Pharmaceutical Inorganic Chemistry Introduction Inorganic Chemistry Studying of
























































- Slides: 79

Pharmaceutical Inorganic Chemistry Introduction

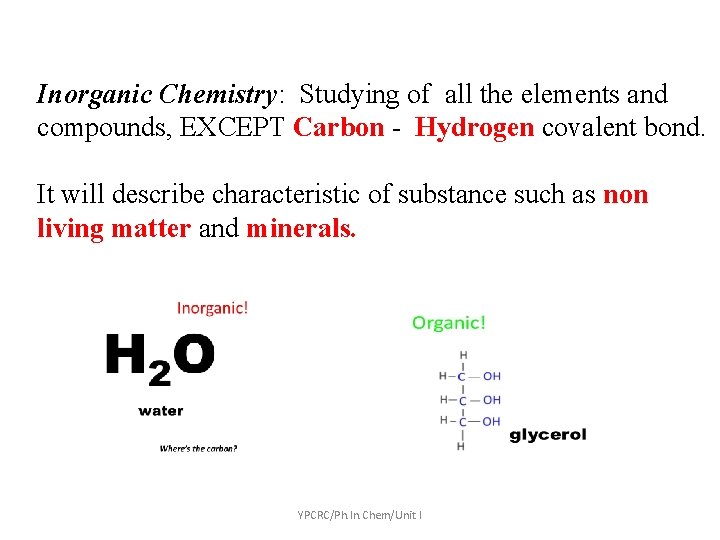
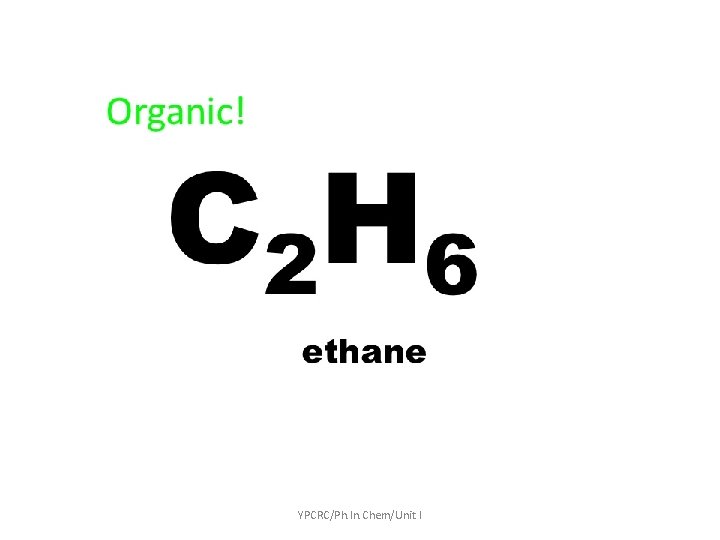
Inorganic Chemistry: Studying of all the elements and compounds, EXCEPT Carbon - Hydrogen covalent bond. It will describe characteristic of substance such as non living matter and minerals. YPCRC/Ph. In. Chem/Unit I

PHARMACEUTICAL CHEMISTRY ? • Is a science that makes use of the general laws of chemistry to study drugs. YPCRC/Ph. In. Chem/Unit I

PHARMACEUTICAL CHEMISTRY MEANS : • Extraction • Isolation • Identification (Pharmacological activity) • Characterisation (Physical/ chemical properties, compositons etc) • Structural elucidation • SAR • Synthesis of new compound YPCRC/Ph. In. Chem/Unit I

• Qulity Control • Conditions of there uses YPCRC/Ph. In. Chem/Unit I

What is Organic & In Organic • Organic chemists traditionally refer to any molecule containing carbon as an organic compound • Inorganic chemistry deals with molecules lacking carbon / • It is the study of the formation, synthesis, and properties of compounds that do not contain carbon -hydrogen bonds. Ex: Sodium chloride (Na. Cl): used as table salt. YPCRC/Ph. In. Chem/Unit I

• Organic materials include wood, paper, textiles, and animal parts (bone, leather). • They also include some natural materials that are not obviously organic (such as coal, fuel and oil, which are derived from plants and animals sources) and • Some synthetic materials (such as plastics, which are made from chemicals extracted from plant and animal products). YPCRC/Ph. In. Chem/Unit I

• INORGANIC MATERIALS: • Inorganic materials include stone, metal, ceramic, and glass, which are all made from rocks or minerals. • Some inorganic materials are found in paper- based formats: photographs contain metallic particles. YPCRC/Ph. In. Chem/Unit I

