ORGANIC CHEMISTRY TOPIC 10 INTRODUCTION 1 ORGANIC CHEMISTRY









- Slides: 25

ORGANIC CHEMISTRY TOPIC 10

INTRODUCTION 1. ORGANIC CHEMISTRY IS ONE OF THE MAJOR BRANCHES OF CHEMISTRY. IT INCLUDES THE STUDY OF: • ALL BIOLOGICAL MOLECULES • ALL FOSSIL FUELS • NEARLY ALL SYNTHETIC MATERIALS • MANY DOMESTIC AND INDUSTRIAL PRODUCTS

ORGANIC COMPOUNDS • AN ORGANIC COMPOUND IS ONE THAT CONTAINS CARBON, AND USUALLY HYDROGEN, IN COVALENTLY BONDED STRUCTURES. OTHER ELEMENTS, SUCH AS OXYGEN, NITROGEN, SULFUR AND CHLORINE , ARE OFTEN ALSO PRESENT. • CARBON IS THE KEY ELEMENT IN ORGANIC COMPOUNDS BECAUSE IT CAN FORM 4 COVALENT BONDS WITH OTHER CARBON ATOMS OR WITH OTHER ELEMENTS, ESPECIALLY HYDROGEN. • CARBON HAS THE ABILITY TO FORM CHAINS AND RINGS; THIS IS KNOWN AS CATENATION

