What is Inorganic Chemistry What is Organic Chemistry




- Slides: 28

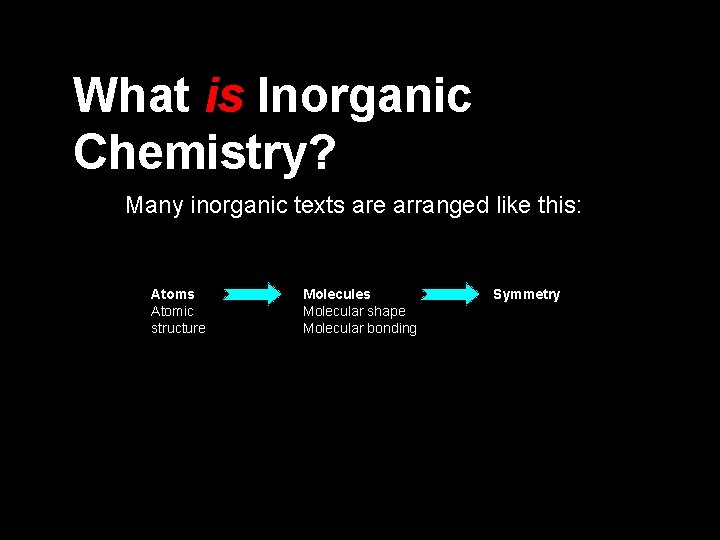
What is Inorganic Chemistry?

What is Organic Chemistry? Most organic texts are arranged like this: Structure and bonding Acids and bases Alkanes- reactions of, stereochemistry Alkenes - reactions of, stereochemistry Alkyl halides - “ Benzene- “ Alcohols - ” Ethers, , epoxides- “ Carbonyls - “ Aldehydes and ketones - “ Carboxylic acids and nitriles -” Amines - “ Carbohydrates - “ Amino acids. Proteins - “ Lipids Heterocycles and nucleic acids

What is Inorganic Chemistry? Many inorganic texts are arranged like this: Atoms Atomic structure Molecules Molecular shape Molecular bonding Symmetry

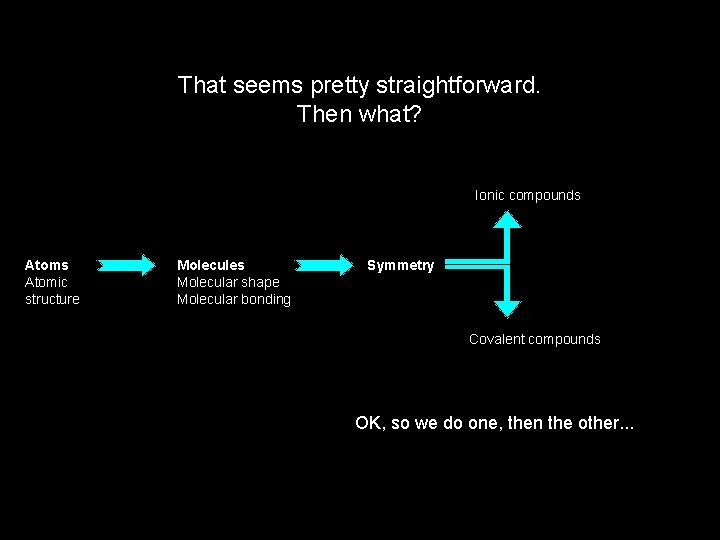
That seems pretty straightforward. Then what? Ionic compounds Atomic structure Molecules Molecular shape Molecular bonding Symmetry Covalent compounds OK, so we do one, then the other. . .