The most common differentiation between organic and inorganic compounds are: • Organic compounds are those that are found in biological system. • Inorganic chemistry describes the characteristics of substances obtained from non living things/ matter and minerals found on earth. YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

PHARMACEUTICAL CHEMISTRY ? • Is a science that makes use of the general laws of chemistry to study drugs, ie, • Their preparations, chemical nature, composition, structure, physical and chemical properties of drugs, structural elucidation, structural modification, Method of quality control and the conditions of their usage. YPCRC/Ph. In. Chem/Unit I

Also includes isolations, Structural activity relationship (SAR), Synthesis, identification and bio chemical changes after drug administration and pharmacological effects. YPCRC/Ph. In. Chem/Unit I

Todays Class: • What is FTIR & NMR and its use in pharmaceutical chemistry ? ( FYI) § Important Aspects of Pharmaceutical chemistry. § Importance of inorganic Pharmaceuticals. § What is Pharmacopoeia ? § What is monograph ? § Pharmacopoeia and its basic concept : YPCRC/Ph. In. Chem/Unit I

Fourier Transform Infrared Spectroscopy (FTIR) : used to identify organic, polymeric & in some cases, inorganic materials. Nuclear Magnetic Resonance (NMR): use in Quality control (QC) & research, for determining the content and purity of a sample as well as its molecular structure. YPCRC/Ph. In. Chem/Unit I

Important Aspects of Pharmaceutical chemistry : • It includes various methods for Extraction, isolation, purification and characterisation of medically active agents and materials from natural sources (vegetable, mineral or animal) which find various uses in the treatment of diseases. • Various synthesis of medicinal agents that could not be obtained from natural sources due the reason of economy, purity or adequate supply of substances obtained from natural sources. YPCRC/Ph. In. Chem/Unit I

• It includes natural substances are converted into products with high therapeutic effect. • It includes determination of the forms of medicinal agent which shows optimum medicinal activity. • It includes determination of chemical and biological incompatibilities among the various ingredients of the prescription. • It helps to establishment of safe and practical standards with respect to both dosage and quality in order to maintain uniform and therapeutical standards for all form of medications. YPCRC/Ph. In. Chem/Unit I

• It improve and promote the use of chemical agents for prevention of illness, cure of diseases , alleviation of pain and search for new therapeutical agents. YPCRC/Ph. In. Chem/Unit I

• Importance of inorganic Pharmaceuticals: • Medicinally useful for their therapeutic purpose. Example: Astringents, antimicrobials, etc. • Useful as pharmaceutical aids. Examples : Bentonite , Talc etc. • To Change the reaction of body fluids. Example antacids, alkalis, mineral acids. YPCRC/Ph. In. Chem/Unit I

• Replacing the normal content of body fluids. Example: sodium, potassium, calcium, etc. . • Useful as reagents to carry out the reactions. Example: catalysts ( Platimum) , oxidising ( KMNO 4) and reducing agents ( Li. Al. H 4). • Examples: Collins reagent, Fenton's reagent, and Grignard reagents • Useful in pharmaceutical analysis. Example: Titrants such as KMNO 4. YPCRC/Ph. In. Chem/Unit I

Overview Introduction Indian Pharmacopoiea British Pharmacopoiea EUROPEAN Pharmacopoiea YPCRC/Ph. In. Chem/Unit I

• Pharmacopoeia: • The word derives from the ancient Greek word • Pharmaco means drug & poeia- to make. YPCRC/Ph. In. Chem/Unit I

Pharmacopoeia Means: • It is a legally binding collection, prepared by a national or regional authority. Contains list of: • Medicinal substances, • Crude drugs, • Formulas. For making preparation of Medicine. YPCRC/Ph. In. Chem/Unit I

• Many of the countries have issued their own pharmacopoeia, to serve as a guide and a book of standards for pharmaceutical preparations to be used in individual country. YPCRC/Ph. In. Chem/Unit I