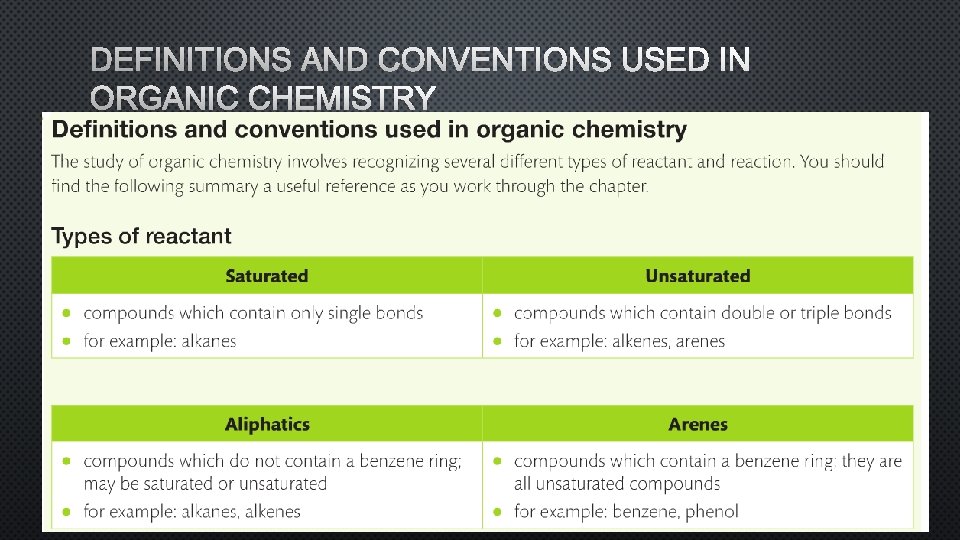
DEFINITIONS AND CONVENTIONS USED IN ORGANIC CHEMISTRY

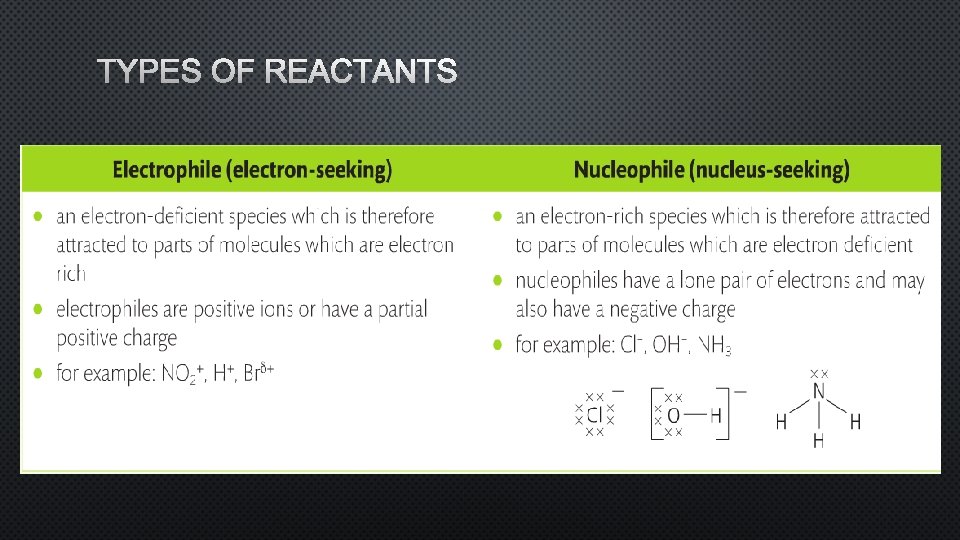
TYPES OF REACTANTS

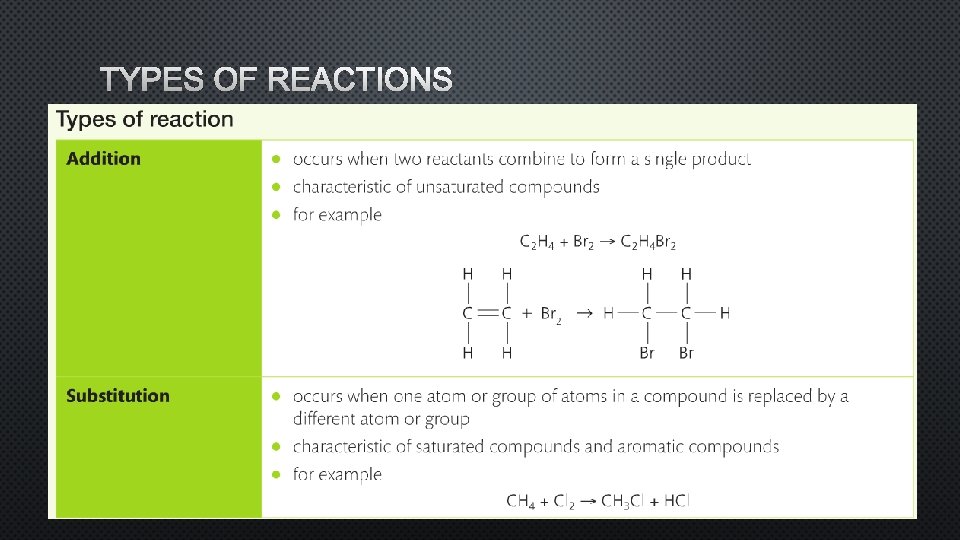
TYPES OF REACTIONS

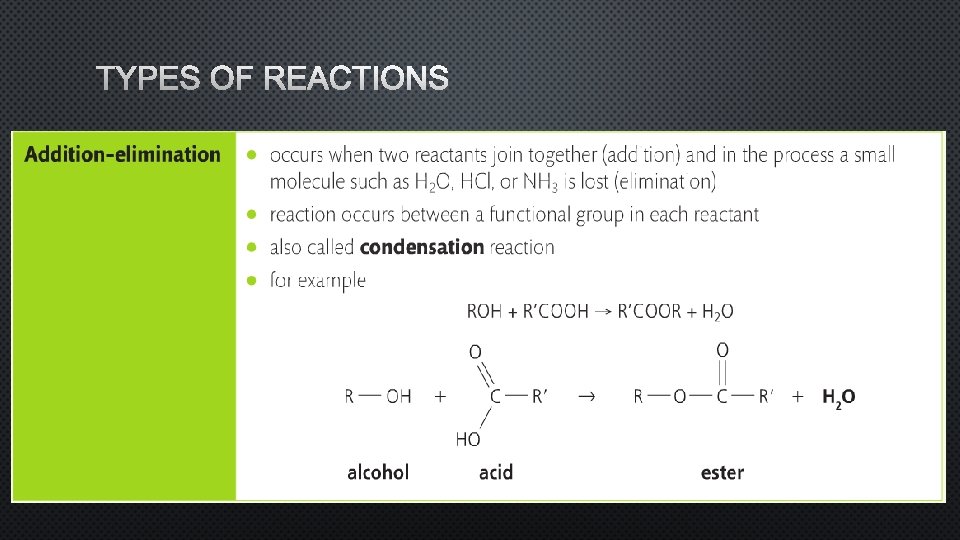
TYPES OF REACTIONS

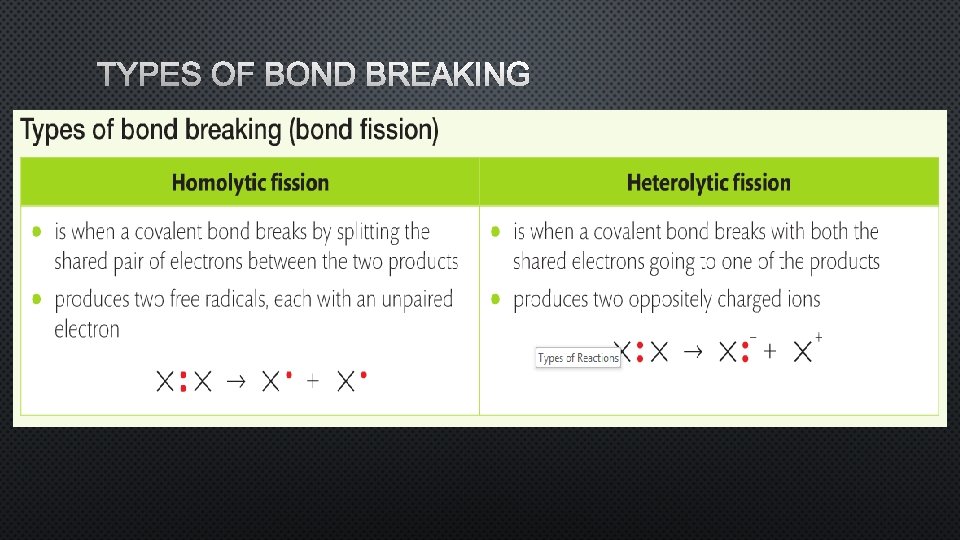
TYPES OF BOND BREAKING