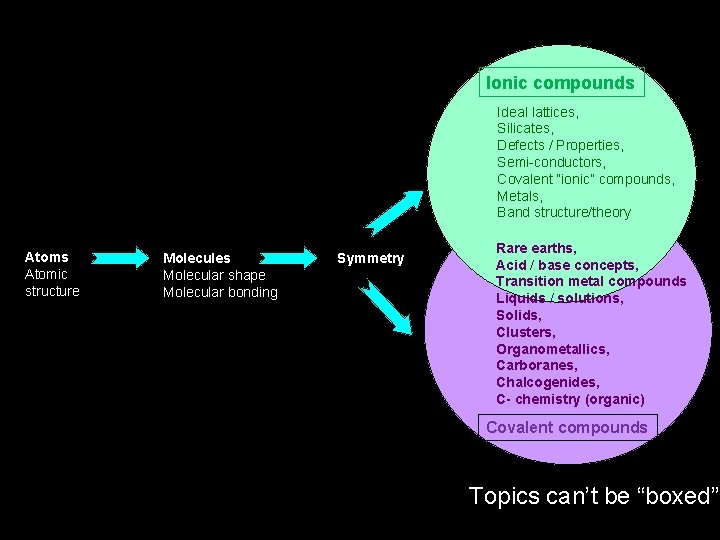
Ionic compounds Ideal lattices, Silicates, Defects / Properties, Semi-conductors, Covalent “ionic” compounds, Metals, Band structure/theory Atoms Atomic structure Molecules Molecular shape Molecular bonding Symmetry Rare earths, Acid / base concepts, Transition metal compounds Liquids / solutions, Solids, Clusters, Organometallics, Carboranes, Chalcogenides, C- chemistry (organic) Covalent compounds Topics can’t be “boxed”

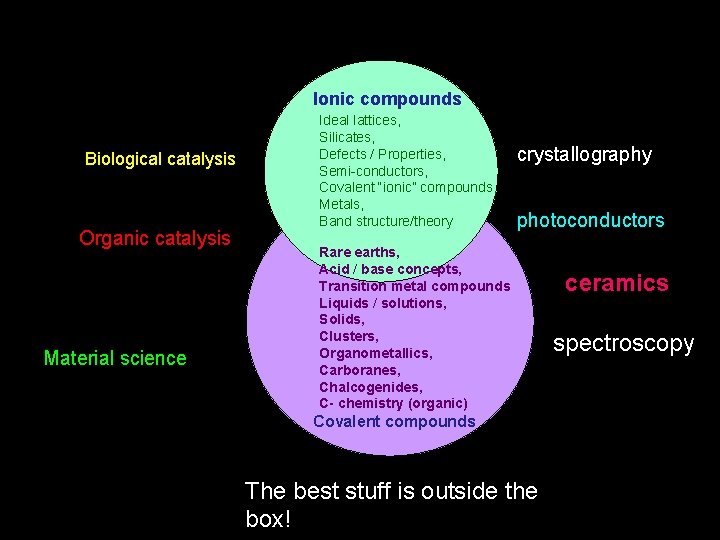
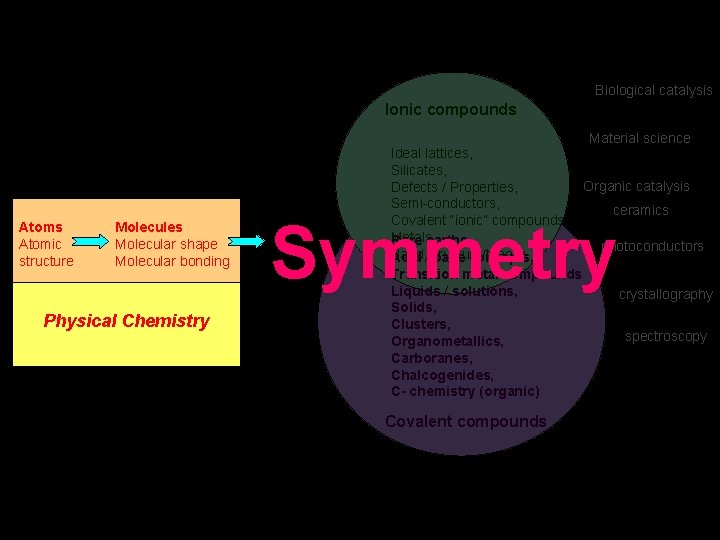
Ionic compounds Biological catalysis Organic catalysis Material science Ideal lattices, Silicates, Defects / Properties, Semi-conductors, Covalent “ionic” compounds, Metals, Band structure/theory crystallography photoconductors Rare earths, Acid / base concepts, Transition metal compounds Liquids / solutions, Solids, Clusters, Organometallics, Carboranes, Chalcogenides, C- chemistry (organic) Covalent compounds The best stuff is outside the box! ceramics spectroscopy