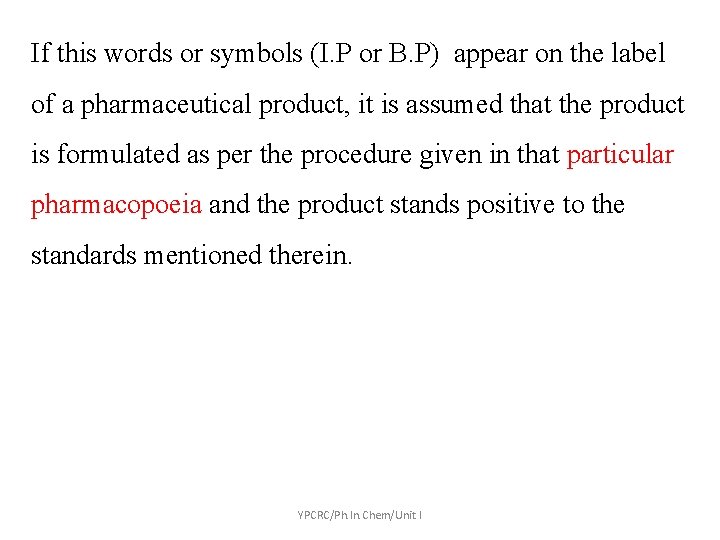
If this words or symbols (I. P or B. P) appear on the label of a pharmaceutical product, it is assumed that the product is formulated as per the procedure given in that particular pharmacopoeia and the product stands positive to the standards mentioned therein. YPCRC/Ph. In. Chem/Unit I

History of pharmacopoeia and its basic concept : • Each country has its own legislation on pharmaceutical preparations. • Which sets standards and required quality index for, medicament, raw materials & preparations employed in manufacture of drugs. • These regulations are presented in separate articles. • General and Specific matters relating to individual drugs are published in the form of a book called a Pharmacopoeia. YPCRC/Ph. In. Chem/Unit I

All Pharmacopoeias consist of the three main sections: 1) Introduction: 2) Monograph: 3) Appendices: YPCRC/Ph. In. Chem/Unit I

Introduction (General notices) : It will point out to pharmaceutical progress, since compare to last edition, it shows the additions / deletions/ or changes done in the current editions. YPCRC/Ph. In. Chem/Unit I

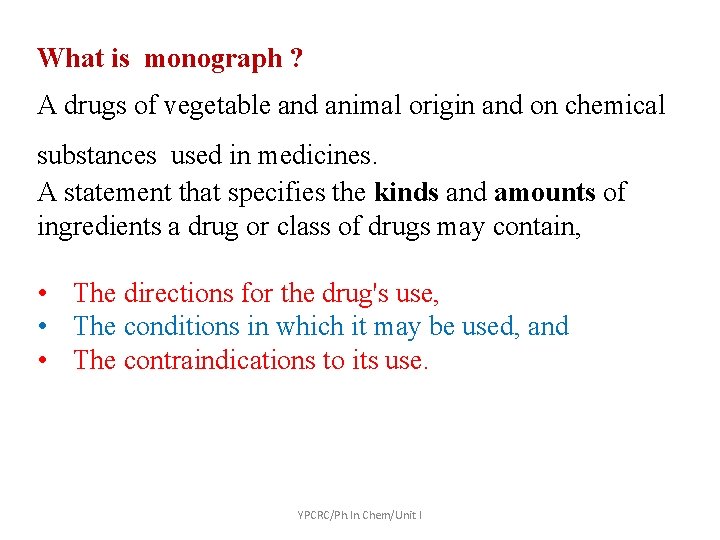
What is monograph ? A drugs of vegetable and animal origin and on chemical substances used in medicines. A statement that specifies the kinds and amounts of ingredients a drug or class of drugs may contain, • The directions for the drug's use, • The conditions in which it may be used, and • The contraindications to its use. YPCRC/Ph. In. Chem/Unit I

Monograph will provide following information OR Description about the drug and pharmaceutical aids: • • • Title Formula and molecular weight Category Doses Description Solubility Standards Identification Limits for impurities Assay YPCRC/Ph. In. Chem/Unit I p. H & Storage

1) Title: Stated in English and refers to the official name of the compound, Eg: Calcium carbonate can also called precipitated chalk. 2) Formula weight and molecular weight: Eg: Magnesium chloride. Water : WT, 202. 30 3) Category: This describes therapeutic or pharmacologic or pharmaceutical application of the compound • Eg: antacid, laxative, astringent etc: 4) Dose: These are the quantities for the guidance to achive desired therapeutic effects in adults. Eg: Calcium carbonate dose 1 to 5 gm. YPCRC/Ph. In. Chem/Unit I

5) Description : This shows the physical properties of the substances like crystalline or amorphous nature, colour, odour, taste, etc… this properties gives preliminary evaluation not standard or test for purity , Eg: Calcium carbonate fine, white microcrystalline powder , odourless and tasteless. 6) Solubility: This is usually given in water, some times in hot water, in alcohol, in glycerol, in solvent ether and sometime in organic solvent, acids or alkalis. This above mentioned method refer to primarily information, but if it is given under the quantitative solubility test , then it taken as standard. YPCRC/Ph. In. Chem/Unit I

