IUPAC Nomenclature of Organic Compounds In Chemistry an




- Slides: 21

IUPAC Nomenclature of Organic Compounds

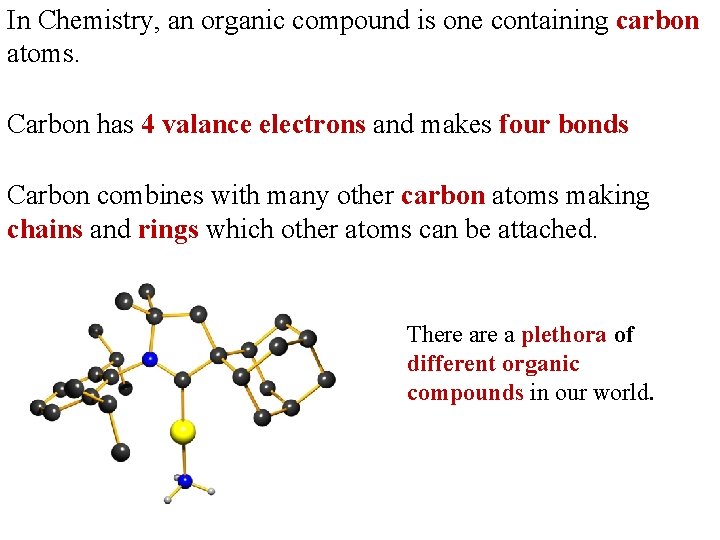
In Chemistry, an organic compound is one containing carbon atoms. Carbon has 4 valance electrons and makes four bonds Carbon combines with many other carbon atoms making chains and rings which other atoms can be attached. There a plethora of different organic compounds in our world.


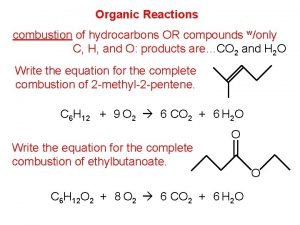
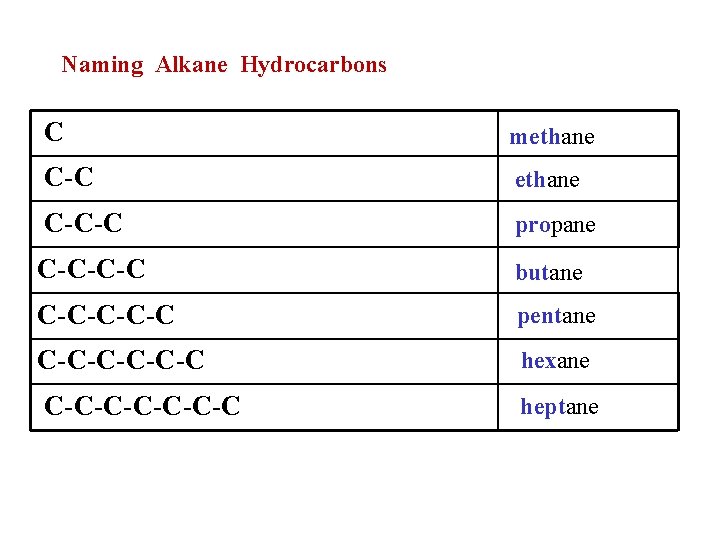
Naming Alkane Hydrocarbons C methane C-C-C propane C-C-C-C butane C-C-C pentane C-C-C-C hexane C-C-C-C heptane

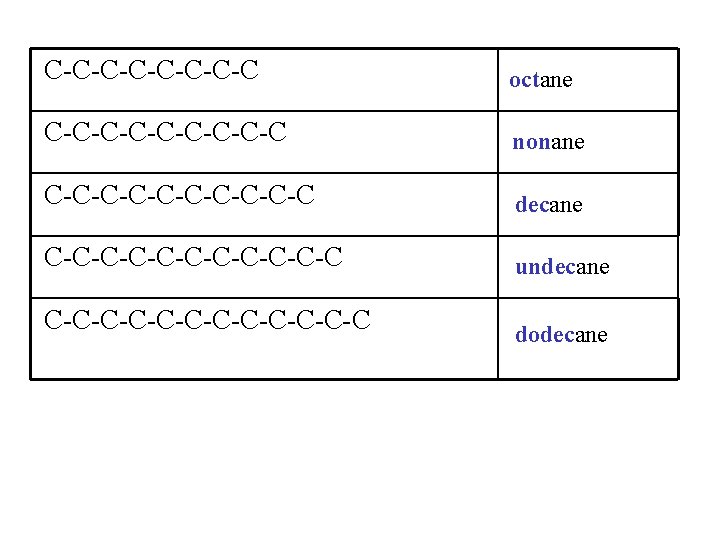
C-C-C-C-C octane C-C-C-C-C nonane C-C-C-C-C-C decane C-C-C-C-C-C undecane C-C-C-C-C-C-C dodecane

