Properties of Organic and Inorganic Compounds Experiment 1






- Slides: 16

Properties of Organic and Inorganic Compounds Experiment 1 Chem 121 Organic Chemistry Laboratory 1

What is ORGANIC CHEMISTRY? 2

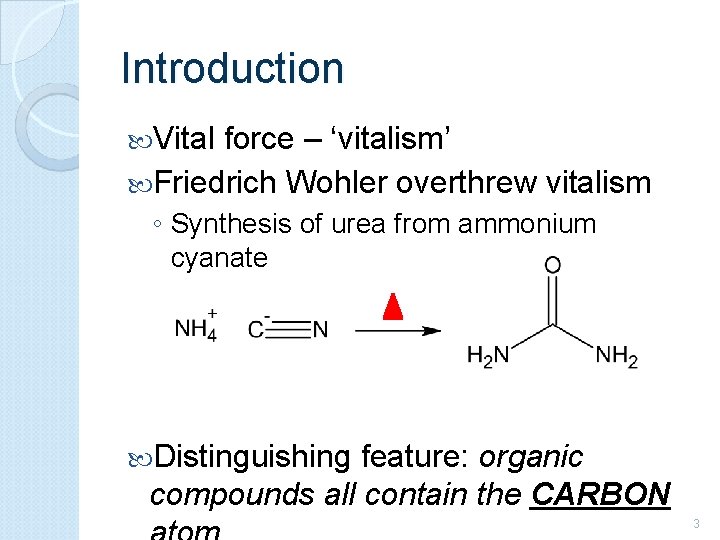
Introduction Vital force – ‘vitalism’ Friedrich Wohler overthrew vitalism ◦ Synthesis of urea from ammonium cyanate Distinguishing feature: organic compounds all contain the CARBON 3

What is ORGANIC chemistry? the study of carbon containing compounds ◦ Other elements in organic compounds: H, O, N, S, P, Cl, Br, I and other transition metals Why Carbon? ◦ Can share four valence electrons ◦ Form strong covalent bonds ◦ Form rings and long chains, e. g. benzene and DNA 4

Definition of terms Ionic compounds: compounds made up of a metal cation and a nonmetal anion ◦ e. g. Na. Cl, KNO 3 Intramolecular forces of attraction: forces existing within molecules that holds the atoms together ◦ e. g. Ionic bond, covalent bond, metallic bond Intermolecular forces of attraction: 5

Intermolecular Forces of Attraction Ion-dipole ◦ Between an ionic compound a polar compound e. g. Na. Cl dissolved in water Dipole-dipole ◦ Between two polar compounds e. g. HCl dissolved in water 6

Hydrogen Bonding ◦ Requirement: H atoms bonded to F, O, N ◦ Strongest intermolecular force ◦ e. g. NH 3 in H 2 O London dispersion forces/van der Waals forces ◦ Between two NONPOLAR compounds ◦ Weakest intermolecular force; present in all organic molecules 7

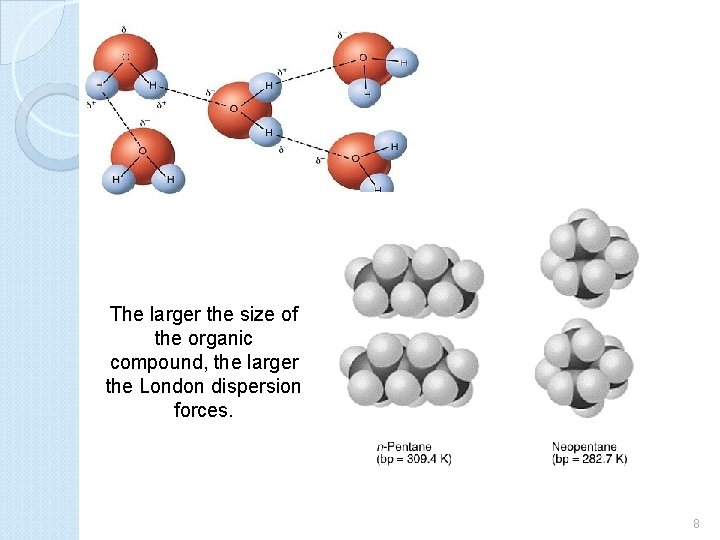
The larger the size of the organic compound, the larger the London dispersion forces. 8

Properties of Organic Compounds Flammable ◦ Due to the C-C bond energies in organic compounds ◦ Energy released is in the form of heat Ethanol vs. Water ◦ Ethanol – produces the distinct blue flame ◦ Water – smothers flame instead of generating one 9

Charring ◦ also known as burning, scorching ◦ organic compounds are sensitive to heat ◦ End result of charring: elemental C Sucrose • disaccharide • common ingredient in sweet foods like ice cream, candy • also works as a food preservative 10