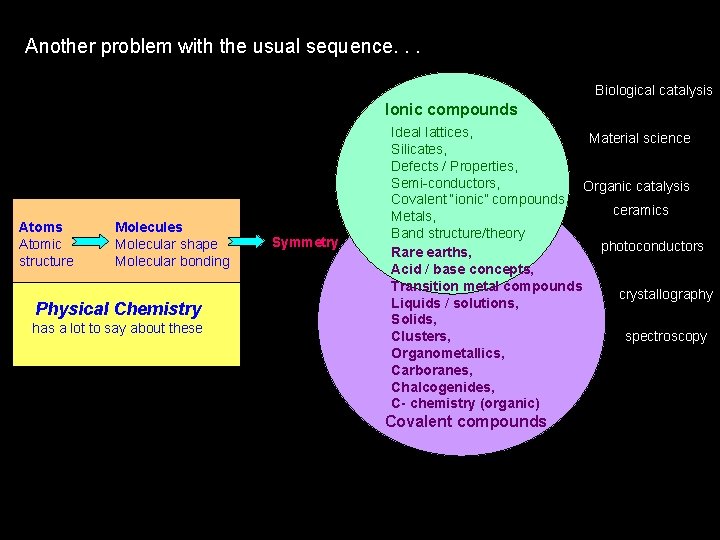
Another problem with the usual sequence. . . Biological catalysis Ionic compounds Atomic structure Molecules Molecular shape Molecular bonding Physical Chemistry has a lot to say about these Symmetry Ideal lattices, Material science Silicates, Defects / Properties, Semi-conductors, Organic catalysis Covalent “ionic” compounds, ceramics Metals, Band structure/theory photoconductors Rare earths, Acid / base concepts, Transition metal compounds crystallography Liquids / solutions, Solids, spectroscopy Clusters, Organometallics, Carboranes, Chalcogenides, C- chemistry (organic) Covalent compounds

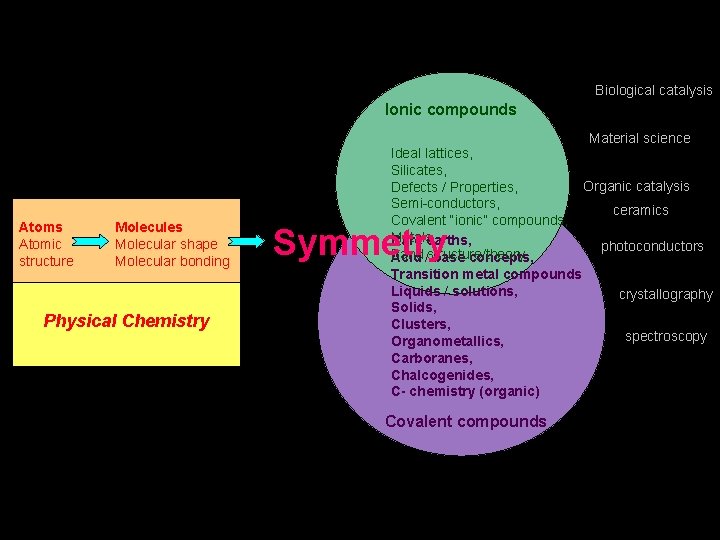
Biological catalysis Ionic compounds Material science Atoms Atomic structure Molecules Molecular shape Molecular bonding Physical Chemistry Ideal lattices, Silicates, Organic catalysis Defects / Properties, Semi-conductors, ceramics Covalent “ionic” compounds, Metals, Rare earths, photoconductors Band Acid /structure/theory base concepts, Transition metal compounds Liquids / solutions, crystallography Solids, Clusters, spectroscopy Organometallics, Carboranes, Chalcogenides, C- chemistry (organic) Symmetry Covalent compounds

Biological catalysis Ionic compounds Material science Atoms Atomic structure Molecules Molecular shape Molecular bonding Physical Chemistry Ideal lattices, Silicates, Organic catalysis Defects / Properties, Semi-conductors, ceramics Covalent “ionic” compounds, Metals, Rare earths, photoconductors Band Acid /structure/theory base concepts, Transition metal compounds Liquids / solutions, crystallography Solids, Clusters, spectroscopy Organometallics, Carboranes, Chalcogenides, C- chemistry (organic) Symmetry Covalent compounds