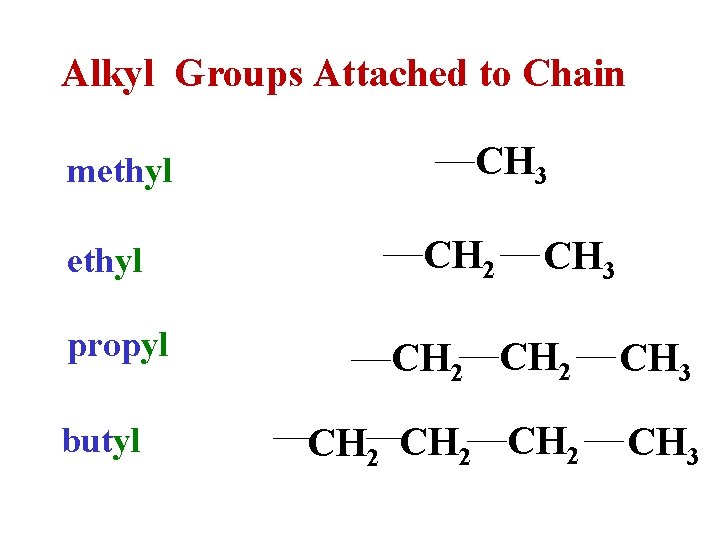
Alkyl Groups Attached to Chain methyl propyl butyl CH 3 CH 2 CH 2 CH 3

Naming Alkanes- hydrocarbons with single bonds

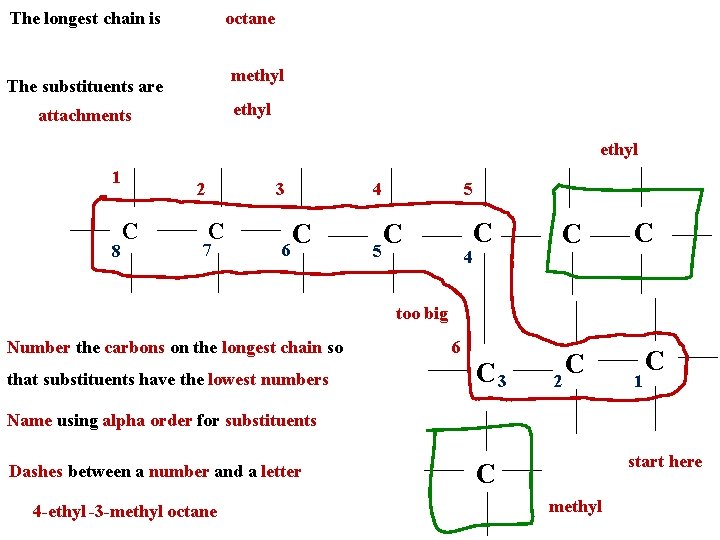
The longest chain is octane methyl The substituents are ethyl attachments ethyl 1 8 2 C 3 C 7 C 6 4 5 5 C 4 C C C 3 C C too big Number the carbons on the longest chain so that substituents have the lowest numbers 6 2 1 C Name using alpha order for substituents Dashes between a number and a letter 4 -ethyl -3 -methyl octane start here C methyl

