Cofactors Cofactors Cofactors are organic or inorganic molecules






- Slides: 35

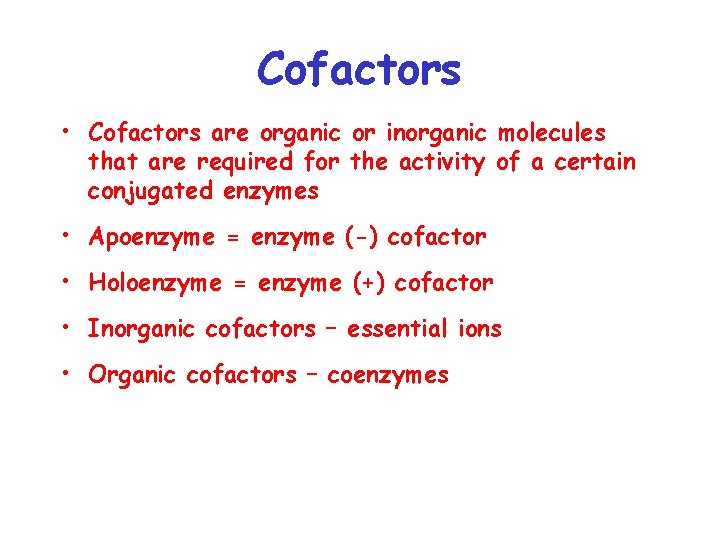
Cofactors

Cofactors • Cofactors are organic or inorganic molecules that are required for the activity of a certain conjugated enzymes • Apoenzyme = enzyme (-) cofactor • Holoenzyme = enzyme (+) cofactor • Inorganic cofactors – essential ions • Organic cofactors – coenzymes

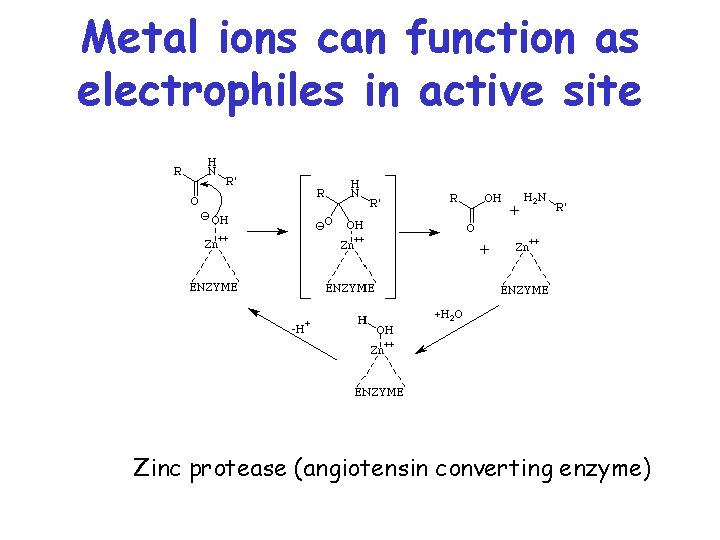
Essential Ion Cofactors • Activator ions – bind reversibly to enzyme and often participate in substrate binding. • Metal ions of metalloenzymes – cations that are tightly bound to enzyme and participate directly in catalysis (Fe, Zn, Cu, Co). • Metal activated enzymes – require or are stimulated by addition of metal ions (i. e. Mg 2+, is required by many ATP requiring enzymes)

Metal ions can function as electrophiles in active site Zinc protease (angiotensin converting enzyme)

Coenzymes Cosubstrates- altered in rxn and regenerated to original structure in subsequent rxn - disassociated from active site - shuttle chemical groups among different enzyme rxns. Prosthetic groups- remains bound to enzyme - must return to original form Both cosubstrates and prosthetic groups supply reactive groups not present on amino acid side chains

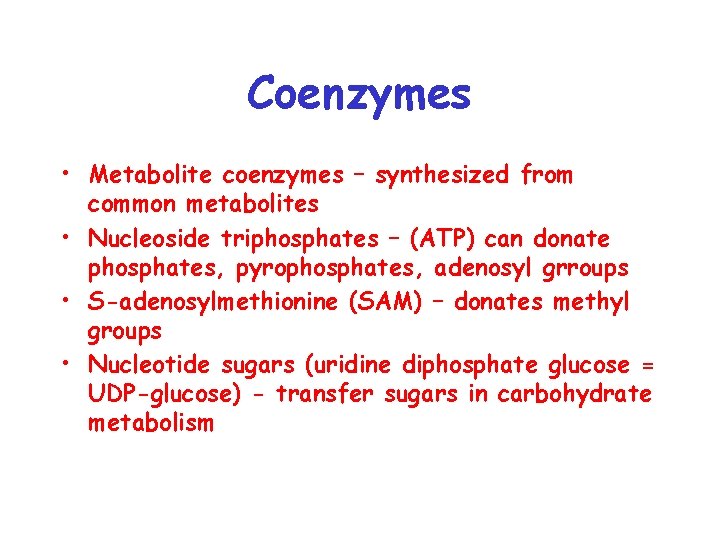
Coenzymes • Metabolite coenzymes – synthesized from common metabolites • Nucleoside triphosphates – (ATP) can donate phosphates, pyrophosphates, adenosyl grroups • S-adenosylmethionine (SAM) – donates methyl groups • Nucleotide sugars (uridine diphosphate glucose = UDP-glucose) - transfer sugars in carbohydrate metabolism

Vitamin derived coenzymes • Must be obtained from diet • Synthesized by microorganisms and plants • Vitamin deficiencies lead to disease state • Most vitamins must be enzymatically transformed to function as a coenzyme

