Organic Chemistry Review Organic Chemistry Review Organic compounds





- Slides: 27

Organic Chemistry Review

Organic Chemistry Review • Organic compounds contain carbon atoms that are bonded to one another in chains, rings, and networks to form a variety of structures (polymers, oils, and other large molecules). • Carbon has four valence electrons and always makes four covalent bonds with other atoms.

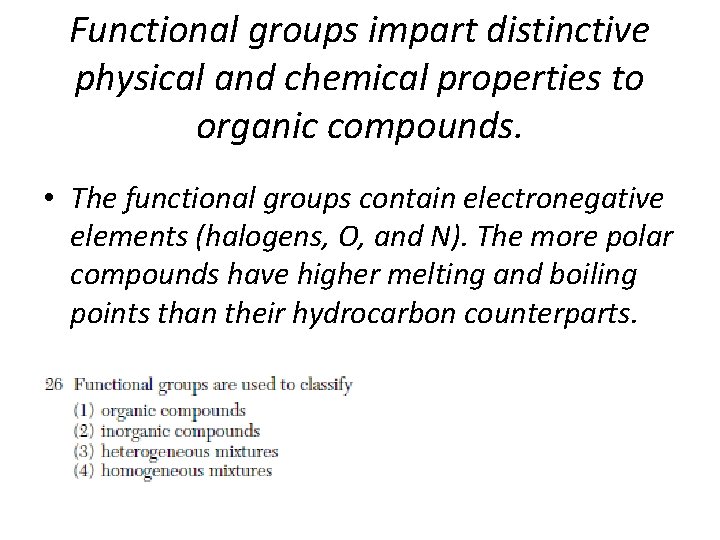
Functional groups impart distinctive physical and chemical properties to organic compounds. • The functional groups contain electronegative elements (halogens, O, and N). The more polar compounds have higher melting and boiling points than their hydrocarbon counterparts.

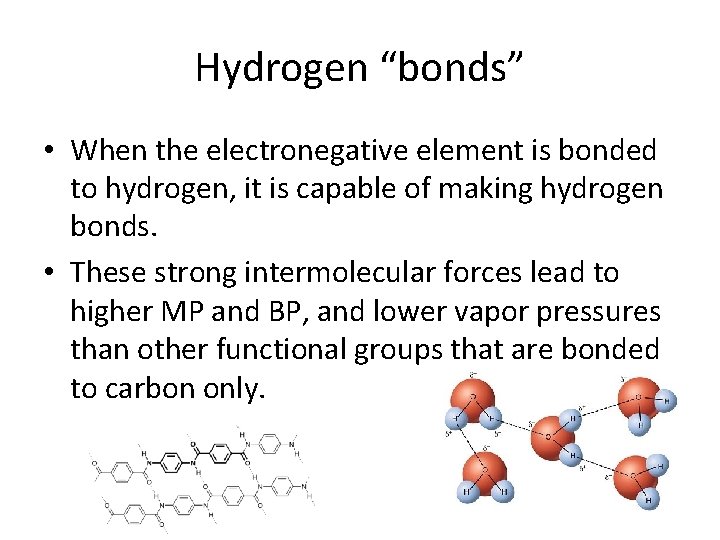
Hydrogen “bonds” • When the electronegative element is bonded to hydrogen, it is capable of making hydrogen bonds. • These strong intermolecular forces lead to higher MP and BP, and lower vapor pressures than other functional groups that are bonded to carbon only.