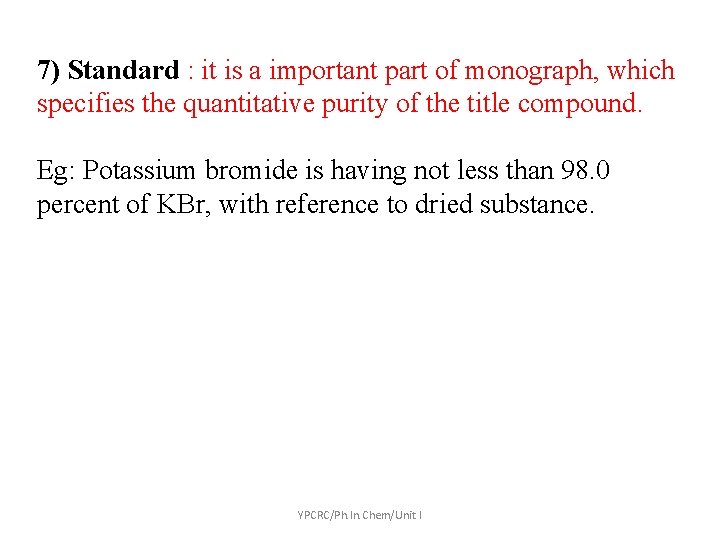
7) Standard : it is a important part of monograph, which specifies the quantitative purity of the title compound. Eg: Potassium bromide is having not less than 98. 0 percent of KBr, with reference to dried substance. YPCRC/Ph. In. Chem/Unit I

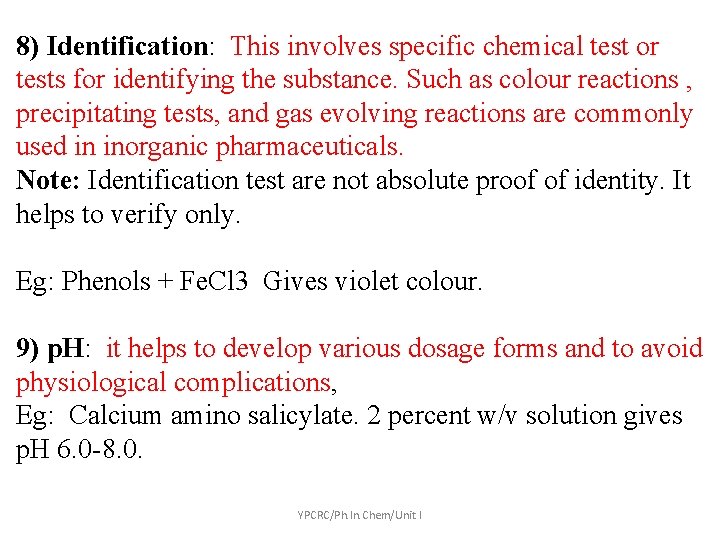
8) Identification: This involves specific chemical test or tests for identifying the substance. Such as colour reactions , precipitating tests, and gas evolving reactions are commonly used in inorganic pharmaceuticals. Note: Identification test are not absolute proof of identity. It helps to verify only. Eg: Phenols + Fe. Cl 3 Gives violet colour. 9) p. H: it helps to develop various dosage forms and to avoid physiological complications, Eg: Calcium amino salicylate. 2 percent w/v solution gives p. H 6. 0 -8. 0. YPCRC/Ph. In. Chem/Unit I

10) Limit test for impurities: 11) Assay: It gives step by step description of chemical analytical method for the active substance. Eg: Titrimetric method. 12) Storage: This is the last term under the monograph. These directions are useful in preserving the activity of the chemical. • Eg: Ferrous fumarate - store in a light resistant container • Insulin injection – store in multiple dose container at a temperature between 2 - 8 degree c. it should not be allowed to freeze. YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

Appendices : Appendix 1: Describes about various apparatus needed for various pharmacopoeial tests and assays, such as Nessler’s cylinders, thermometers etc. . Appendix 3: Describes various chemical testes and assays Appendix 5 : Some physical tests and determinations like loss on drying, determination of p. H. etc YPCRC/Ph. In. Chem/Unit I