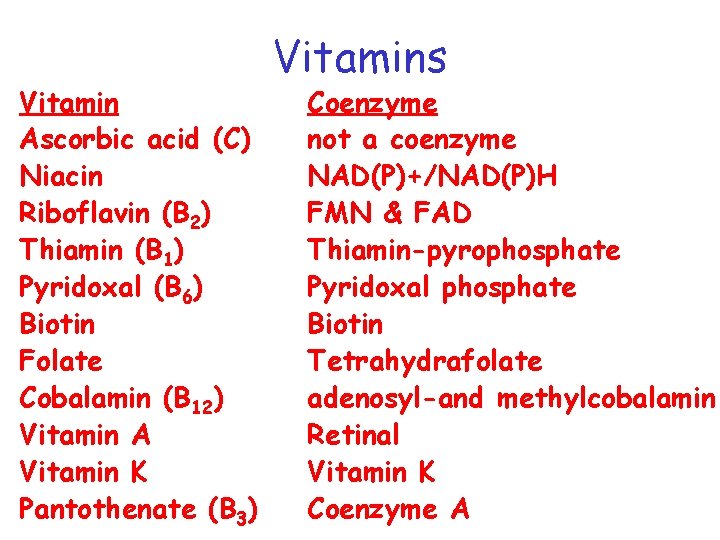
Vitamin Ascorbic acid (C) Niacin Riboflavin (B 2) Thiamin (B 1) Pyridoxal (B 6) Biotin Folate Cobalamin (B 12) Vitamin A Vitamin K Pantothenate (B 3) Vitamins Coenzyme not a coenzyme NAD(P)+/NAD(P)H FMN & FAD Thiamin-pyrophosphate Pyridoxal phosphate Biotin Tetrahydrafolate adenosyl-and methylcobalamin Retinal Vitamin K Coenzyme A

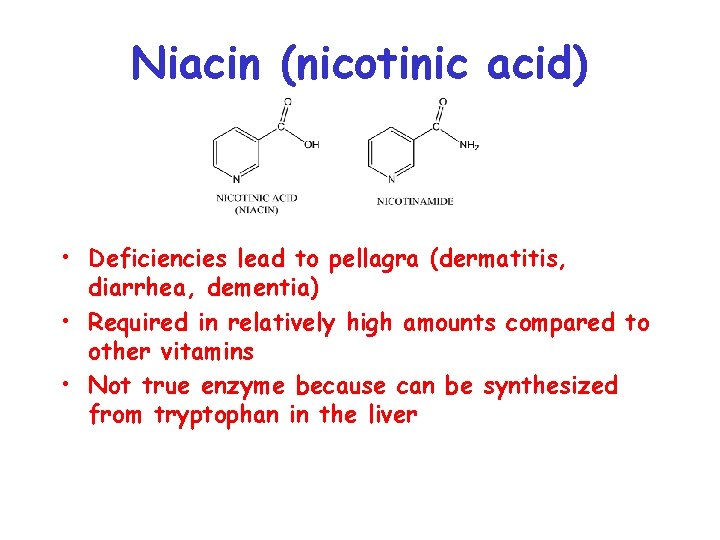
Niacin (nicotinic acid) • Deficiencies lead to pellagra (dermatitis, diarrhea, dementia) • Required in relatively high amounts compared to other vitamins • Not true enzyme because can be synthesized from tryptophan in the liver

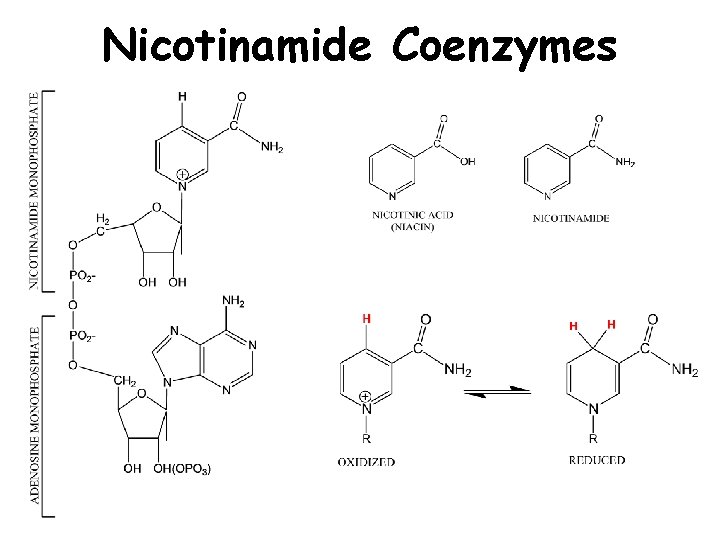
Nicotinamide Coenzymes

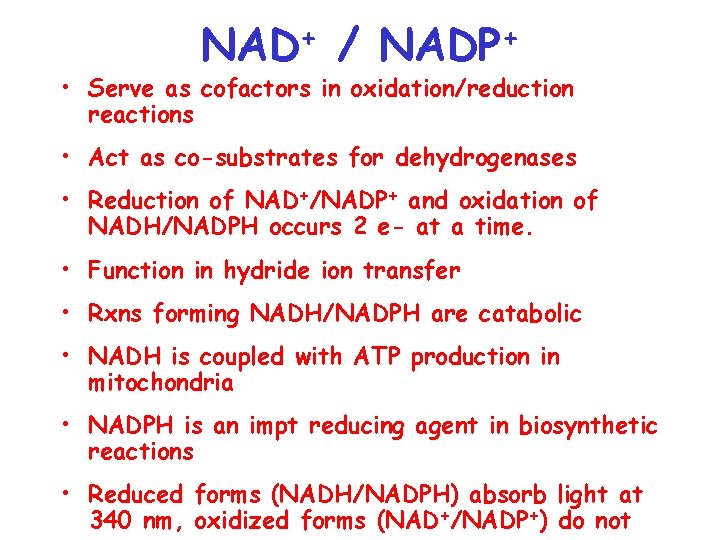
+ NAD / + NADP • Serve as cofactors in oxidation/reduction reactions • Act as co-substrates for dehydrogenases • Reduction of NAD+/NADP+ and oxidation of NADH/NADPH occurs 2 e- at a time. • Function in hydride ion transfer • Rxns forming NADH/NADPH are catabolic • NADH is coupled with ATP production in mitochondria • NADPH is an impt reducing agent in biosynthetic reactions • Reduced forms (NADH/NADPH) absorb light at 340 nm, oxidized forms (NAD+/NADP+) do not