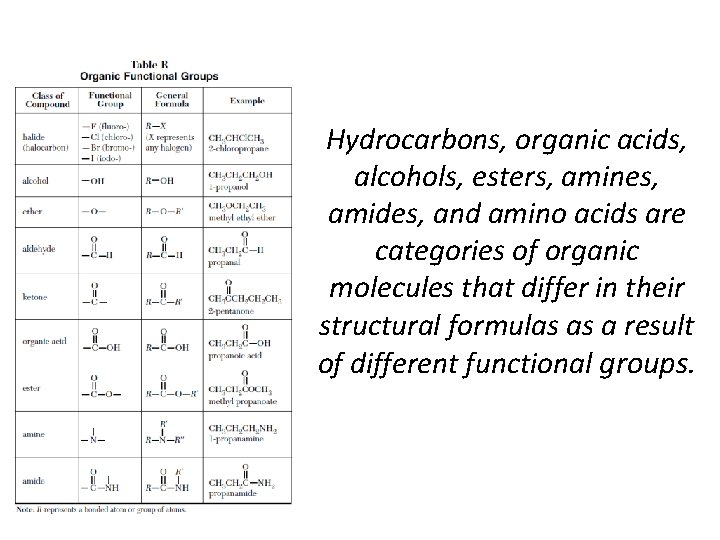
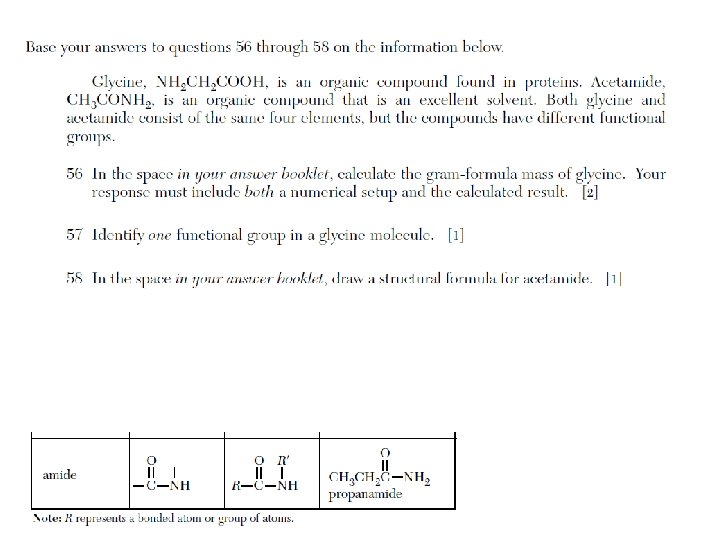
Hydrocarbons, organic acids, alcohols, esters, amines, amides, and amino acids are categories of organic molecules that differ in their structural formulas as a result of different functional groups.

What you need to be able to do… • You need to be able to use Tables P, Q and R with ease to get the organic questions. • Identifying functional groups, naming compounds, recognizing saturated and unsaturated hydrocarbons, especially in reactions; these are skills you need to have.

What You Will See • Note that you will see full structural formulas and condensed structural formulas. • Table R has partially condensed formulas. You need to be able to discern between these different ways of describing molecules: -CHO aldehyde -COOH acid -CO- ketone -COOC- ester


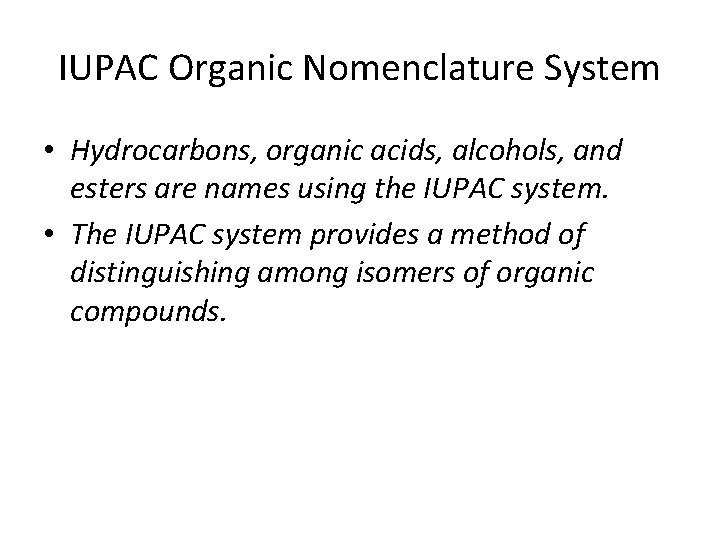
IUPAC Organic Nomenclature System • Hydrocarbons, organic acids, alcohols, and esters are names using the IUPAC system. • The IUPAC system provides a method of distinguishing among isomers of organic compounds.