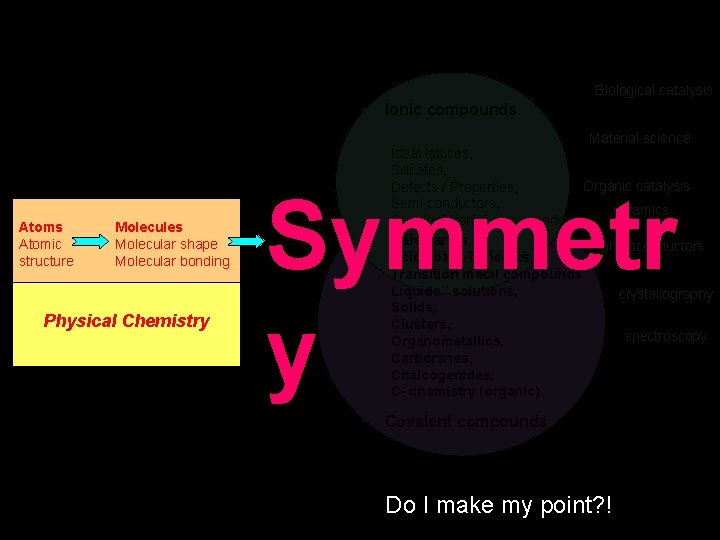
Biological catalysis Ionic compounds Material science Atoms Atomic structure Molecules Molecular shape Molecular bonding Physical Chemistry Ideal lattices, Silicates, Organic catalysis Defects / Properties, Semi-conductors, ceramics Covalent “ionic” compounds, Metals, Rare earths, photoconductors Band Acid /structure/theory base concepts, Transition metal compounds Liquids / solutions, crystallography Solids, Clusters, spectroscopy Organometallics, Carboranes, Chalcogenides, C- chemistry (organic) Symmetr y Covalent compounds Do I make my point? !

How I see Inorganic Chemistry. . .