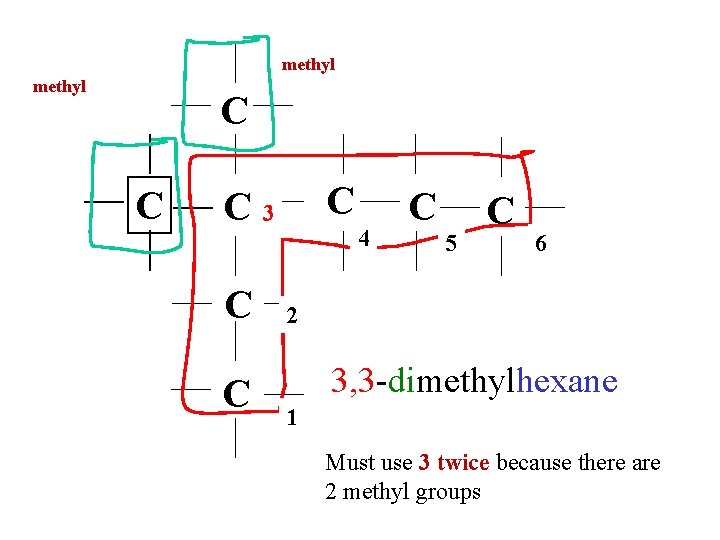
methyl C C 3 C C 4 C 5 C 6 2 3, 3 -dimethylhexane 1 Must use 3 twice because there are 2 methyl groups

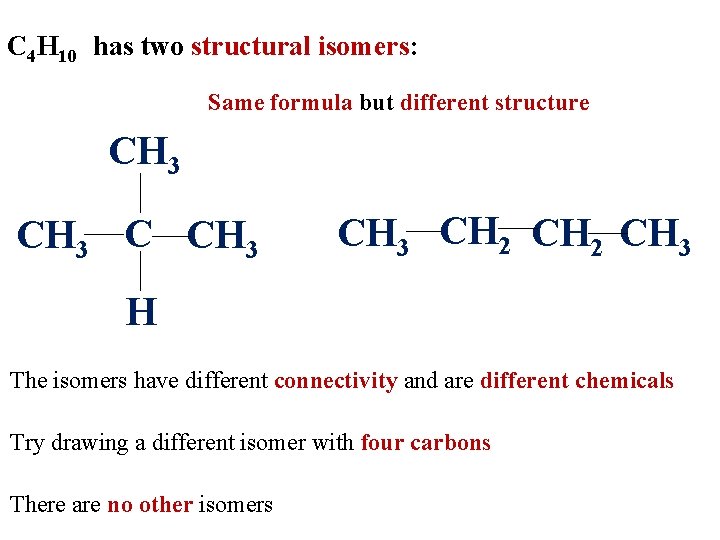
C 4 H 10 has two structural isomers: Same formula but different structure CH 3 CH 2 CH 3 H The isomers have different connectivity and are different chemicals Try drawing a different isomer with four carbons There are no other isomers

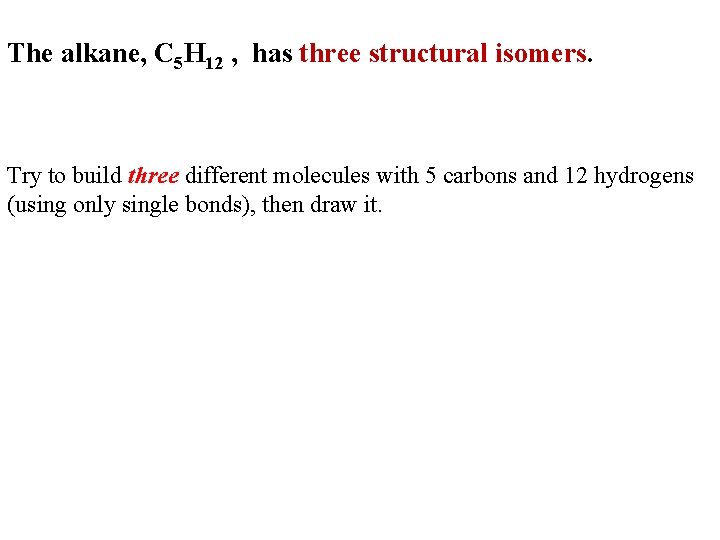
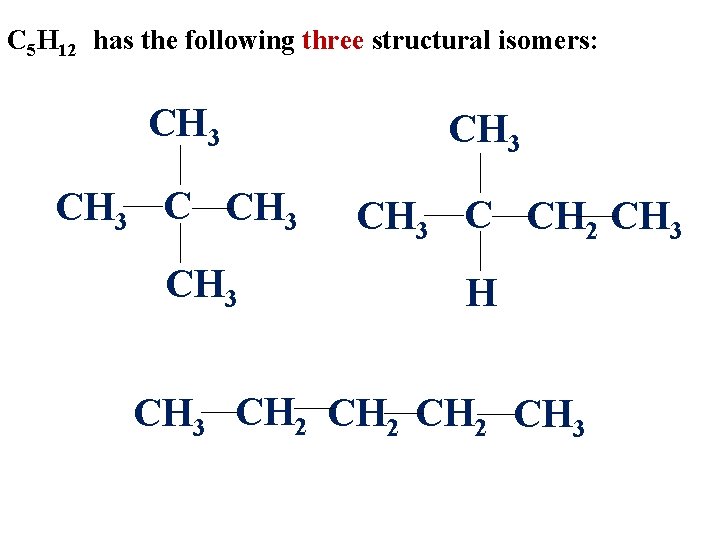
The alkane, C 5 H 12 , has three structural isomers. Try to build three different molecules with 5 carbons and 12 hydrogens (using only single bonds), then draw it.