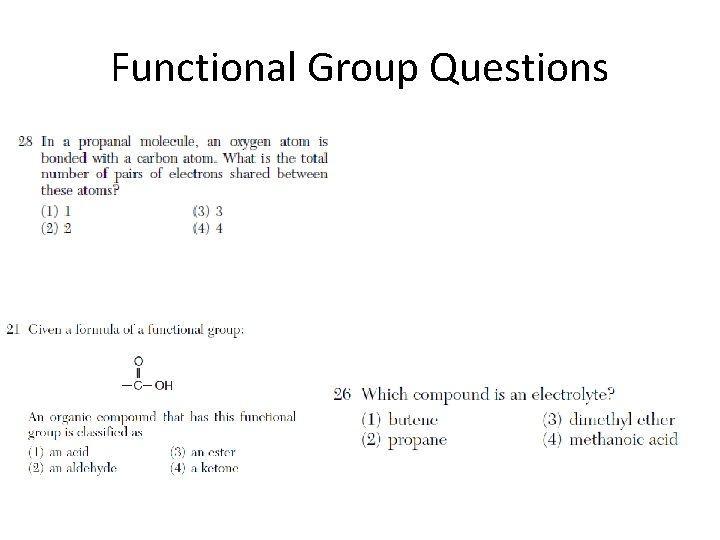
Functional Group Questions


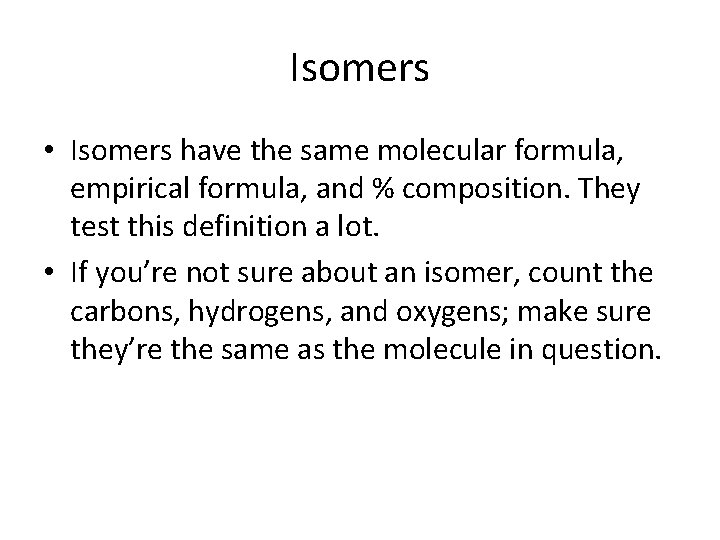
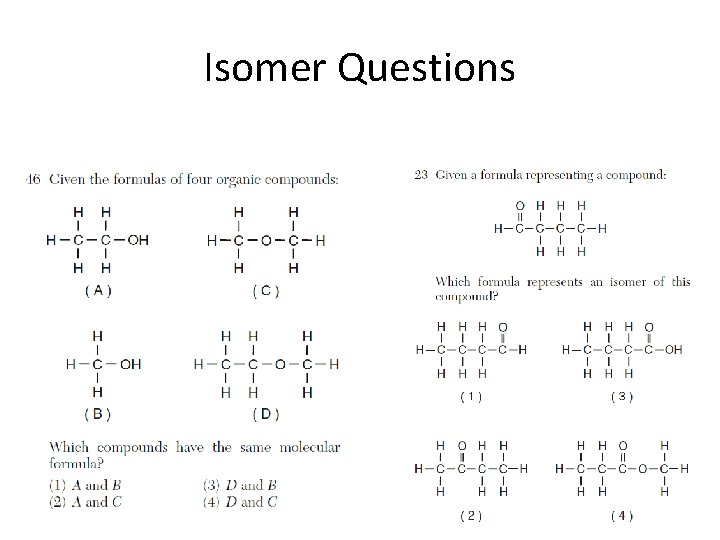
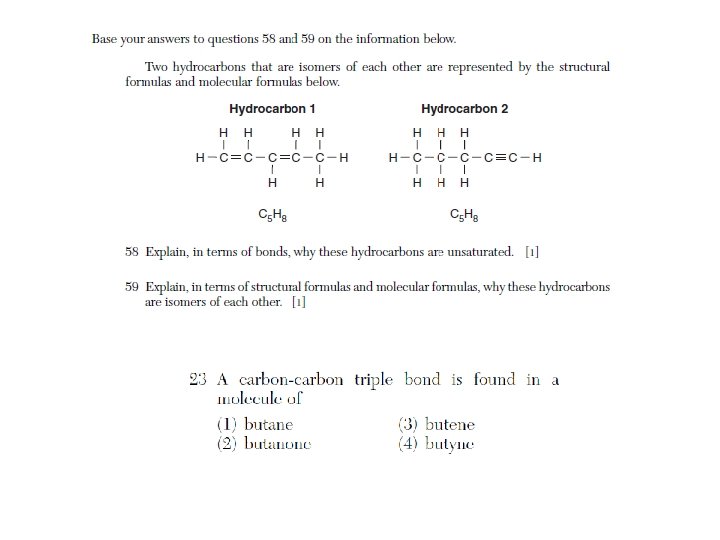
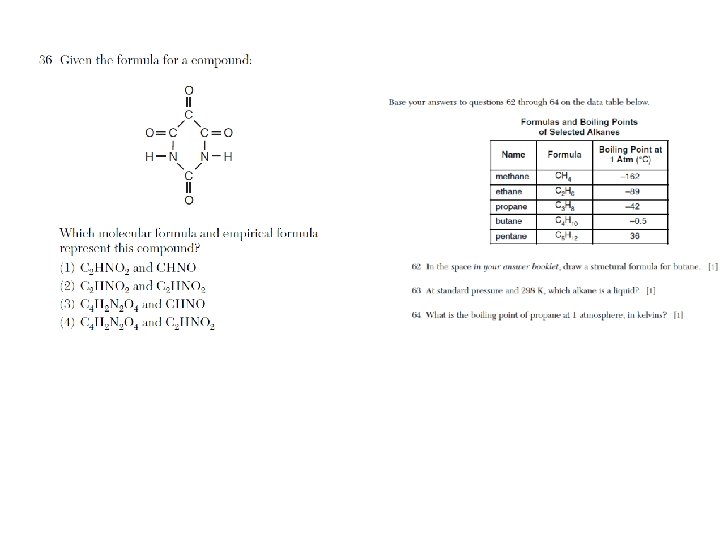
Isomers • Isomers have the same molecular formula, empirical formula, and % composition. They test this definition a lot. • If you’re not sure about an isomer, count the carbons, hydrogens, and oxygens; make sure they’re the same as the molecule in question.