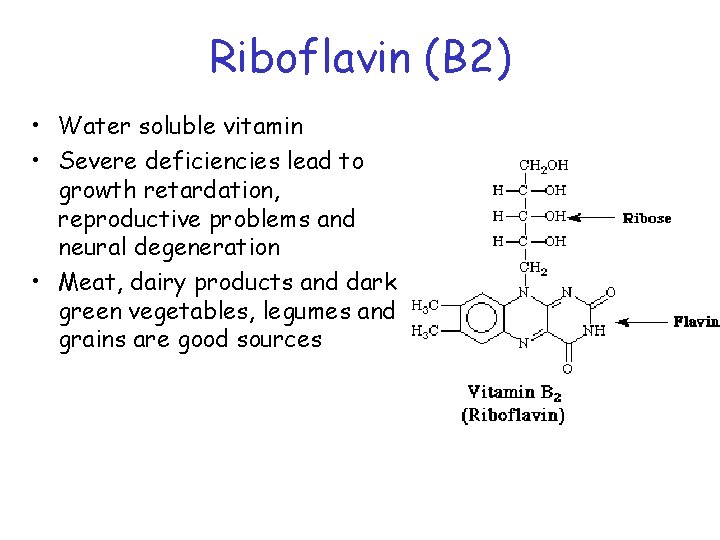
Riboflavin (B 2) • Water soluble vitamin • Severe deficiencies lead to growth retardation, reproductive problems and neural degeneration • Meat, dairy products and dark green vegetables, legumes and grains are good sources

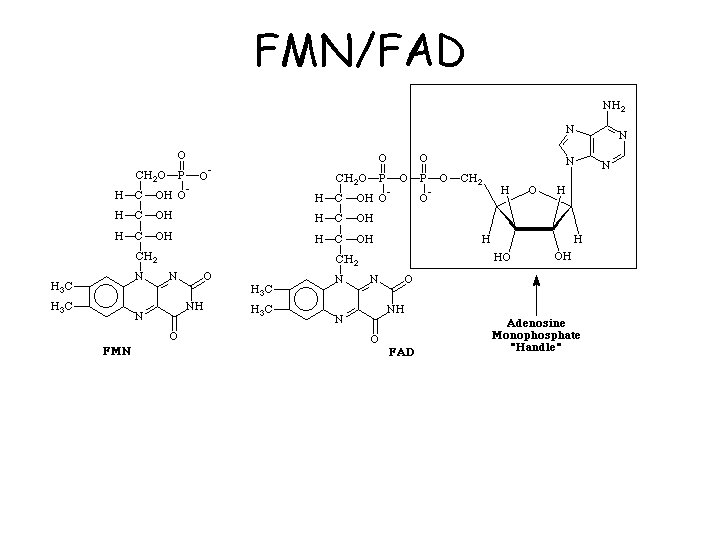
FMN/FAD

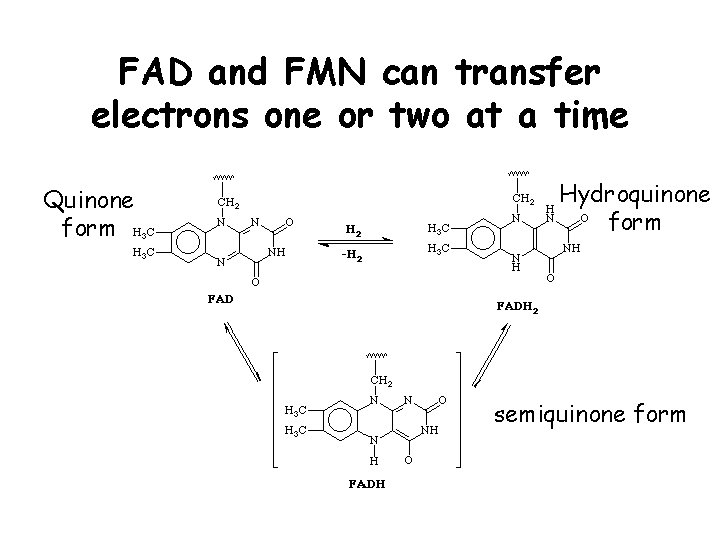
FAD and FMN can transfer electrons one or two at a time Quinone form Hydroquinone form semiquinone form