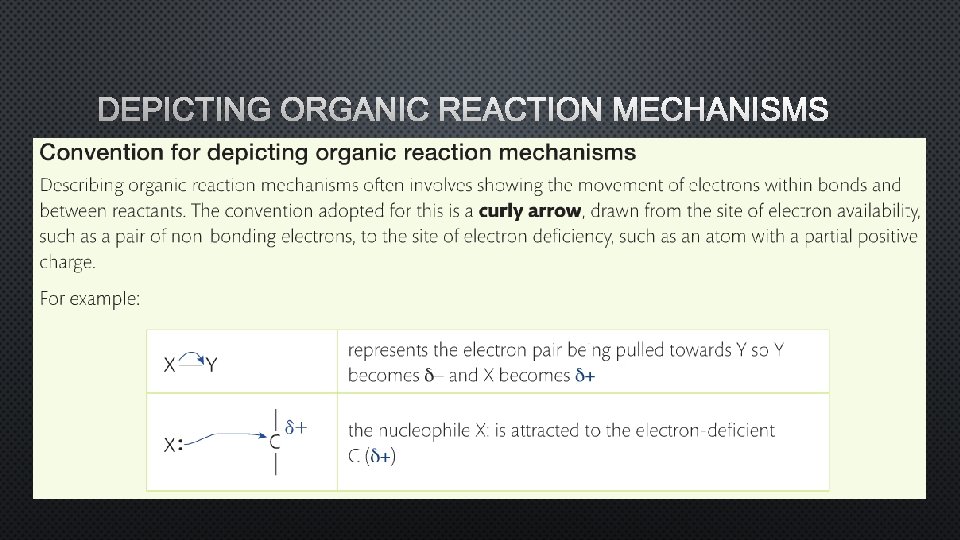
DEPICTING ORGANIC REACTION MECHANISMS

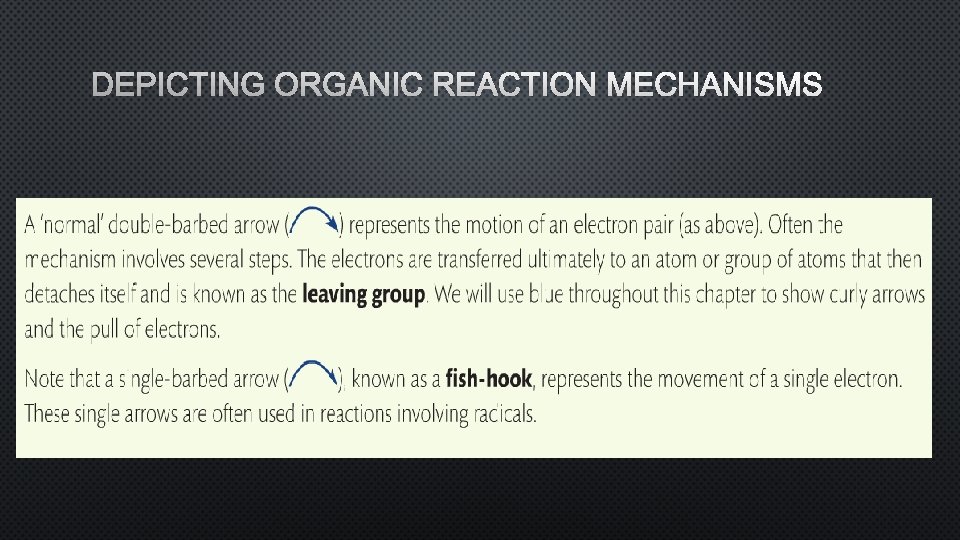
DEPICTING ORGANIC REACTION MECHANISMS

CLASSIFICATION SYSTEM • BECAUSE THERE ARE SO MANY ORGANIC MOLECULES (AT LEAST 10 MILLION DIFFERENT ORGANIC MOLECULES), IT IS USEFUL TO CLASSIFY THEM INTO FAMILIES ACCORDING TO THEIR PROPERTIES. • ORGANIC COMPOUNDS