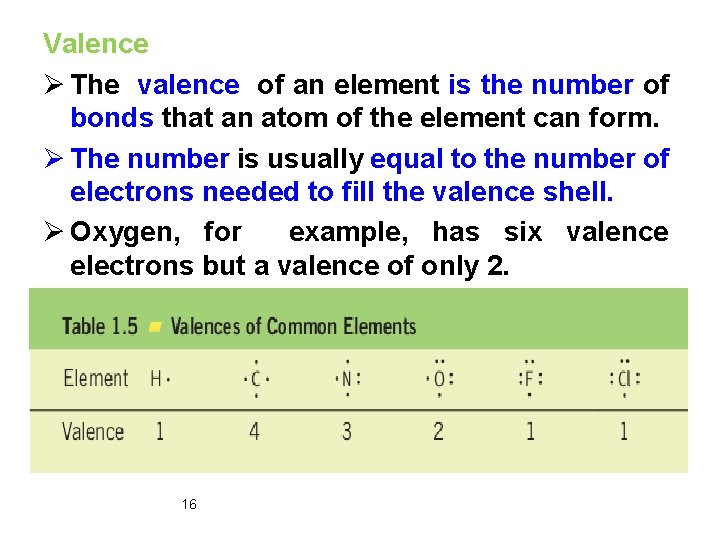
Valence Ø The valence of an element is the number of bonds that an atom of the element can form. Ø The number is usually equal to the number of electrons needed to fill the valence shell. Ø Oxygen, for example, has six valence electrons but a valence of only 2. 16

Isomerism Ø The molecular formula of a substance gives the number of different atoms present. Ø The structural formula indicates how those atoms are arranged. Ø Isomers are molecules with the same number and kinds of atoms but different arrangements of the atoms. 17

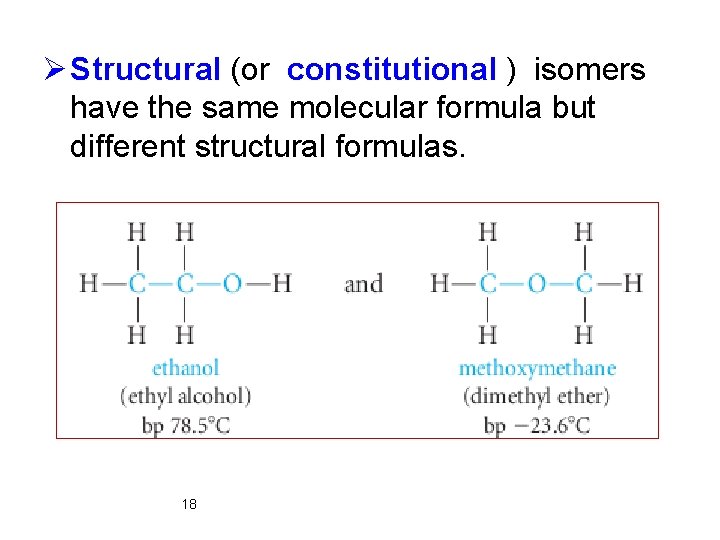
Ø Structural (or constitutional ) isomers have the same molecular formula but different structural formulas. 18