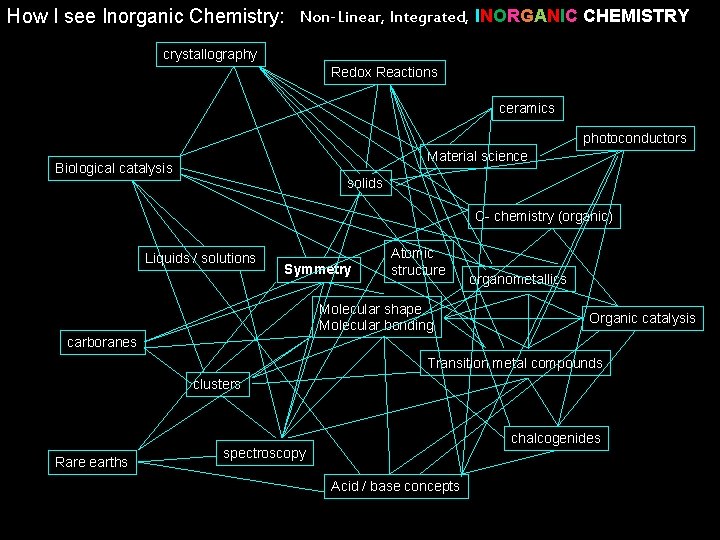
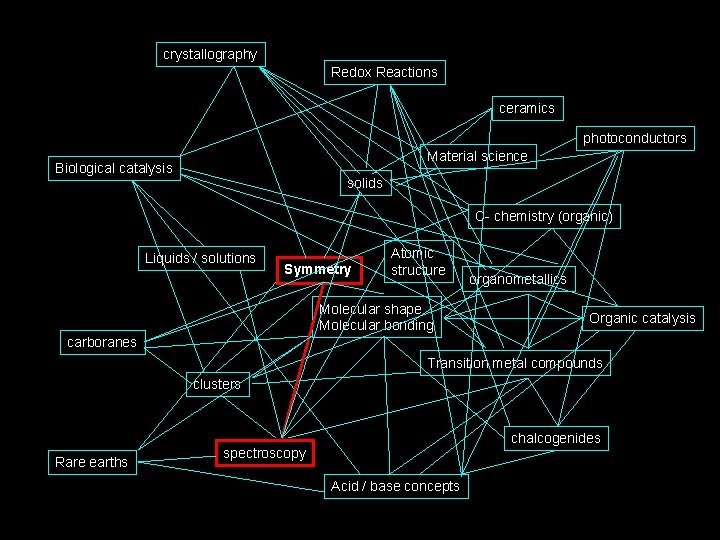
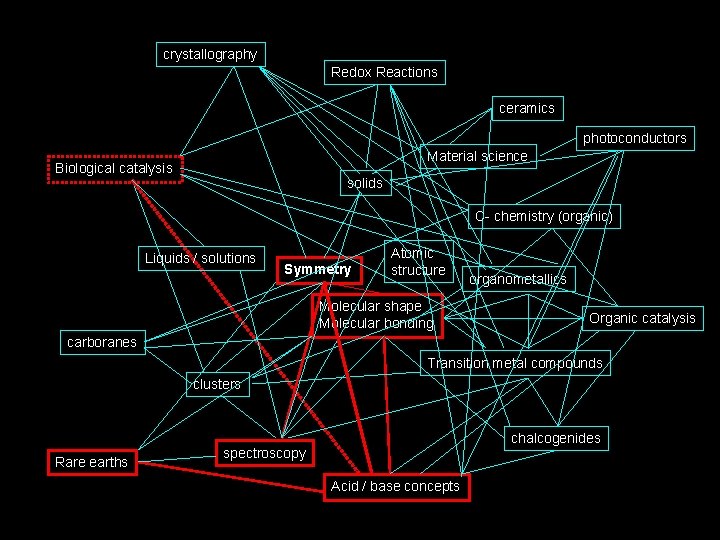
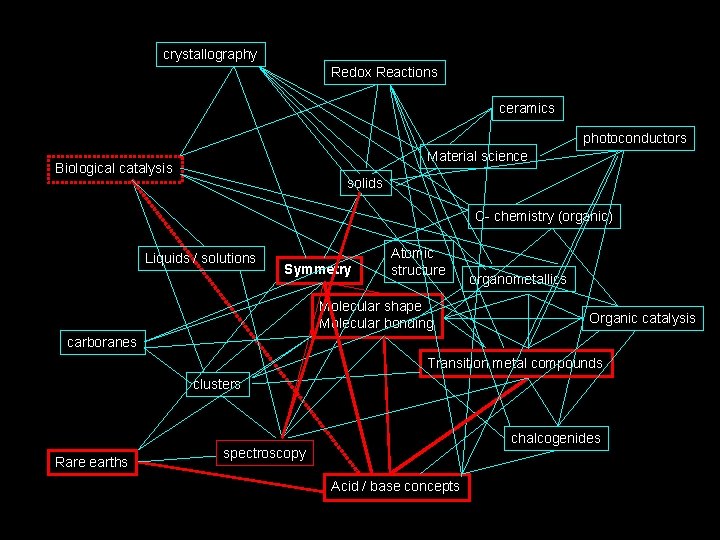
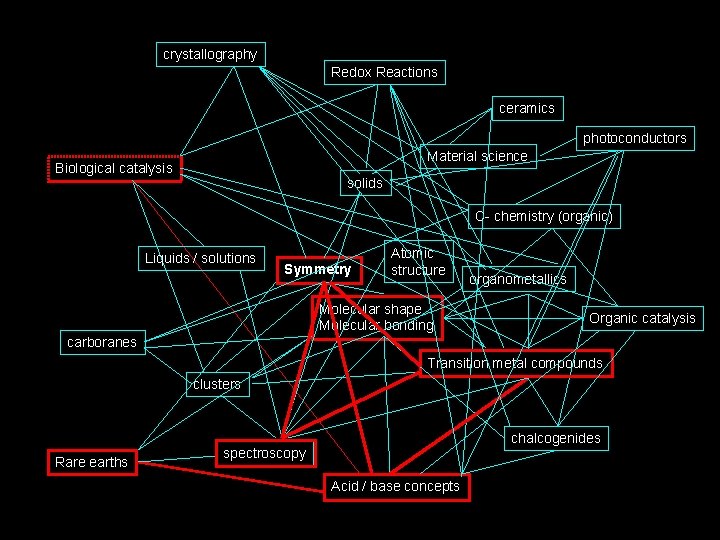
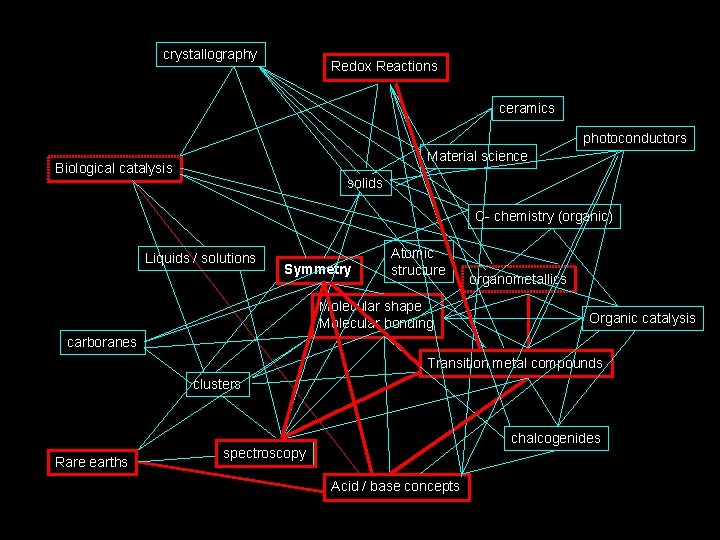