ARE CLASSIFIED INTO FAMILIES OF COMPOUNDS CALLED HOMOLOGOUS SERIES.

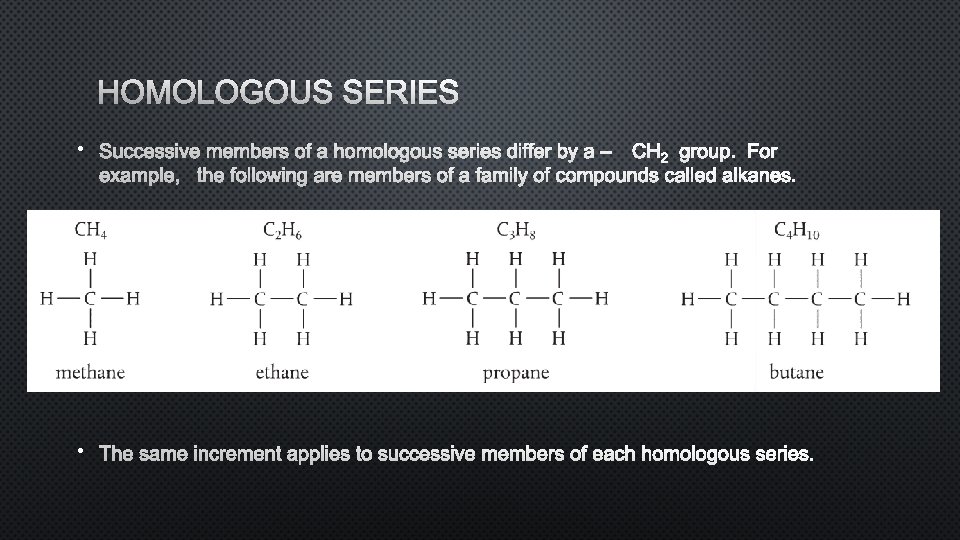
HOMOLOGOUS SERIES • SUCCESSIVE MEMBERS OF A HOMOLOGOUS SERIES DIFFER BY A C – H 2 GROUP. FOR EXAMPLE, THE FOLLOWING ARE MEMBERS OF A FAMILY OF COMPOUNDS CALLED ALKANES. • THE SAME INCREMENT APPLIES TO SUCCESSIVE MEMBERS OF EACH HOMOLOGOUS SERIES.