Appendix 6: Useful direction on cleaning glassware. Appendix 7: Reagents and solutions needed for various tests and assays, their method of preparation, standards etc. Appendix 9: Describe the names and symbols used in the pharmacopoeia for weights and measures and of elements and their atomic weight have been described. YPCRC/Ph. In. Chem/Unit I

General Rules : 1. Only the pharmaceuticals which are commonly and currently use are included in the pharmacopeia 2. The substance which are found to be undesirable from past experience and which are currently not in use are excluded. 3. The chemicals are commercially available in pure state and which are commonly used for other purpose are excluded YPCRC/Ph. In. Chem/Unit I

4. The chemicals which has been used for external application and internal consumption by human beings, are form the part of pharmacopeia. 5. In the pharmacopoeia only minimum standard for a chemical are prescribed. 6. Only those mentioned in the pharmacopoeia are considered official as per the legislation of the country. YPCRC/Ph. In. Chem/Unit I

INDIAN PHARMACOPOEIA (I, P) YPCRC/Ph. In. Chem/Unit I

Publication of I. P on regular basis is an important mandate of IPC aimed at improvement of health of the people to ensuring the, • Quality • Safety • Efficacy Of the medicine YPCRC/Ph. In. Chem/Unit I

The latest Edition of Indian Pharmacopoeia, IP-2018 has been released on 29 th September 2017 by Sh. C. K. Mishra. It is published by the Indian Pharmacopoeia Commission (IPC) on behalf of the Ministry of Health & Family Welfare, Government of India in fulfillment of the requirements of the Drugs and Cosmetics Act, 1940 and the Rules thereunder. “It intends to help in the licensing of manufacturing, inspection and distribution of medicines. ” YPCRC/Ph. In. Chem/Unit I

IP-2018 has been brought out in 4 Volumes incorporating 220 new monographs • (Chemical Monographs (170), • Herbal Monographs (15), • Blood and Blood-related products (10), • Vaccines and Immunosera for Human use monographs (02), • Radiopharmaceutical monographs (03), • Biotechnology Derived Therapeutic Products (06), YPCRC/Ph. In. Chem/Unit I

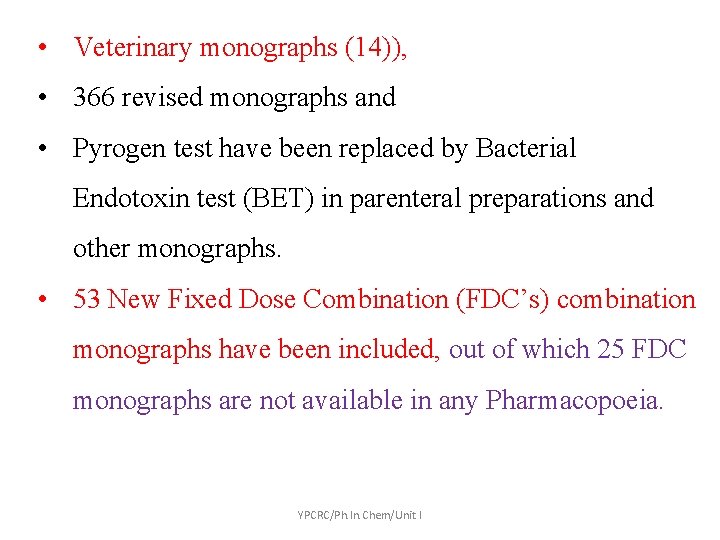
• Veterinary monographs (14)), • 366 revised monographs and • Pyrogen test have been replaced by Bacterial Endotoxin test (BET) in parenteral preparations and other monographs. • 53 New Fixed Dose Combination (FDC’s) combination monographs have been included, out of which 25 FDC monographs are not available in any Pharmacopoeia. YPCRC/Ph. In. Chem/Unit I

The National pharmacopoeia of India is known as pharmacopoeia of india (I. P). What is national pharmacopoeia / I. P tells : • A national pharmacopoeia is a government publication which shows lists of various drugs and formulae for medical products with description, and tests along with standard to which they must confirm. YPCRC/Ph. In. Chem/Unit I

• National pharmacopoeia is a legal and official book of standards and • The information about the drug given in the pharmacopoeia is considered authoritative and valid. YPCRC/Ph. In. Chem/Unit I

• In India – pre independence days, • British Pharmacopoeia, was employed as the official book of standards. YPCRC/Ph. In. Chem/Unit I

• The process of publishing the first Indian Pharmacopoeia started in the year 1944 under the chairmanship of Col. R. N. Chopra • In 1948 government of India appointed an Indian Pharmacopeia committee for preparing ‘Pharmacopeia of India’. YPCRC/Ph. In. Chem/Unit I