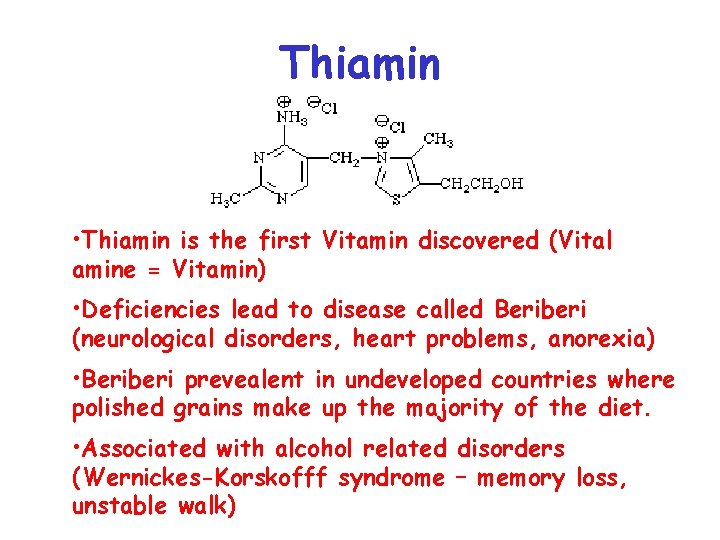
Thiamin • Thiamin is the first Vitamin discovered (Vital amine = Vitamin) • Deficiencies lead to disease called Beriberi (neurological disorders, heart problems, anorexia) • Beriberi prevealent in undeveloped countries where polished grains make up the majority of the diet. • Associated with alcohol related disorders (Wernickes-Korskofff syndrome – memory loss, unstable walk)

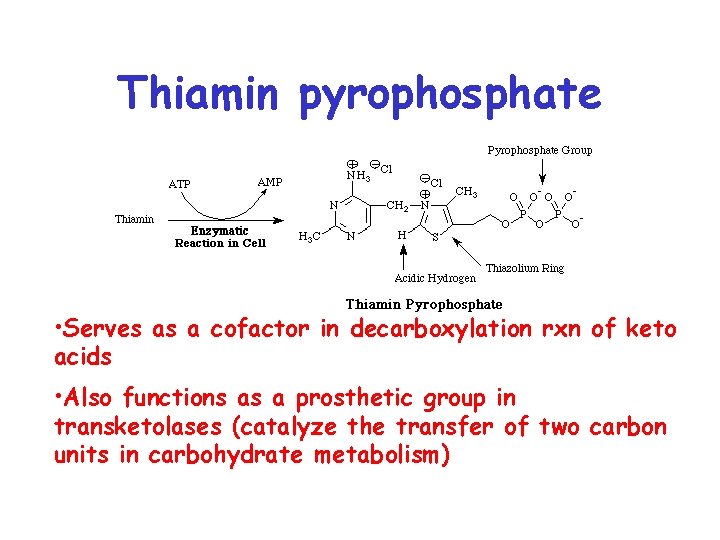
Thiamin pyrophosphate • Serves as a cofactor in decarboxylation rxn of keto acids • Also functions as a prosthetic group in transketolases (catalyze the transfer of two carbon units in carbohydrate metabolism)

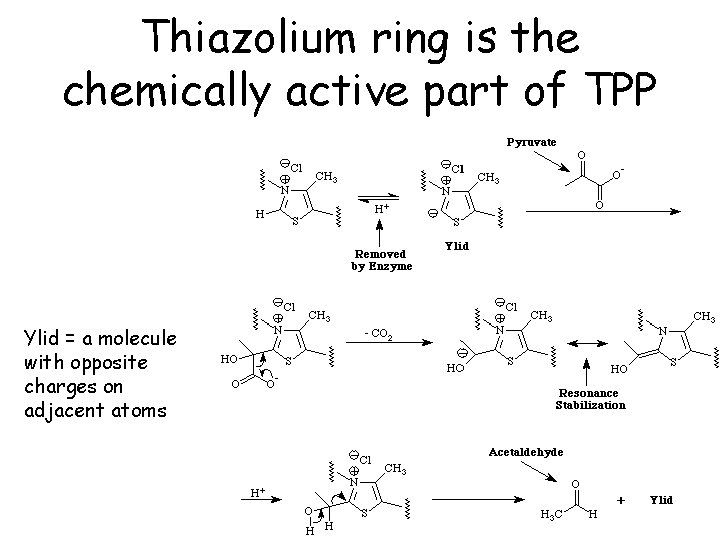
Thiazolium ring is the chemically active part of TPP Ylid = a molecule with opposite charges on adjacent atoms

Pyridoxal

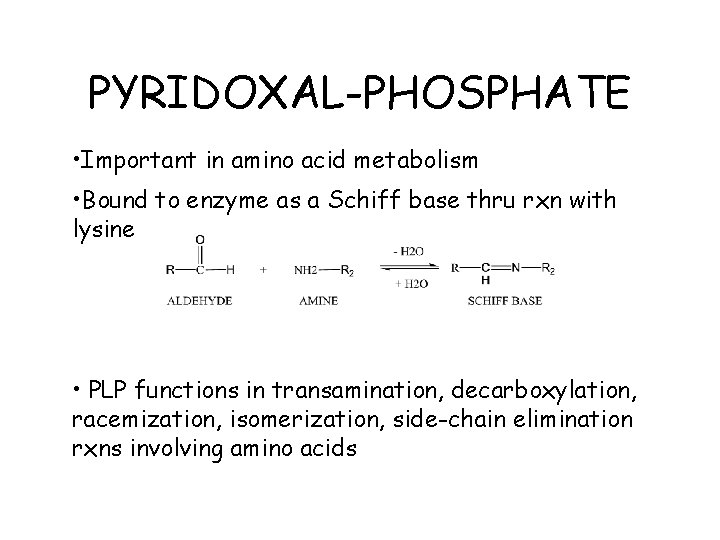
PYRIDOXAL-PHOSPHATE • Important in amino acid metabolism • Bound to enzyme as a Schiff base thru rxn with lysine • PLP functions in transamination, decarboxylation, racemization, isomerization, side-chain elimination rxns involving amino acids