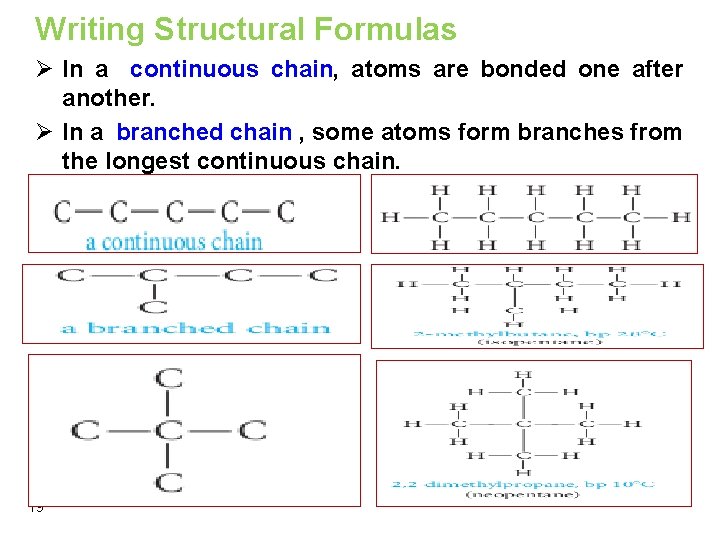
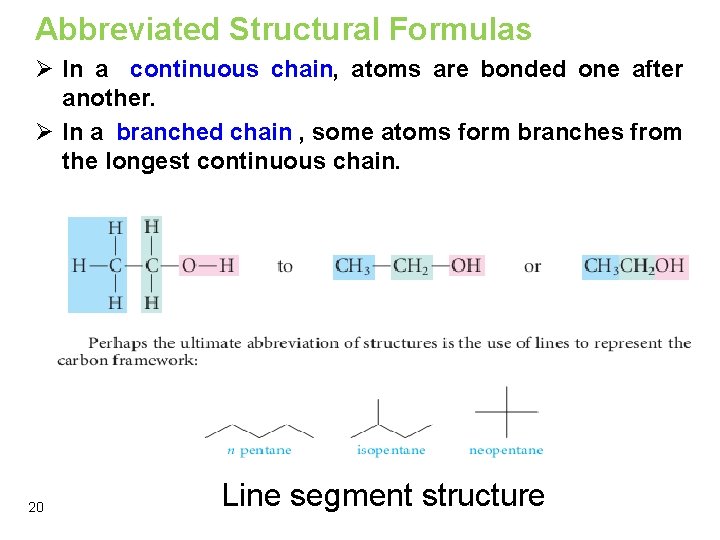
Writing Structural Formulas Ø In a continuous chain, atoms are bonded one after another. Ø In a branched chain , some atoms form branches from the longest continuous chain. 19

Abbreviated Structural Formulas Ø In a continuous chain, atoms are bonded one after another. Ø In a branched chain , some atoms form branches from the longest continuous chain. 20 Line segment structure