Legal and official book published by IPC • IPC (Indian pharmacopoeia commission) established in 1945 • Central Indian Pharmacopoeia Laboratory • Head quarter ( Ghaziabad ) UP • IPC controlled by ministry of Health and family welfare • IP is written in English and all the Monograph written in Latin YPCRC/Ph. In. Chem/Unit I

• IP contains a collection authoritative procedures for analysis and specifications for drugs. • IP prescribes standards for identity, purity and strength of drugs essentially required from health care view point of human beings and animals YPCRC/Ph. In. Chem/Unit I

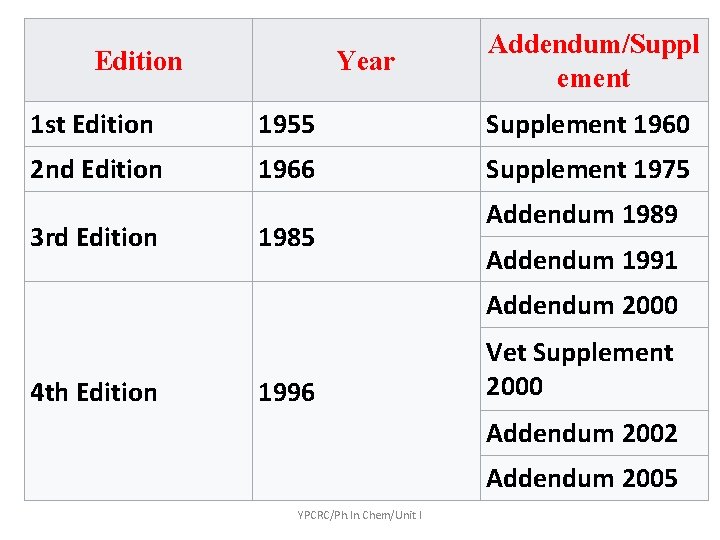
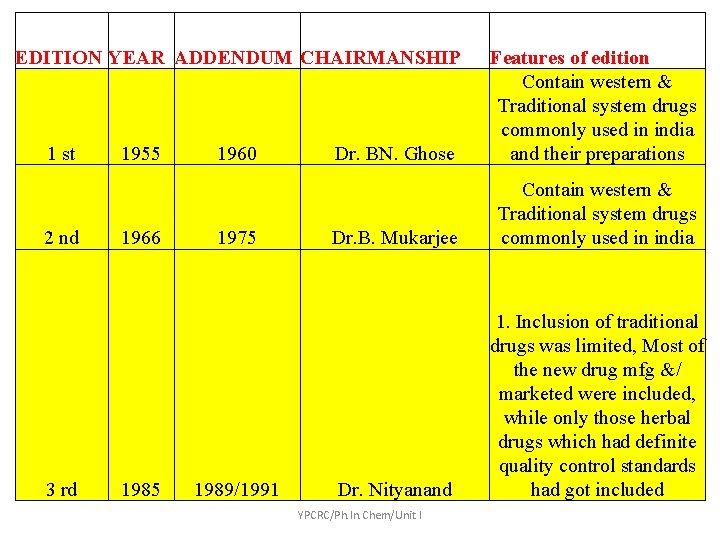
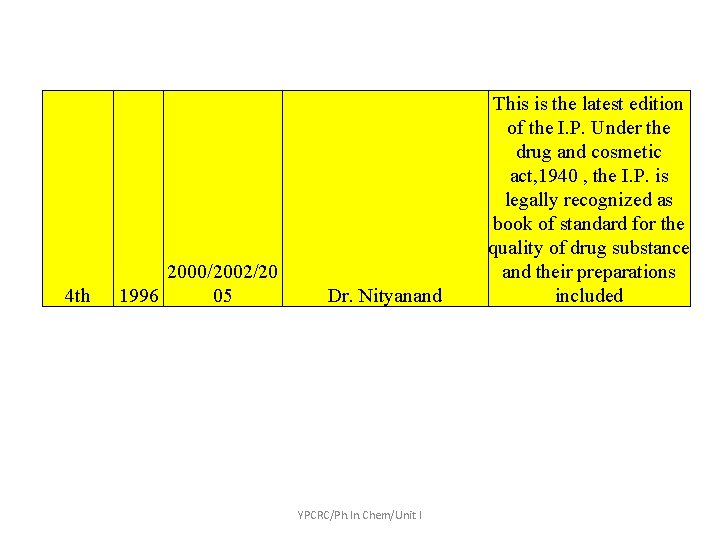
Edition Year Addendum/Suppl ement 1 st Edition 1955 Supplement 1960 2 nd Edition 1966 Supplement 1975 3 rd Edition 1985 Addendum 1989 Addendum 1991 Addendum 2000 4 th Edition 1996 Vet Supplement 2000 Addendum 2002 Addendum 2005 YPCRC/Ph. In. Chem/Unit I