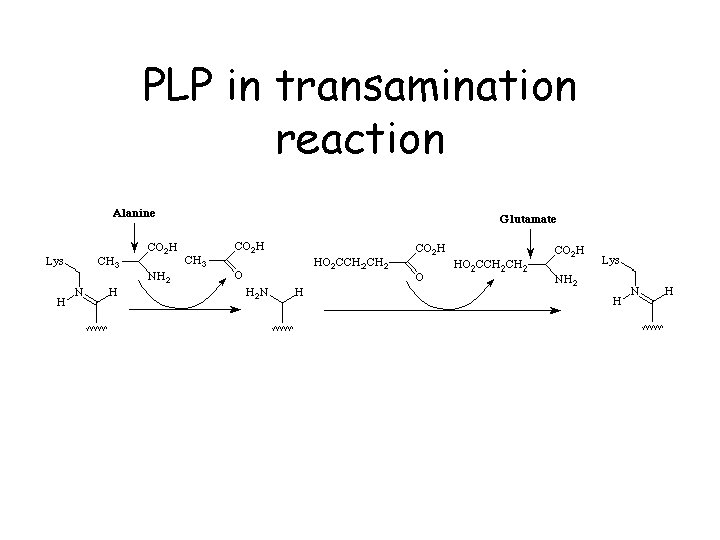
PLP in transamination reaction

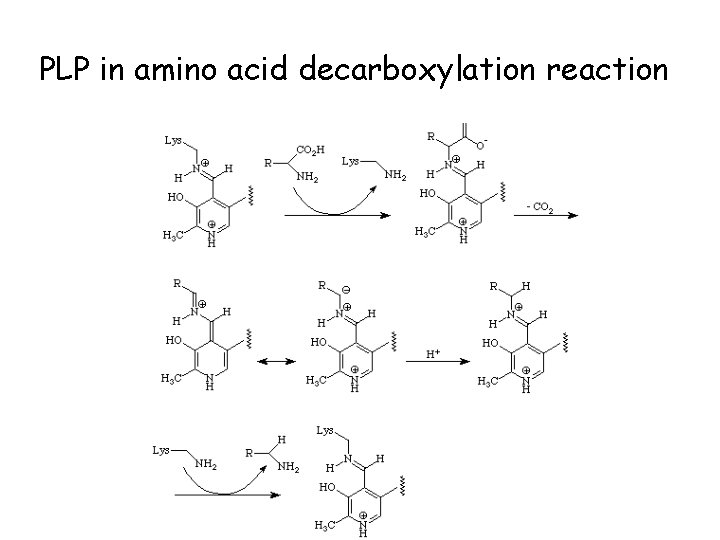
PLP in amino acid decarboxylation reaction

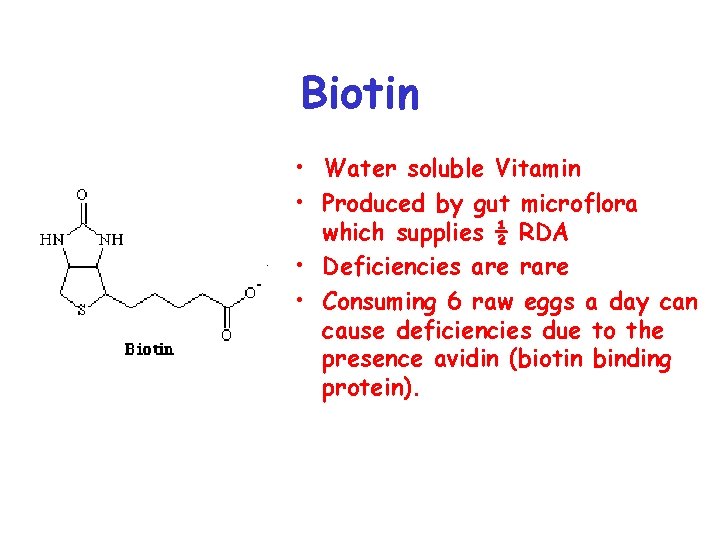
Biotin • Water soluble Vitamin • Produced by gut microflora which supplies ½ RDA • Deficiencies are rare • Consuming 6 raw eggs a day can cause deficiencies due to the presence avidin (biotin binding protein).

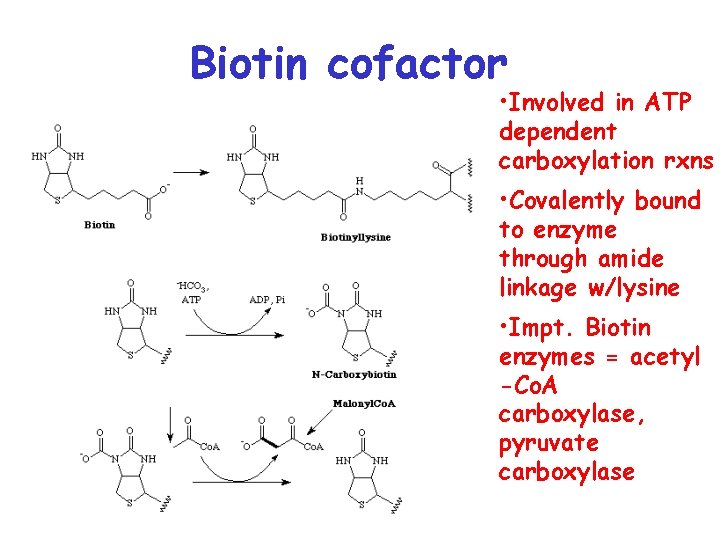
Biotin cofactor • Involved in ATP dependent carboxylation rxns • Covalently bound to enzyme through amide linkage w/lysine • Impt. Biotin enzymes = acetyl -Co. A carboxylase, pyruvate carboxylase