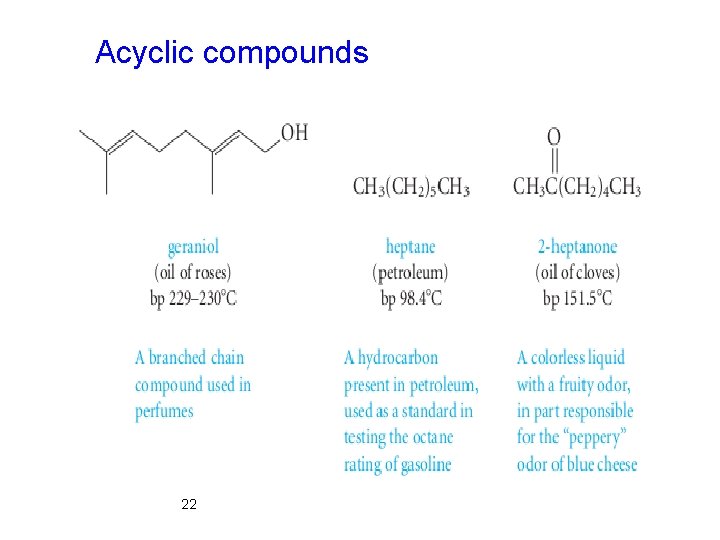
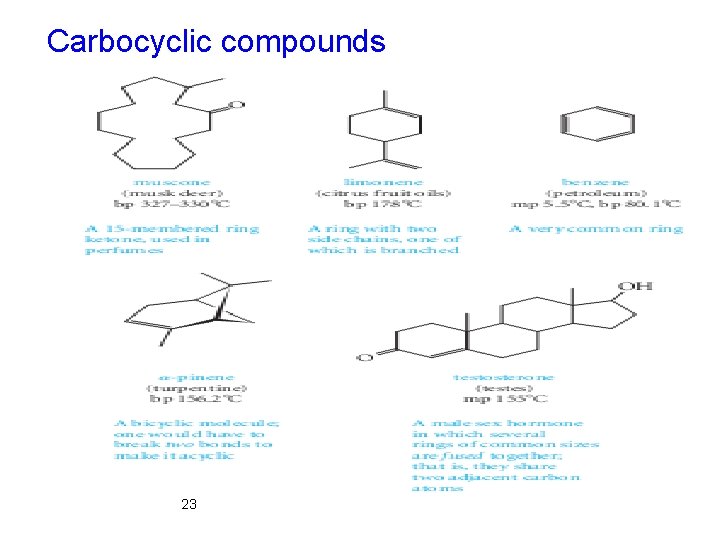
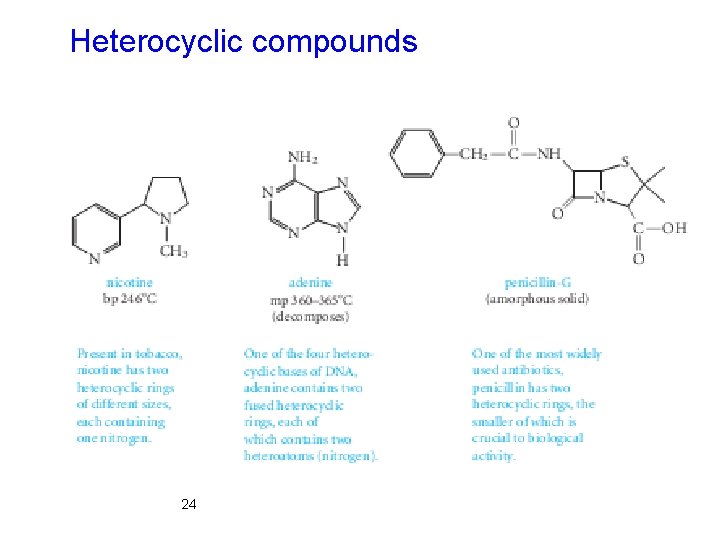
Classification Framework According to Molecular Ø The three main classes of molecular frameworks for organic structures are acyclic, carbocyclic, and heterocyclic compounds. Ø Acyclic compounds contain no rings. Ø Carbocyclic compounds contain rings of carbon atoms. Ø Heterocyclic compounds have rings containing at least one atom that is not carbon. 21

Acyclic compounds 22

Carbocyclic compounds 23

Heterocyclic compounds 24

Allotropic Forms of Carbons • Three forms are know: • Graphite: soft, dark black solid with good electrical conduction. • Diamond: formed over long geologic time periods, when graphite is subjected to extreme underground pressures. Diamond is clear, and is the hardest natural substance known. • Fullerenes: the most common being C 60 have shapes similar to soccer balls 25