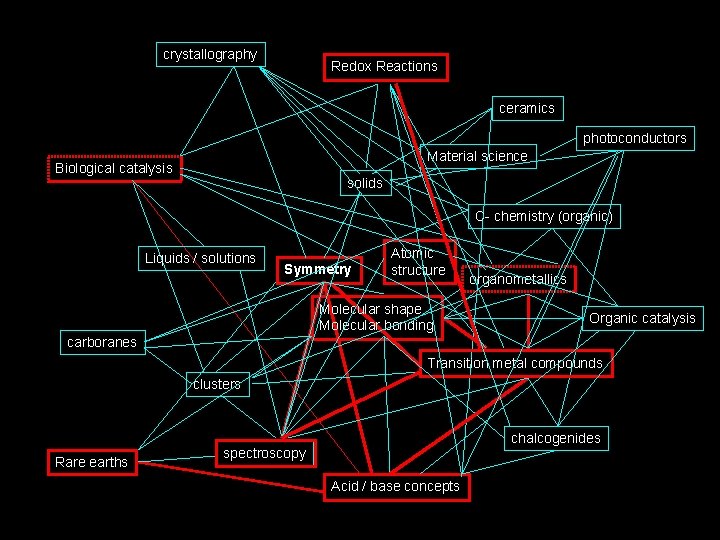
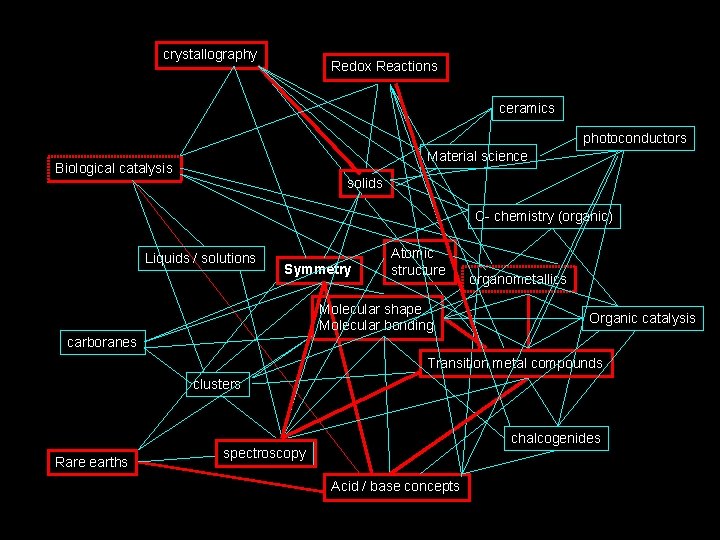
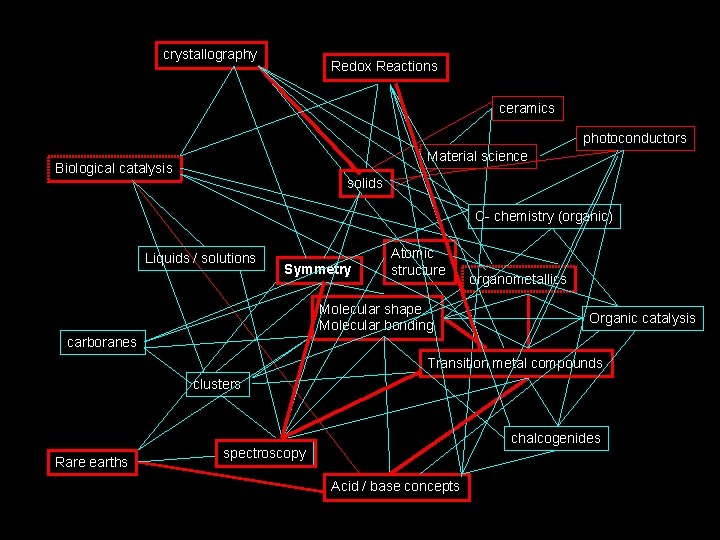
How I see Inorganic Chemistry: Non-Linear, Integrated, INORGANIC CHEMISTRY crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