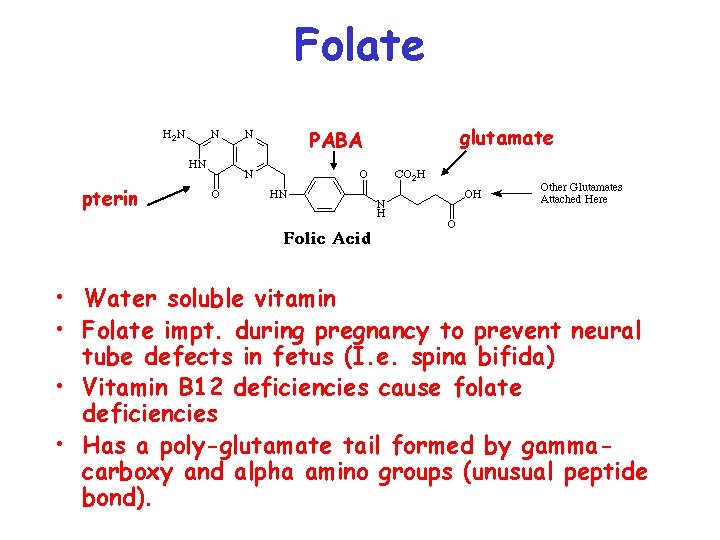
Folate PABA glutamate pterin • Water soluble vitamin • Folate impt. during pregnancy to prevent neural tube defects in fetus (I. e. spina bifida) • Vitamin B 12 deficiencies cause folate deficiencies • Has a poly-glutamate tail formed by gammacarboxy and alpha amino groups (unusual peptide bond).

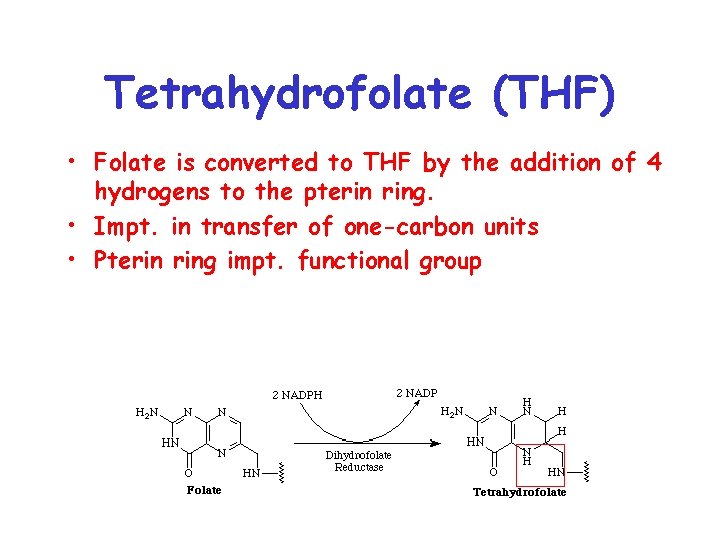
Tetrahydrofolate (THF) • Folate is converted to THF by the addition of 4 hydrogens to the pterin ring. • Impt. in transfer of one-carbon units • Pterin ring impt. functional group

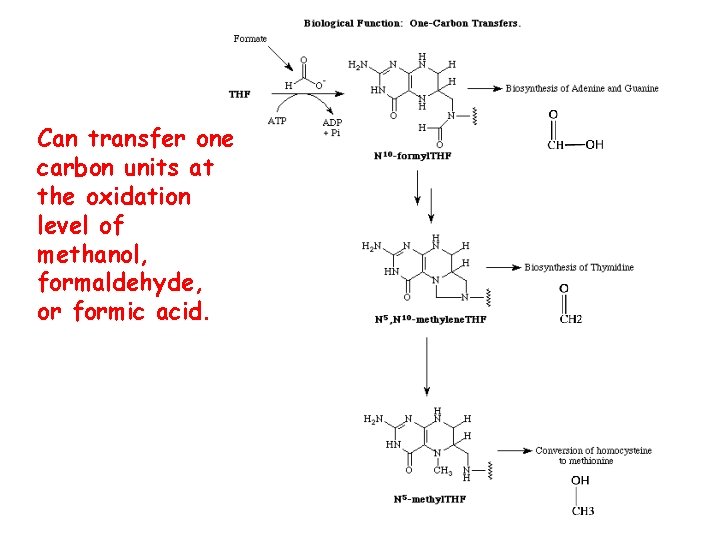
Can transfer one carbon units at the oxidation level of methanol, formaldehyde, or formic acid.

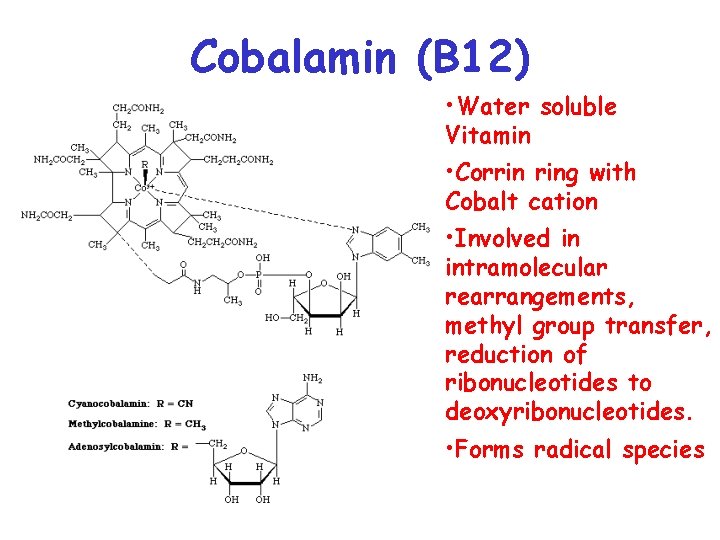
Cobalamin (B 12) • Water soluble Vitamin • Corrin ring with Cobalt cation • Involved in intramolecular rearrangements, methyl group transfer, reduction of ribonucleotides to deoxyribonucleotides. • Forms radical species