C 5 H 12 has the following three structural isomers: CH 3 CH 3 C CH 2 CH 3 H CH 3 CH 2 CH 3

Practice Questions

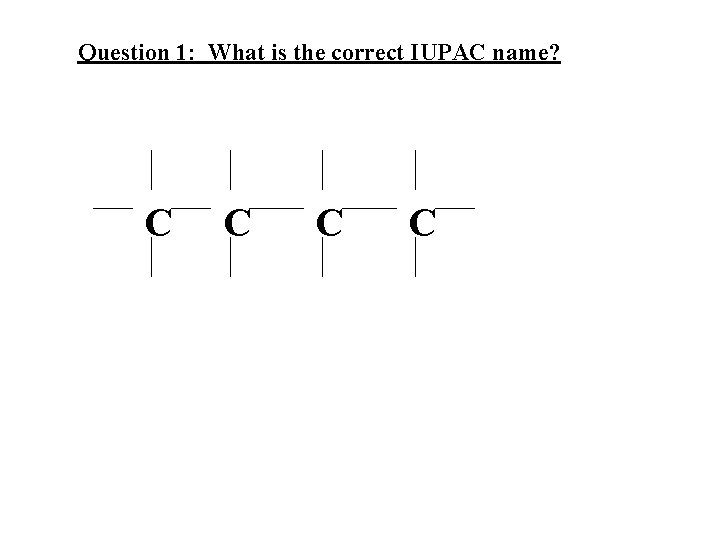
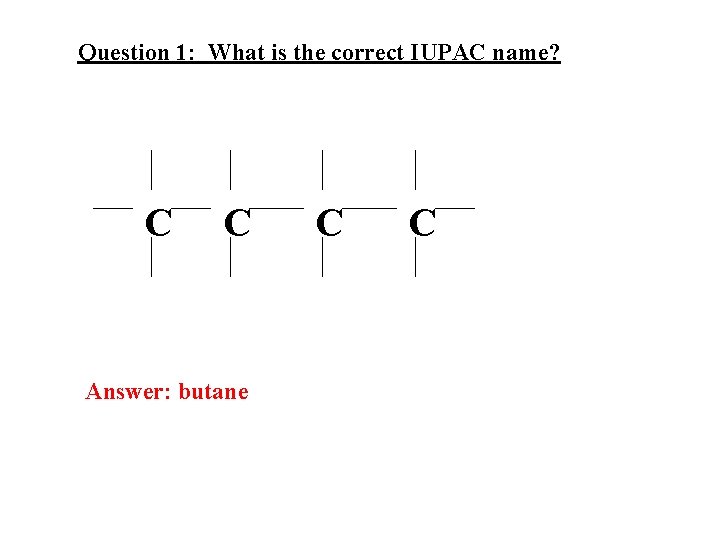
Question 1: What is the correct IUPAC name? C C