HOMOLOGOUS SERIES • MEMBERS OF A HOMOLOGOUS SERIES CAN BE REPRESENTED BY THE SAME GENERAL FORMULA. FOR EXAMPLE, IN ALKANES, THE GENERAL FORMULA IS C n H 2 n + 2. • IN ALCOHOLS, THE GENERAL FORMULA IS C n H 2 n + 1 OH, FOR EXAMPLE,

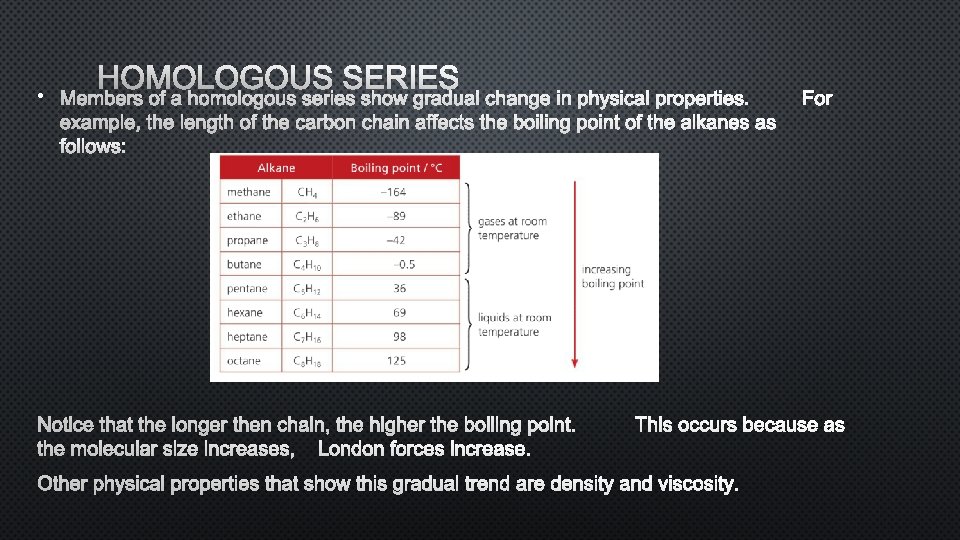
HOMOLOGOUS SERIES • MEMBERS OF A HOMOLOGOUS SERIES SHOW GRADUAL CHANGE IN PHYSICAL PROPERTIES. FOR EXAMPLE, THE LENGTH OF THE CARBON CHAIN AFFECTS THE BOILING POINT OF THE ALKANES AS FOLLOWS: NOTICE THAT THE LONGER THEN CHAIN, THE HIGHER THE BOILING POINT. THIS OCCURS BECAUSE AS THE MOLECULAR SIZE INCREASES, LONDON FORCES INCREASE. OTHER PHYSICAL PROPERTIES THAT SHOW THIS GRADUAL TREND ARE DENSITY AND VISCOSITY.