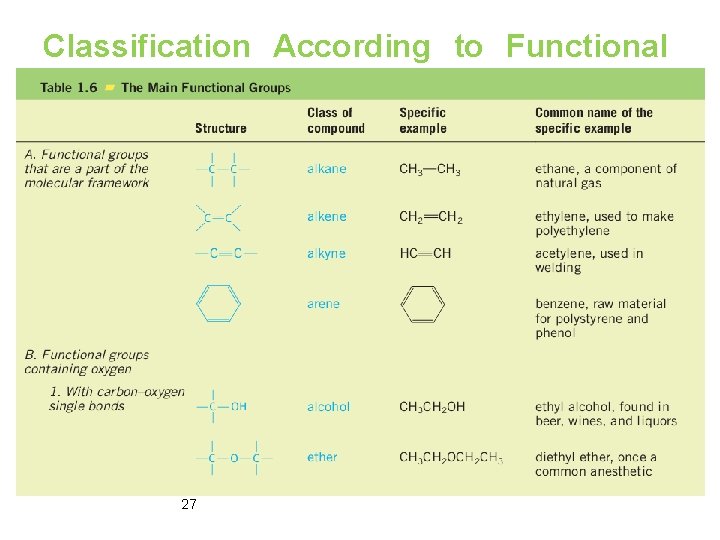
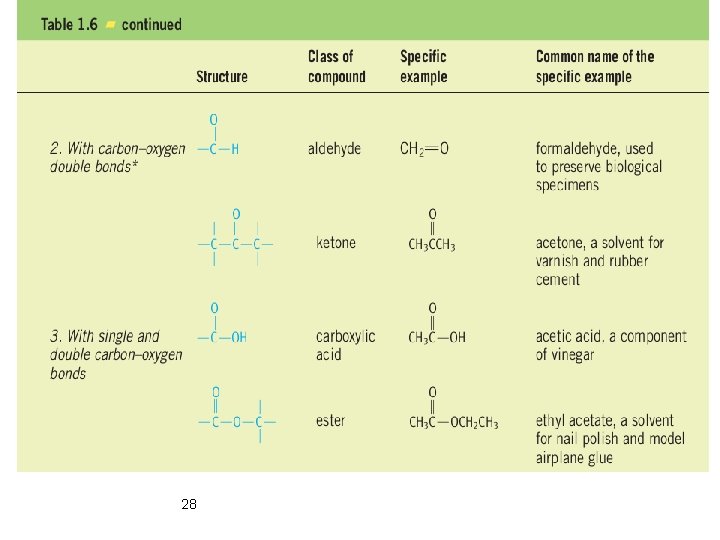
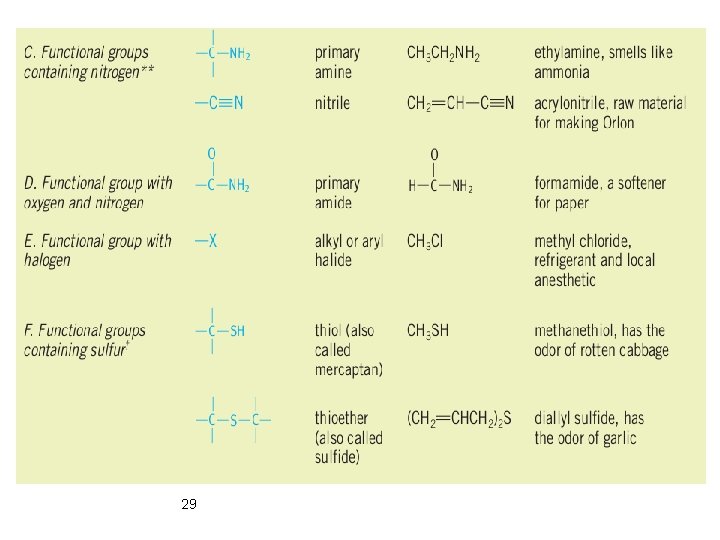
Classification According to Functional Group Ø Functional groups are groups of atoms that have characteristic chemical properties regardless of the molecular framework to which they are attached. 26

Classification According to Functional Group Ø Functional groups are groups of atoms that have characteristic chemical properties regardless of the molecular framework to which they are attached. 27

28

29
 Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Introduction to organic chemistry
Introduction to organic chemistry Pericyclic
Pericyclic Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 organic chemistry
Chapter 7 organic chemistry Entane
Entane Analytical chemistry chapter 1
Analytical chemistry chapter 1 Halohydrin formation
Halohydrin formation Father of organic chemistry
Father of organic chemistry Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry Chemistry organic
Chemistry organic Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Nomenclature of ethers
Nomenclature of ethers Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Leveling effect organic chemistry
Leveling effect organic chemistry Naming organic compounds
Naming organic compounds Organic chemistry lab report sample
Organic chemistry lab report sample Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis Met et prop but
Met et prop but Alkane cracking
Alkane cracking But prop
But prop



















