Isomer Questions

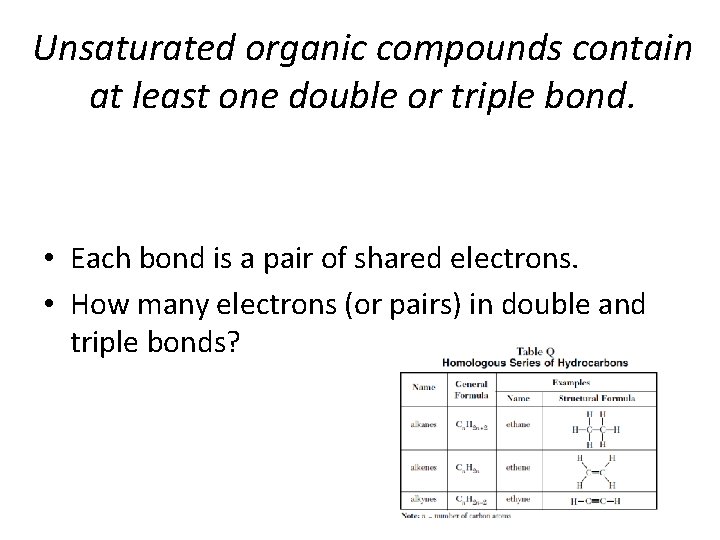
Unsaturated organic compounds contain at least one double or triple bond. • Each bond is a pair of shared electrons. • How many electrons (or pairs) in double and triple bonds?