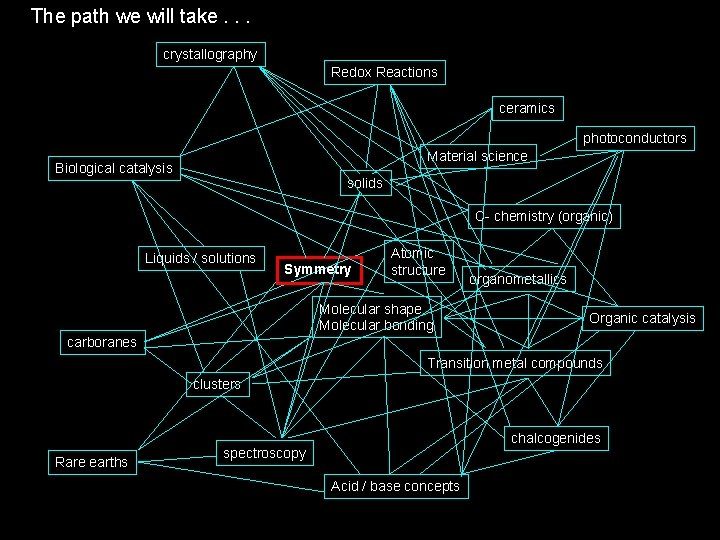
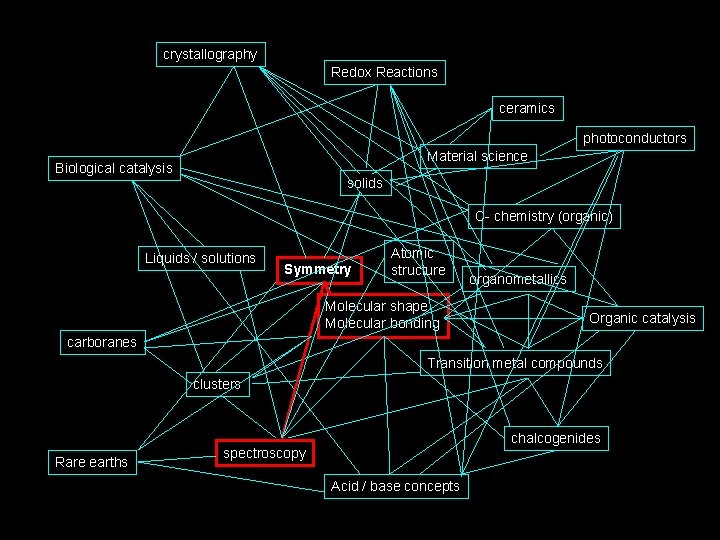
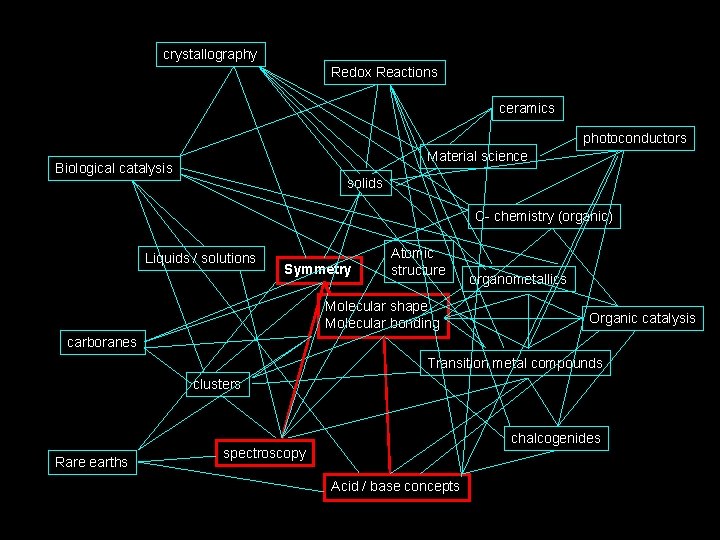
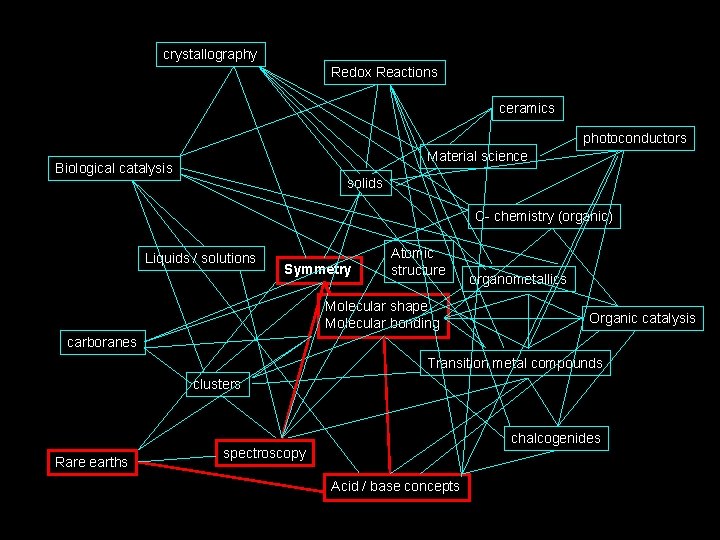
The path we will take. . . crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

