Inorganic Chemistry Inorganic Chemistry Three most common elements






- Slides: 11

Inorganic Chemistry

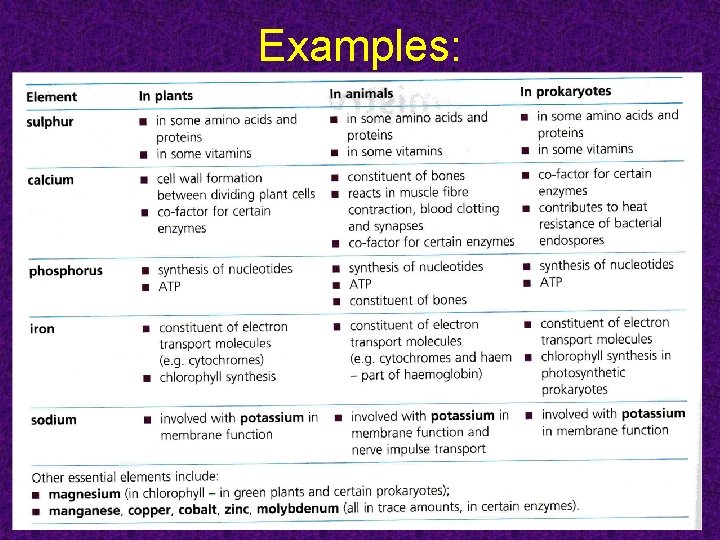
Inorganic Chemistry • • • Three most common elements of life are carbon, hydrogen, and oxygen Other elements needed by living organisms include nitrogen, sulfur, phosphorus, iron, calcium, sodium and potassium C, O, H, and N make up 96% of living matter

Examples:

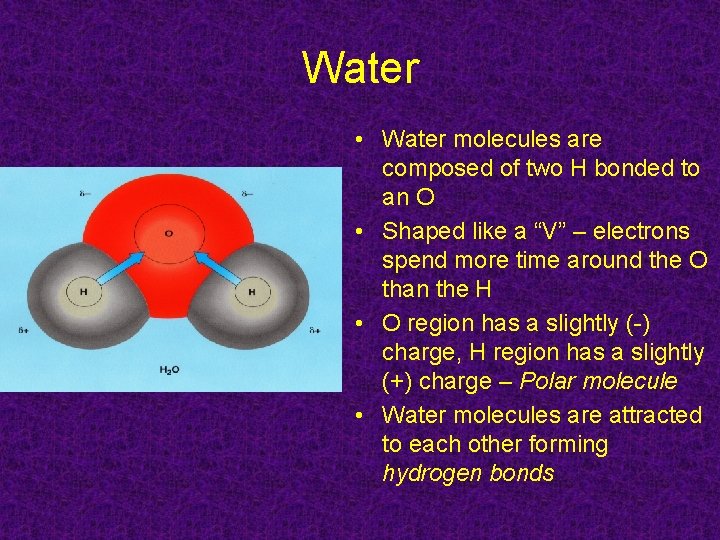
Water • Water molecules are composed of two H bonded to an O • Shaped like a “V” – electrons spend more time around the O than the H • O region has a slightly (-) charge, H region has a slightly (+) charge – Polar molecule • Water molecules are attracted to each other forming hydrogen bonds

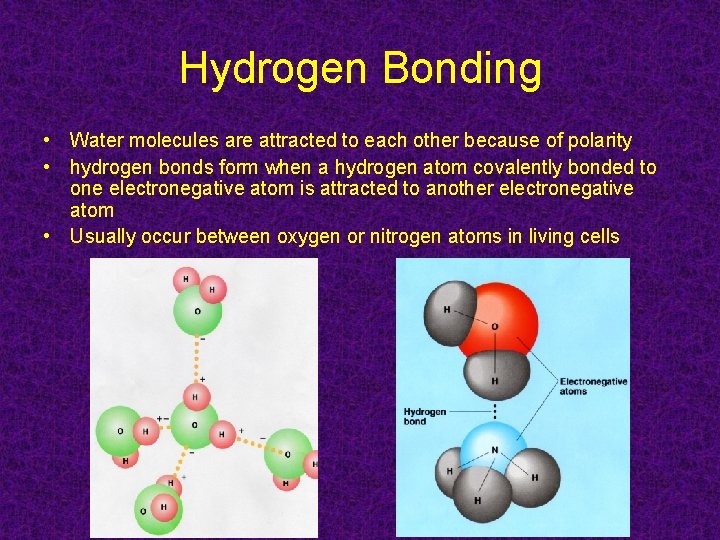
Hydrogen Bonding • Water molecules are attracted to each other because of polarity • hydrogen bonds form when a hydrogen atom covalently bonded to one electronegative atom is attracted to another electronegative atom • Usually occur between oxygen or nitrogen atoms in living cells

Why is Water so Important to Life? • All life is dependent on water (65 – 95% of all living tissue is water) • Many of water’s unique properties are due to hydrogen bonding

Why is Water so Important to Life? • Water is an excellent solvent because of polarity – dissolves a wide variety of substances – Ionic compounds and polar molecules dissolve easily in water – hydrophilic – Uncharged and nonpolar molecules do not dissolve in water – hydrophobic

Why is Water so Important to Life? • Thermal Properties of Water - Water moderates the effects of temperature – water does not change temperature easily 1. Water has high specific heat – much heat energy has to be added to get water to heat up (to break H bonds) • Ex. Bodies of water are slow to heat even when the air temperature is high

Why is Water so Important to Life? 2. Water has high heat of vaporization – much energy is needed to change liquid water to water vapor (because of H bonds) – good for cooling • Sweating to cool 3. Water has high heat of fusion – much energy has to be removed to get water to cool down

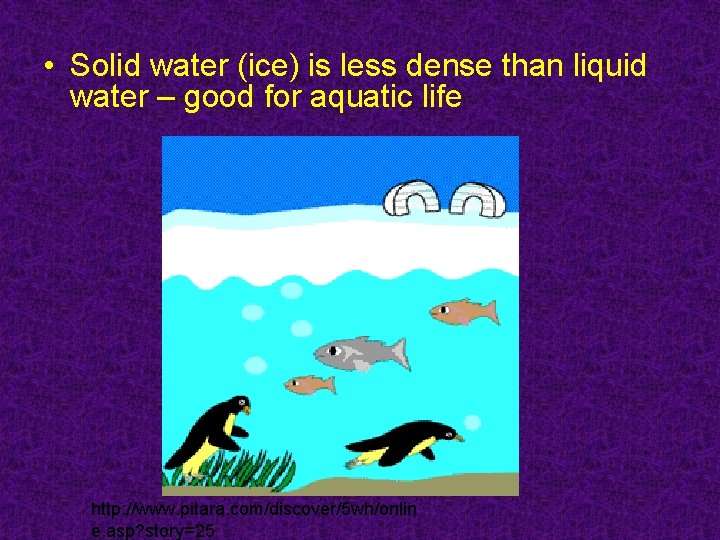
• Solid water (ice) is less dense than liquid water – good for aquatic life http: //www. pitara. com/discover/5 wh/onlin e. asp? story=25

• Water molecules tend to stick together – high cohesion and adhesion – water molecules tend to stick to each other (cohesion) and other substance (adhesion) • important for water transport in plants – Surface tension – outermost molecules of water form hydrogen bonds with water molecules below them – gives water high surface tension (ex. Water strider insects)