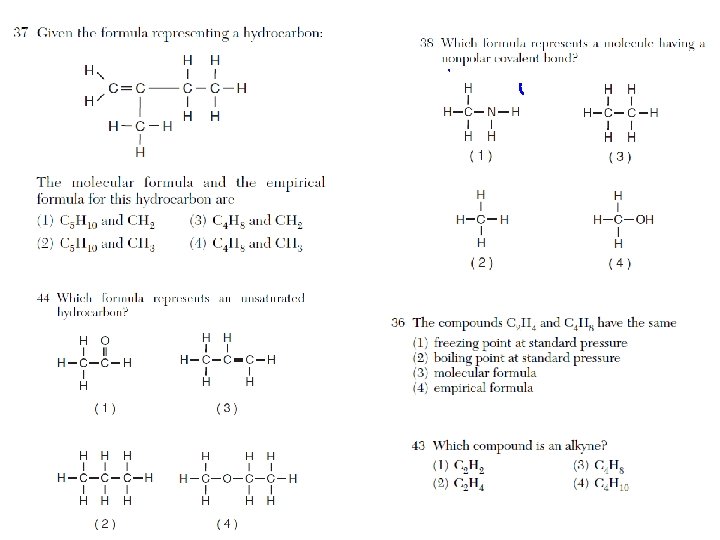
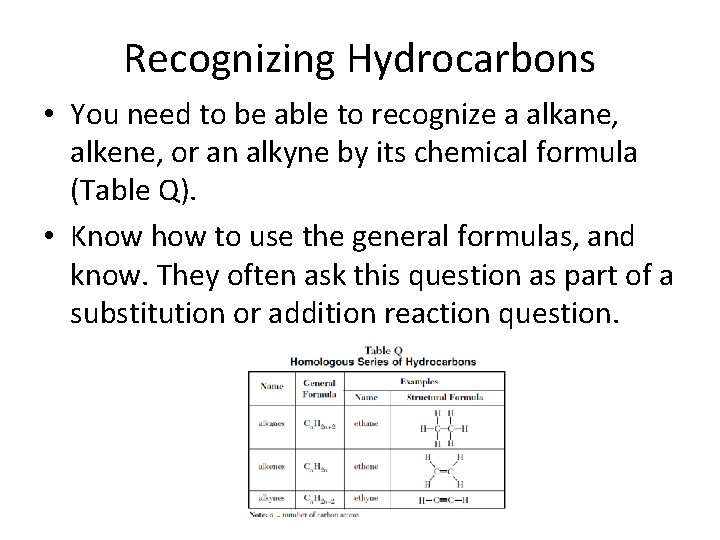
Recognizing Hydrocarbons • You need to be able to recognize a alkane, alkene, or an alkyne by its chemical formula (Table Q). • Know how to use the general formulas, and know. They often ask this question as part of a substitution or addition reaction question.