crystallography Redox Reactions ceramics photoconductors Material science Biological catalysis solids C- chemistry (organic) Liquids / solutions Symmetry Atomic structure Molecular shape Molecular bonding organometallics Organic catalysis carboranes Transition metal compounds clusters Rare earths chalcogenides spectroscopy Acid / base concepts

What is Inorganic Chemistry?

What is Inorganic Chemistry? “well, whatever it is, it starts with symmetry. ” “… and what’s the big deal about this symmetry concept anyway? ” Symmetry is the answer “… and if symmetry is so important, why hasn’t it shown up by now? ”

Group Theory: the parent concept for symmetry (as described by a physical chemist in some quantum text from 5. 1950’s) Group Theory (by a physical chemist whose name is forgotten) The subject of this chapter is one of the most enthralling within the domain of quantum mechanics, for not only does it simplify calculations, but also it reveals the underlying connection between apparently disparate phenomena. Whole regions of study may be unified in terms of its concepts. Angular momentum is a facet of group theory; so too are the properties of the harmonic oscillator. Turn the subject over and there appears the basis of the conservation of linear momentum and energy. Another facet classifiesthe elementary particles. ; another illuminates the structure of transition metal complexes. The subject glitters with power and achievements, and one feels that (wo)man’s quest for understanding in terms of symmetry receives its vindication in Group Theory.

Why is Symmetry useful? ~ for Spectroscopy ~ for Classifying Structure ~ for Bonding And to … Learn how to see differently…. .
 Inorganic vs organic chemistry
Inorganic vs organic chemistry Is ch4o organic or inorganic
Is ch4o organic or inorganic C10h22 organic or inorganic
C10h22 organic or inorganic Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Organic vs inorganic
Organic vs inorganic Oxalic acid is organic or inorganic
Oxalic acid is organic or inorganic Organic and inorganic cofactors
Organic and inorganic cofactors Organic vs inorganic compounds
Organic vs inorganic compounds Organic vs inorganic
Organic vs inorganic Meaning of the word organic
Meaning of the word organic Difference between organic and inorganic
Difference between organic and inorganic Difference between organic and inorganic growth
Difference between organic and inorganic growth Importance of organic compounds
Importance of organic compounds Organic vs inorganic
Organic vs inorganic Organic and inorganic cofactors
Organic and inorganic cofactors Composition of smear layer
Composition of smear layer Organic and inorganic nutrients
Organic and inorganic nutrients Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Ib chemistry organic chemistry
Ib chemistry organic chemistry Hard soft acid base theory
Hard soft acid base theory Importance of inorganic chemistry
Importance of inorganic chemistry Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Introduction to inorganic chemistry
Introduction to inorganic chemistry Halohydrin formation
Halohydrin formation Organic chemistry
Organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry stuart warren
Organic chemistry stuart warren Kiliani fischer synthesis
Kiliani fischer synthesis Ario organic chemistry
Ario organic chemistry