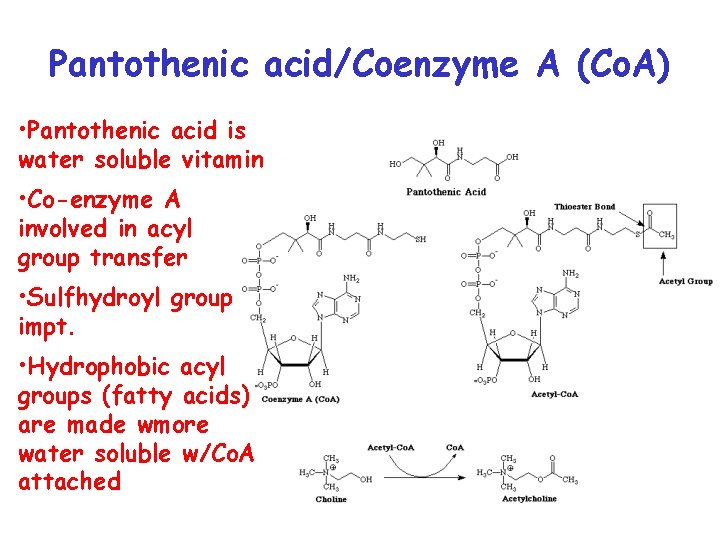
Pantothenic acid/Coenzyme A (Co. A) • Pantothenic acid is water soluble vitamin • Co-enzyme A involved in acyl group transfer • Sulfhydroyl group impt. • Hydrophobic acyl groups (fatty acids) are made wmore water soluble w/Co. A attached

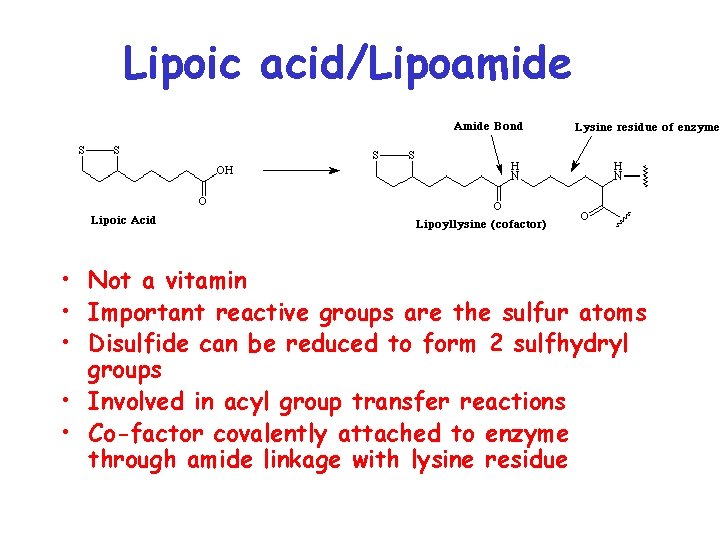
Lipoic acid/Lipoamide • Not a vitamin • Important reactive groups are the sulfur atoms • Disulfide can be reduced to form 2 sulfhydryl groups • Involved in acyl group transfer reactions • Co-factor covalently attached to enzyme through amide linkage with lysine residue

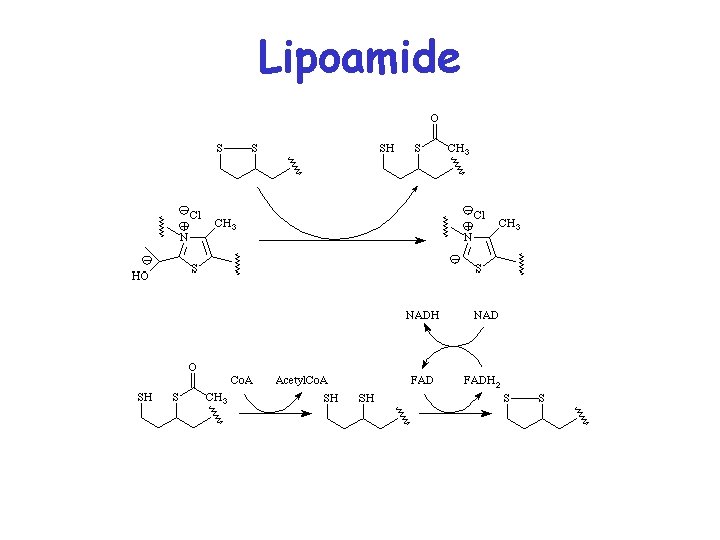
Lipoamide

Fat soluble Vitamins • Vitamin A (retinol) derived from bcarotene impt for vision, regulation of gene expression during cell differentiation, teratogenic • Vitamin D – impt in Ca absorption, regulates intestinal absorption and deposition in bones • Vitamin E – antioxidant