Question 1: What is the correct IUPAC name? C C Answer: butane C C

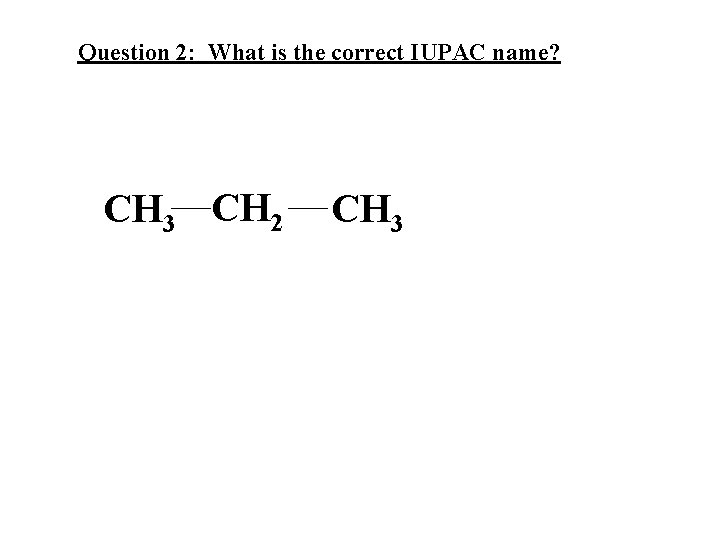
Question 2: What is the correct IUPAC name? CH 3 CH 2 CH 3

Question 2: What is the correct IUPAC name? CH 3 CH 2 Answer: propane CH 3

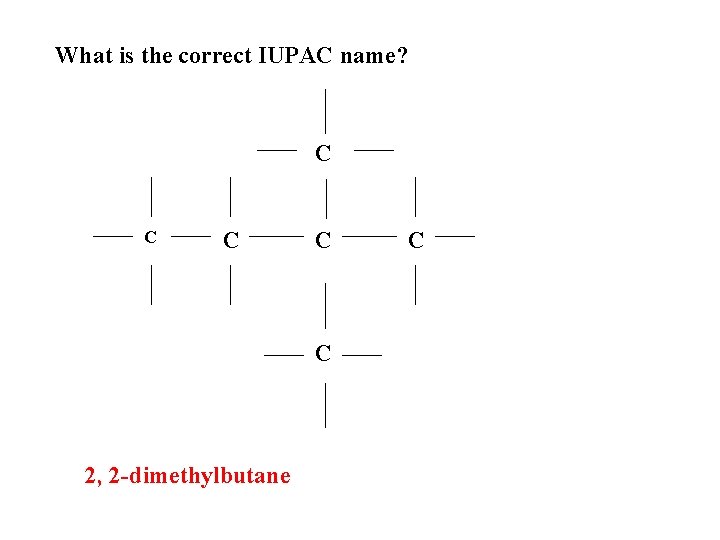
What is the correct IUPAC name? C C C 2, 2 -dimethylbutane C

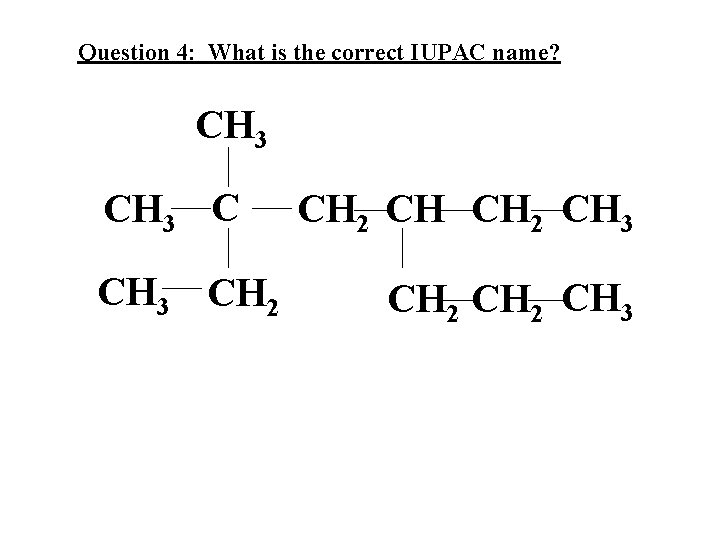
Question 4: What is the correct IUPAC name? CH 3 CH 2 CH CH 2 CH 3