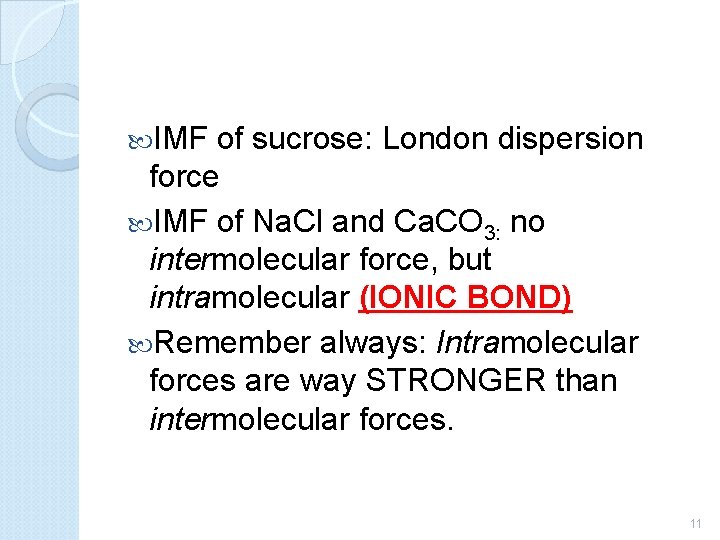
IMF of sucrose: London dispersion force IMF of Na. Cl and Ca. CO 3: no intermolecular force, but intramolecular (IONIC BOND) Remember always: Intramolecular forces are way STRONGER than intermolecular forces. 11

Solubility ◦ relies on the intermolecular forces of organic compounds ◦ ‘like dissolves like’ ◦ Polar solvents dissolve in polar solutes. ◦ Nonpolar solvents dissolve in nonpolar solutes. ◦ Organic compounds = mostly nonpolar It only follows that most organic compounds are soluble in organic solvents. 12

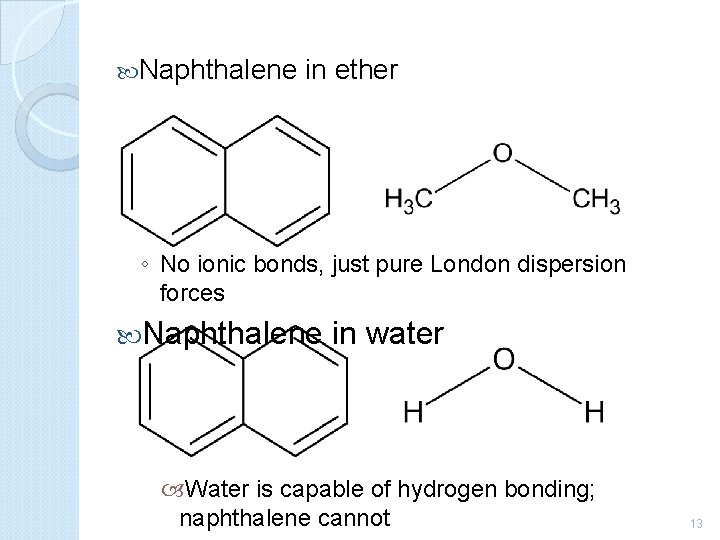
Naphthalene in ether ◦ No ionic bonds, just pure London dispersion forces Naphthalene in water Water is capable of hydrogen bonding; naphthalene cannot 13

Electrical Conductivity Electrical conductivity is only possible when a compound contains charged particles (i. e. an electrolyte) ◦ e. g. Na. Cl, Na. NO 3 are electrolytes Since most organic compounds are molecular, not ionic, it does not conduct electricity. 14

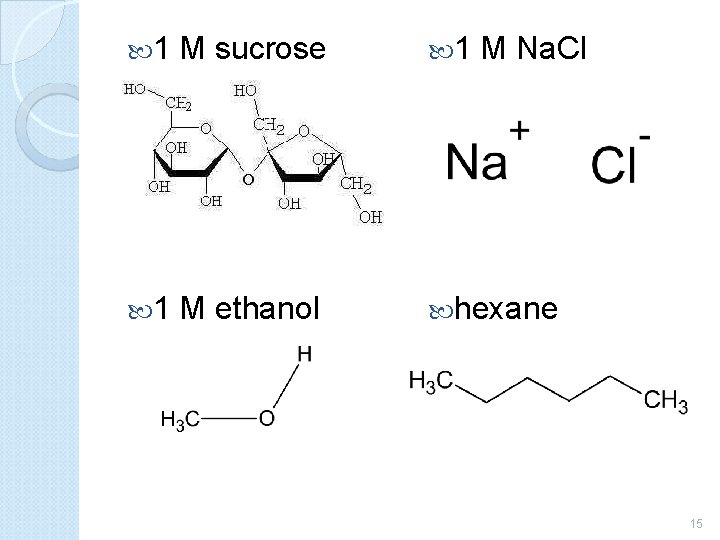
1 M sucrose 1 M Na. Cl 1 M ethanol hexane 15

Summary Organic chemistry is the study of carbon compounds. Organic compounds have the ff properties: ◦ Flammable ◦ Combustible ◦ Immiscble in polar solvents like water ◦ Non-electrolytes; do not conduct electricity 16
 Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Importance of organic chemistry
Importance of organic chemistry Difference between organic and inorganic
Difference between organic and inorganic Organic vs inorganic compounds
Organic vs inorganic compounds Organic versus inorganic compounds
Organic versus inorganic compounds Organic and inorganic cofactors
Organic and inorganic cofactors Organic and inorganic cofactors
Organic and inorganic cofactors Organic vs inorganic growth
Organic vs inorganic growth Organic and inorganic nutrients
Organic and inorganic nutrients Thickness of smear layer
Thickness of smear layer Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Organic vs inorganic
Organic vs inorganic Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Unsaturated hydrocarbon
Unsaturated hydrocarbon Asam oksalat rumus kimia
Asam oksalat rumus kimia Organic vs inorganic
Organic vs inorganic