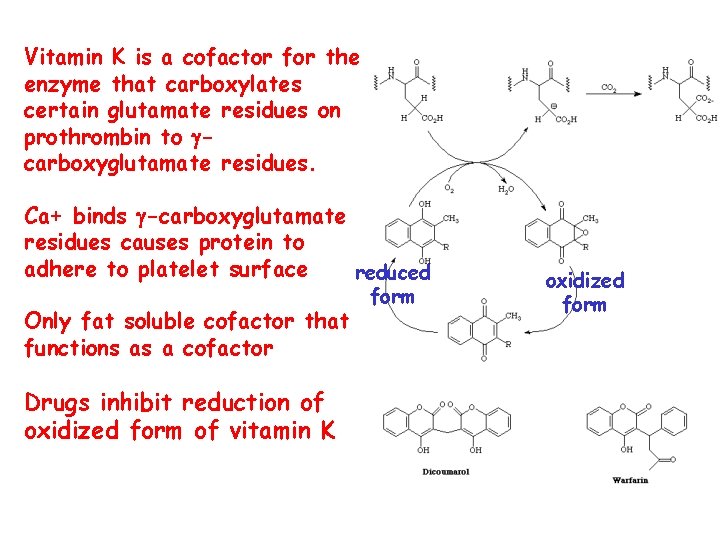
Vitamin K is a cofactor for the enzyme that carboxylates certain glutamate residues on prothrombin to gcarboxyglutamate residues. Ca+ binds g-carboxyglutamate residues causes protein to adhere to platelet surface reduced Only fat soluble cofactor that functions as a cofactor Drugs inhibit reduction of oxidized form of vitamin K form oxidized form

Ubiquinone/Plastoquinone • Lipid soluble electron carriers. • Impt in electron transport chains • Can accept or donate electrons one or two at a time (See page 223 of text)

Protein coenzymes • Usually small proteins • Active groups are either prosthetic groups or part of protein backbone • Participate in group transfer and oxidation/reduction rxns • acyl carrier protein • biotin carboxyl carrier protein

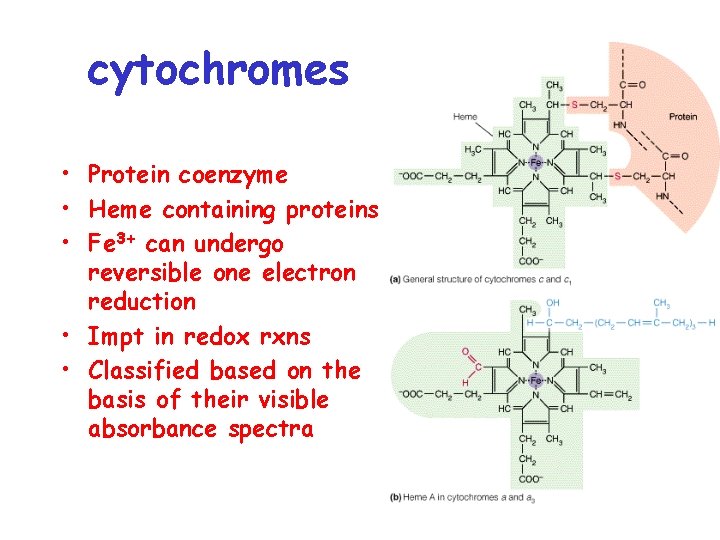
cytochromes • Protein coenzyme • Heme containing proteins • Fe 3+ can undergo reversible one electron reduction • Impt in redox rxns • Classified based on the basis of their visible absorbance spectra
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Organic and inorganic cofactors
Organic and inorganic cofactors Organic and inorganic cofactors
Organic and inorganic cofactors Is ch4o organic or inorganic
Is ch4o organic or inorganic Mikael ferm
Mikael ferm Asam oksalat rumus kimia
Asam oksalat rumus kimia Organic vs inorganic
Organic vs inorganic Organic vs inorganic compounds
Organic vs inorganic compounds Smear layer definicion
Smear layer definicion Organic vs inorganic
Organic vs inorganic Importance of organic compounds
Importance of organic compounds Difference between organic and inorganic growth
Difference between organic and inorganic growth Organic and inorganic compounds experiment
Organic and inorganic compounds experiment What is inorganic matter
What is inorganic matter What is the difference between organic and inorganic
What is the difference between organic and inorganic Organic and inorganic nutrients
Organic and inorganic nutrients Organic vs inorganic chemistry
Organic vs inorganic chemistry Organic vs inorganic
Organic vs inorganic Unsaturated hydrocarbon
Unsaturated hydrocarbon Four types of organic molecules
Four types of organic molecules Are unsaturated fats solid at room temperature
Are unsaturated fats solid at room temperature Vitamin cofactors
Vitamin cofactors Determinants by cofactor expansion
Determinants by cofactor expansion Carimazole
Carimazole Cofactor
Cofactor Inorganic polymer
Inorganic polymer Nascent soap method
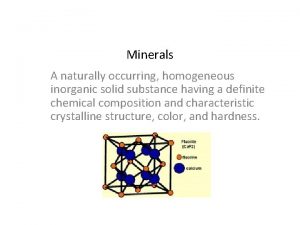
Nascent soap method Naturally occurring inorganic solid
Naturally occurring inorganic solid Solvent in chemical reactions
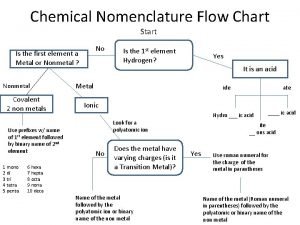
Solvent in chemical reactions Chemical nomenclature
Chemical nomenclature Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Importance of inorganic chemistry in pharmacy
Importance of inorganic chemistry in pharmacy Air pollution class 9
Air pollution class 9 Inorganic nomenclature flow chart
Inorganic nomenclature flow chart Homogeneous inorganic substances
Homogeneous inorganic substances Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air