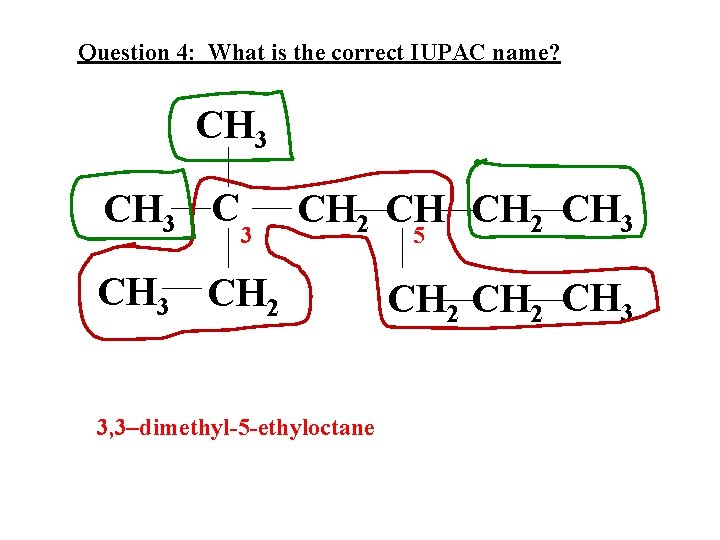
Question 4: What is the correct IUPAC name? CH 3 C 3 CH 2 CH 3 CH 2 3, 3–dimethyl-5 -ethyloctane 5 CH 2 CH 3

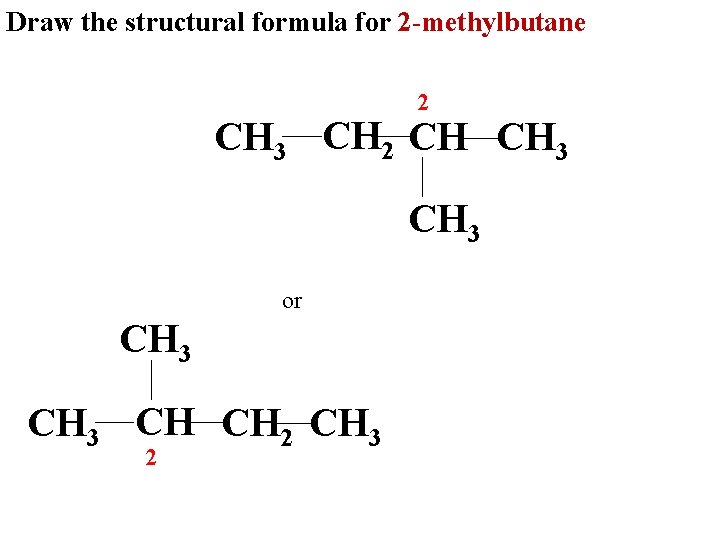
Draw the structural formula for 2 -methylbutane 2 CH 3 CH 2 CH CH 3 or CH 3 CH CH 2 CH 3 2
 How to name compounds in organic chemistry
How to name compounds in organic chemistry C-c-c-c-c chemistry
C-c-c-c-c chemistry Organic chemistry nomenclature
Organic chemistry nomenclature Which compound is inorganic
Which compound is inorganic Physical properties of esters
Physical properties of esters Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Basic organic nomenclature packet
Basic organic nomenclature packet Heterocyclic compounds nomenclature
Heterocyclic compounds nomenclature Fused ring nomenclature
Fused ring nomenclature Nomenclature of binary ionic compounds
Nomenclature of binary ionic compounds Coordination compound nomenclature
Coordination compound nomenclature Stamatitis
Stamatitis Dumas method
Dumas method Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic halogen compounds
Organic halogen compounds Organic compounds
Organic compounds Condensed formula
Condensed formula Families of organic compounds
Families of organic compounds What is the classification of organic compounds
What is the classification of organic compounds Organic chemistry chapter 1
Organic chemistry chapter 1 Vitamin classification chart
Vitamin classification chart