Organic and Inorganic Matter The word organic means

- Slides: 6

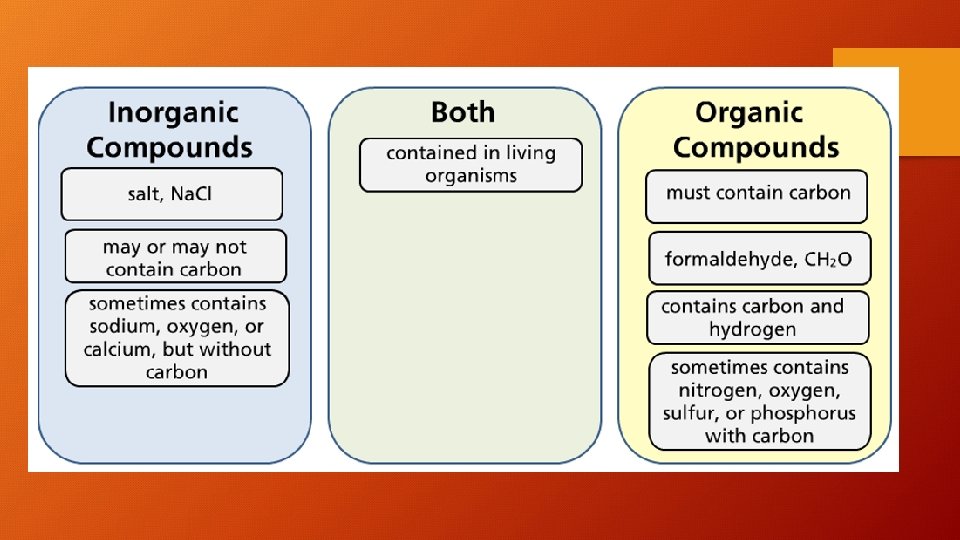
Organic and Inorganic Matter • The word "organic" means something very different in chemistry than it does when you're talking about produce and food. • Organic compounds and inorganic compounds form the basis of chemistry. The primary difference between organic compounds and inorganic compounds is that organic compounds always contain carbon while most inorganic compounds do not contain carbon. Also, nearly all organic compounds contain carbon-hydrogen or C-H bonds. Note, containing carbon is not sufficient for a compound to be considered organic! Look for both carbon and hydrogen.

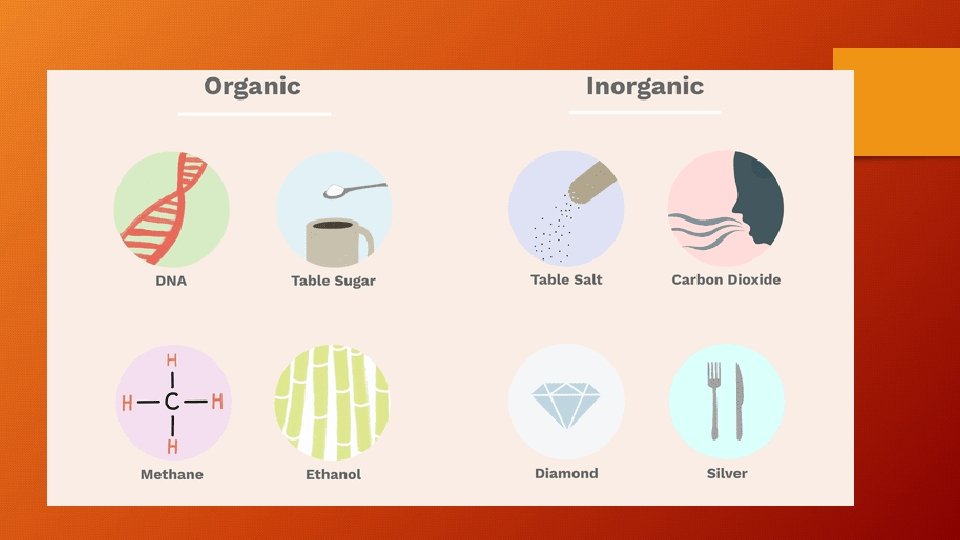
Examples of Organic Compounds or Molecules • Molecules associated with living organisms are organic. These include nucleic acids, fats, sugars, proteins, enzymes and hydrocarbon fuels. All organic molecules contain carbon, nearly all contain hydrogen, and many also contain oxygen. DNA table sugar or sucrose, C 12 H 22 O 11 benzene, C 6 H 6 methane, CH 4 ethanol or grain alcohol, C 2 H 6 O

Examples of Inorganic Compounds • Inorganics include salts, metals, substances made from single elements and any other compounds that don't contain carbon bonded to hydrogen. Some inorganic molecules do, in fact, contain carbon. table salt or sodium chloride, Na. Cl carbon dioxide, CO 2 diamond (pure carbon) silver sulfur

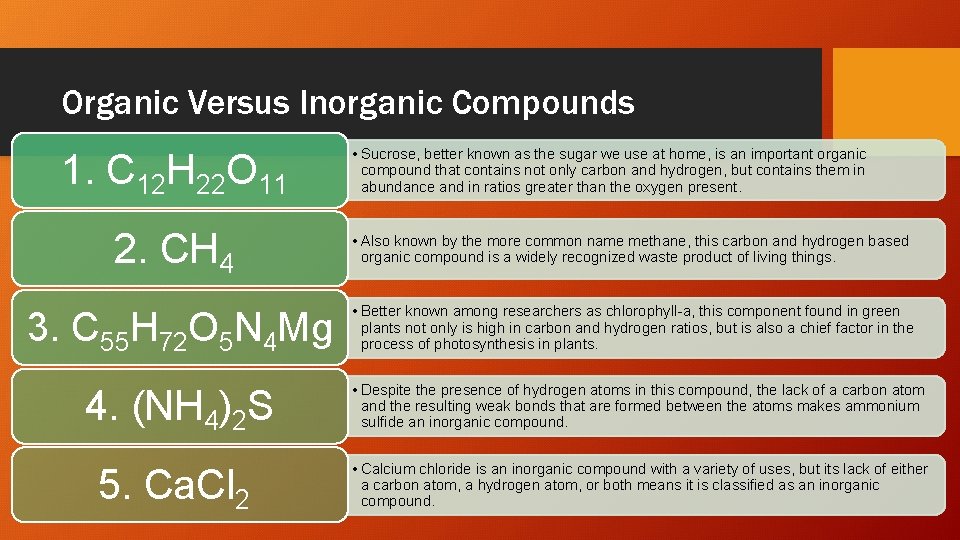
Organic Versus Inorganic Compounds 1. C 12 H 22 O 11 • Sucrose, better known as the sugar we use at home, is an important organic compound that contains not only carbon and hydrogen, but contains them in abundance and in ratios greater than the oxygen present. 2. CH 4 • Also known by the more common name methane, this carbon and hydrogen based organic compound is a widely recognized waste product of living things. 3. C 55 H 72 O 5 N 4 Mg • Better known among researchers as chlorophyll-a, this component found in green plants not only is high in carbon and hydrogen ratios, but is also a chief factor in the process of photosynthesis in plants. 4. (NH 4)2 S • Despite the presence of hydrogen atoms in this compound, the lack of a carbon atom and the resulting weak bonds that are formed between the atoms makes ammonium sulfide an inorganic compound. 5. Ca. Cl 2 • Calcium chloride is an inorganic compound with a variety of uses, but its lack of either a carbon atom, a hydrogen atom, or both means it is classified as an inorganic compound.