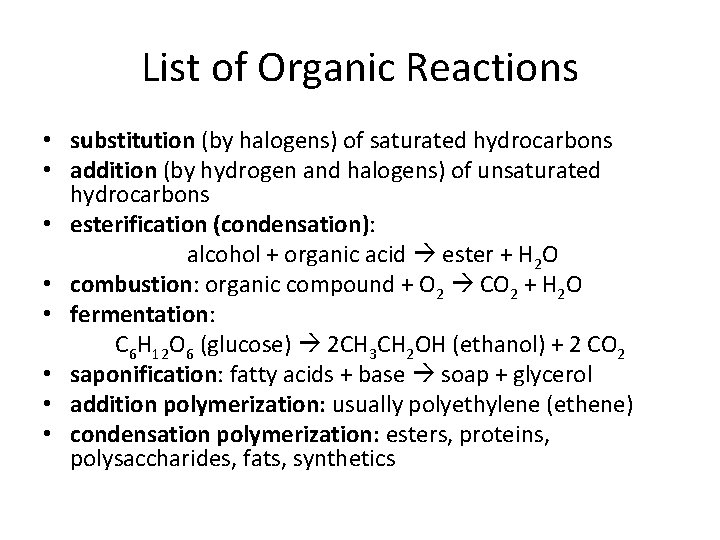
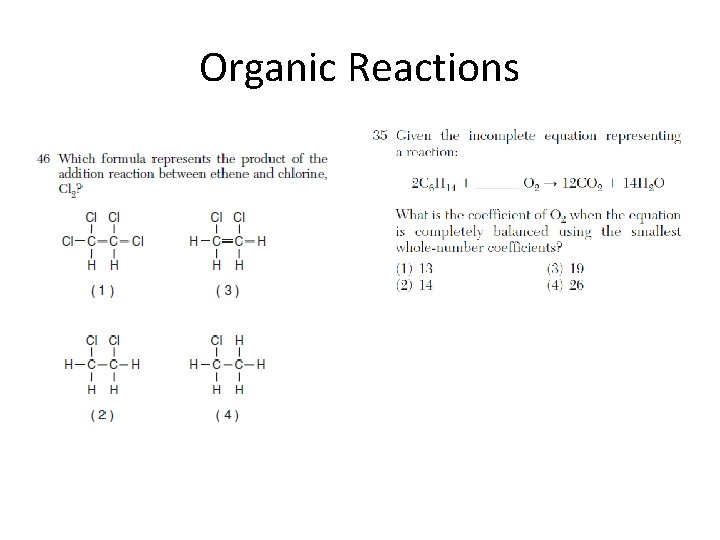
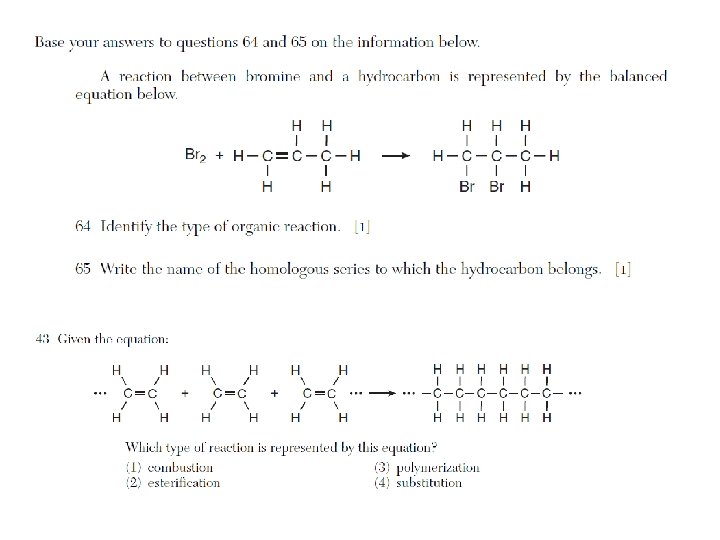
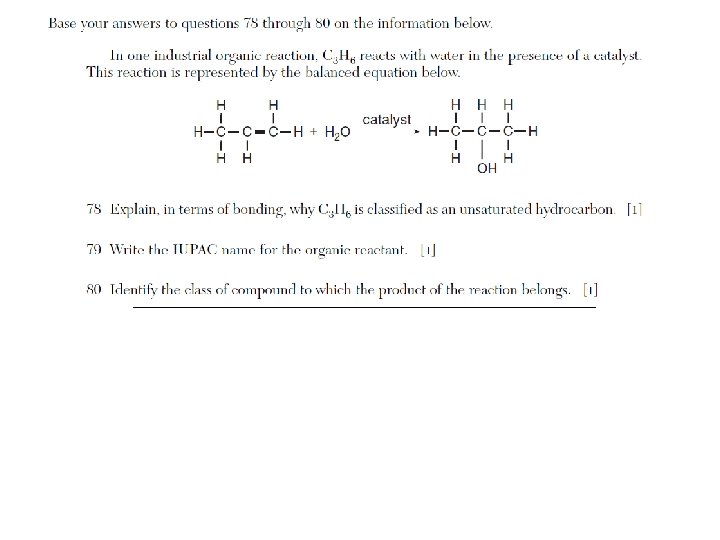
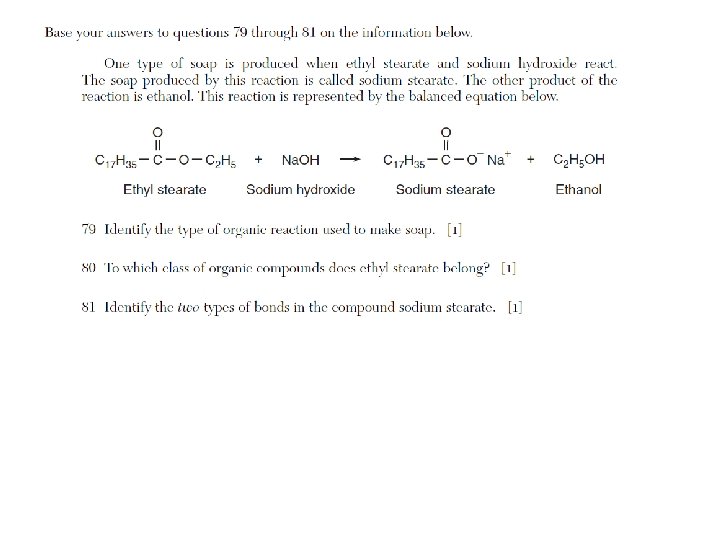
Addition, hydrogenation, substitution, polymerization, esterification, fermentation, saponification, oxidation, and combustion are examples of organic reactions. • Addition is probably the most popular reaction they test with, followed by esterification.

List of Organic Reactions • substitution (by halogens) of saturated hydrocarbons • addition (by hydrogen and halogens) of unsaturated hydrocarbons • esterification (condensation): alcohol + organic acid ester + H 2 O • combustion: organic compound + O 2 CO 2 + H 2 O • fermentation: C 6 H 12 O 6 (glucose) 2 CH 3 CH 2 OH (ethanol) + 2 CO 2 • saponification: fatty acids + base soap + glycerol • addition polymerization: usually polyethylene (ethene) • condensation polymerization: esters, proteins, polysaccharides, fats, synthetics

Organic Reactions