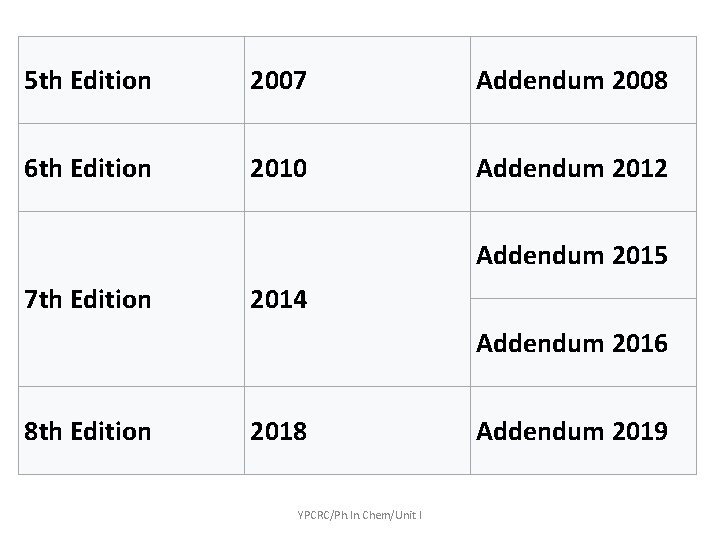
5 th Edition 2007 Addendum 2008 6 th Edition 2010 Addendum 2012 Addendum 2015 7 th Edition 2014 Addendum 2016 8 th Edition 2018 YPCRC/Ph. In. Chem/Unit I Addendum 2019

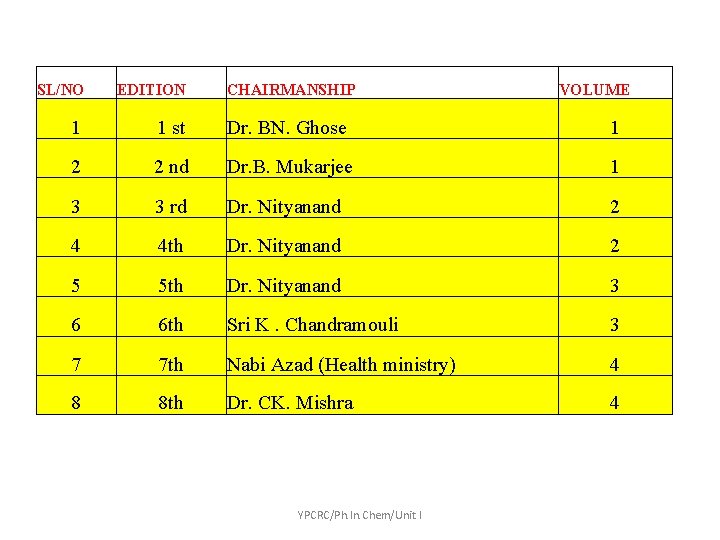
SL/NO EDITION CHAIRMANSHIP 1 1 st Dr. BN. Ghose 1 2 2 nd Dr. B. Mukarjee 1 3 3 rd Dr. Nityanand 2 4 4 th Dr. Nityanand 2 5 5 th Dr. Nityanand 3 6 6 th Sri K. Chandramouli 3 7 7 th Nabi Azad (Health ministry) 4 8 8 th Dr. CK. Mishra 4 YPCRC/Ph. In. Chem/Unit I VOLUME

EDITION YEAR ADDENDUM CHAIRMANSHIP 1 st 2 nd 3 rd 1955 1966 1985 1960 1975 1989/1991 Dr. BN. Ghose Features of edition Contain western & Traditional system drugs commonly used in india and their preparations Dr. B. Mukarjee Contain western & Traditional system drugs commonly used in india Dr. Nityanand 1. Inclusion of traditional drugs was limited, Most of the new drug mfg &/ marketed were included, while only those herbal drugs which had definite quality control standards had got included YPCRC/Ph. In. Chem/Unit I

4 th 2000/2002/20 1996 05 Dr. Nityanand YPCRC/Ph. In. Chem/Unit I This is the latest edition of the I. P. Under the drug and cosmetic act, 1940 , the I. P. is legally recognized as book of standard for the quality of drug substance and their preparations included

• On 15 th December 1820, the first united state pharmacopoeia (U. S. P) was released. • In 1864, the first British Pharmacopoeia (B. P) was published with inclusion of mono graphs on, Benzoic acid, Gallic acid , tartaric acid , camphor , lactose, etc. . YPCRC/Ph. In. Chem/Unit I

British Pharmacopoeia (B. P) • The reason for appearance of a (B. P) is regards to the medical act of 1858, • which need of publication of a book having a list of medicines and compounds, manner of preparing them together, with standard weight and measures. • The British Pharmacopoeia (BP) is the national pharmacopoeia of the United Kingdom. YPCRC/Ph. In. Chem/Unit I

• The British Pharmacopoeia is an important statutory component in the control of medicines. • Along with the British National Formulary (BNF), it defines the UK's pharmaceutical standards. • In 1907 the British Pharmacopoeia was supplemented by the British Pharmaceutical Codex, which gave information on drugs and other pharmaceutical substances not included in the BP, and provided standards for these. YPCRC/Ph. In. Chem/Unit I

History • The first edition of the British Pharmacopoeia was published in 1864 • Next 2 nd edition in 1867, an addendum in 1874. • 3 rd edition in 1885, further addendum in 1890. • 4 th edition in 1898. • 5 th edition until 1914 YPCRC/Ph. In. Chem/Unit I

• After publication of B. P 1914, their was found a huge gap on technical complexity of the drug specifications was increased. Different kind of changes to be done in pharmacopoeia. • From 6 edition 1932 , on words it was recommended that the B. P to be revised every 10 years onces. • In 7 th edition of 1948, generic name were provided for substances newly introduced into the medicine and • Method of analysis, disintegration tests for tablets and sterilization methods were expanded. YPCRC/Ph. In. Chem/Unit I

• Many new monographs related to penicillin's & sex hormones were included. • During this time it was decided that the normal interval between new editions should be 5 instead of 10 YEARS. Due to rapid progress in the development of pharmaceutical and pharmacological has been made. • Next new edition appear in 1953 ( the title of drug and preparation were given in English instead of Latin). • In this edition Capsules , constituted as new group. The implant methods for sex hormones and their standards were described. YPCRC/Ph. In. Chem/Unit I

• Next new 9 th edition on 1958 was having 160 new monograph (Tranquilising drugs and spectrophotometric analysis were added. • Tenth addition appeared on 1963. • 13 th edition of B. P. in 1980. YPCRC/Ph. In. Chem/Unit I

• Currently the B. P start publishing into two volumes due to an expansion of drug and information. • Volume 1 deals with medicinal and pharmaceutical substances and also include Infrared reference spectra needed for the identification of materials. • Volume 2 contains sections on formulated preparations, blood products, immunological products, radio pharmaceutical preparation and surgical materials. YPCRC/Ph. In. Chem/Unit I

Editions • The current edition of the British Pharmacopoeia comprises six volumes which contain nearly 3, 000 monographs for drug substances, excipients and formulated preparation • Items used exclusively in veterinary medicine in the UK are included in the BP YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

Editions • The European Pharmacopoeia is a pharmacopoeia that aims to provide common quality standards throughout Europe to control the quality of medicines and the substances used to manufacture them. YPCRC/Ph. In. Chem/Unit I

YPCRC/Ph. In. Chem/Unit I

• contains more than 2220 monographs and 340 general chapters. YPCRC/Ph. In. Chem/Unit I

United States Pharmacopoeia: Dr, Lyman spalding of New York proposed a plan to the medical society of the country at New York for the publishing of National Pharmacopoeia. 1 st edition published on 1820 “Which was having 217 drugs “ Subsequent edition was published after a gap of 10 years. 9 th edition was published in 1905. “Which tells 25 degree C as the standard temperature for specific gravity and solubility statements”. YPCRC/Ph. In. Chem/Unit I

• The 1940 convention directed that the pharmacopoeia must be revised every 5 years. • On July 5, 1974, unification of USP and NF (National formulary) ws announced. • All the drugs and drug products were covered in USP whereas NF is devoted exclusively to pharmaceutical ingredients YPCRC/Ph. In. Chem/Unit I

Thank You YPCRC/Ph. In. Chem/Unit I