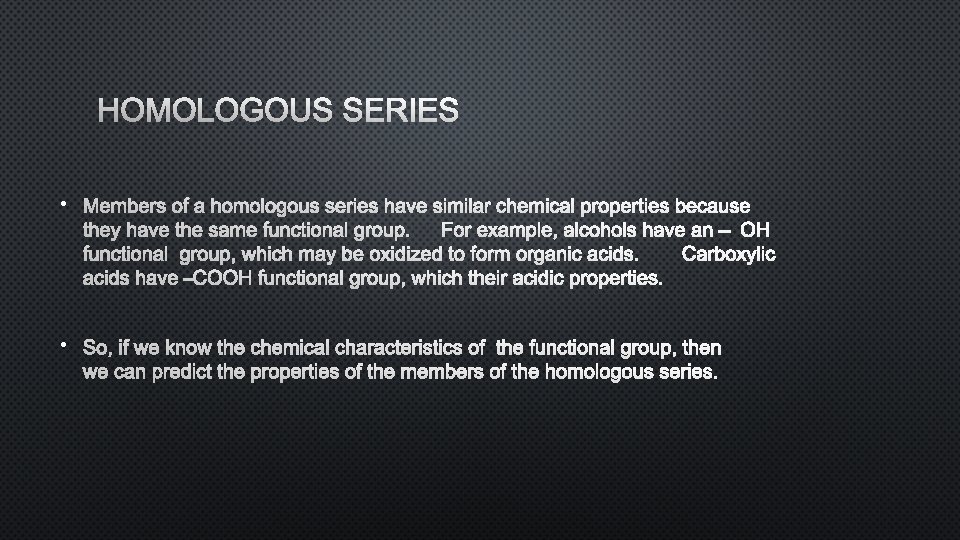
HOMOLOGOUS SERIES • MEMBERS OF A HOMOLOGOUS SERIES HAVE SIMILAR CHEMICAL PROPERTIES BECAUSE THEY HAVE THE SAME FUNCTIONAL GROUP. FOR EXAMPLE, ALCOHOLS HAVE AN – OH FUNCTIONAL GROUP, WHICH MAY BE OXIDIZED TO FORM ORGANIC ACIDS. CARBOXYLIC ACIDS HAVE –COOH FUNCTIONAL GROUP, WHICH THEIR ACIDIC PROPERTIES. • SO, IF WE KNOW THE CHEMICAL CHARACTERISTICS OF THE FUNCTIONAL GROUP, THEN WE CAN PREDICT THE PROPERTIES OF THE MEMBERS OF THE HOMOLOGOUS SERIES.

HOMOLOGOUS SERIES

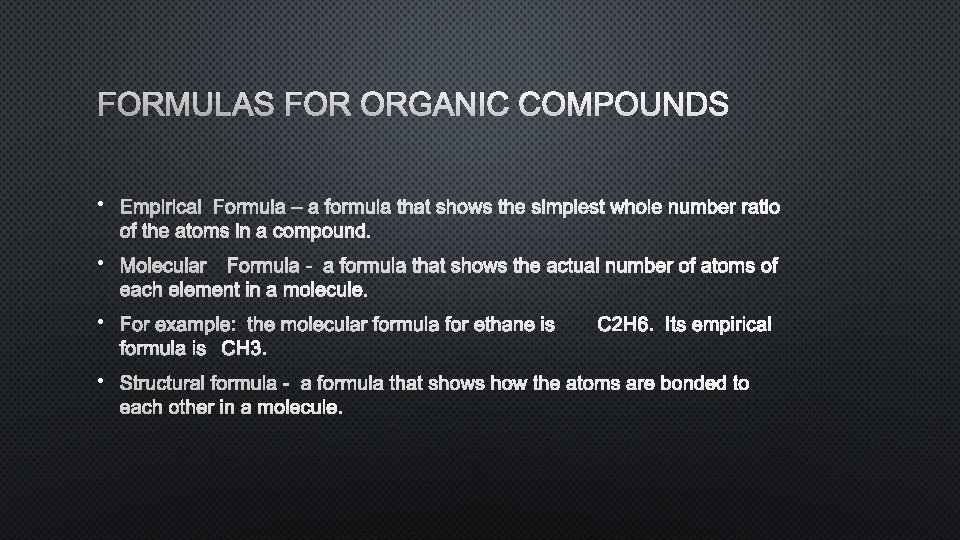
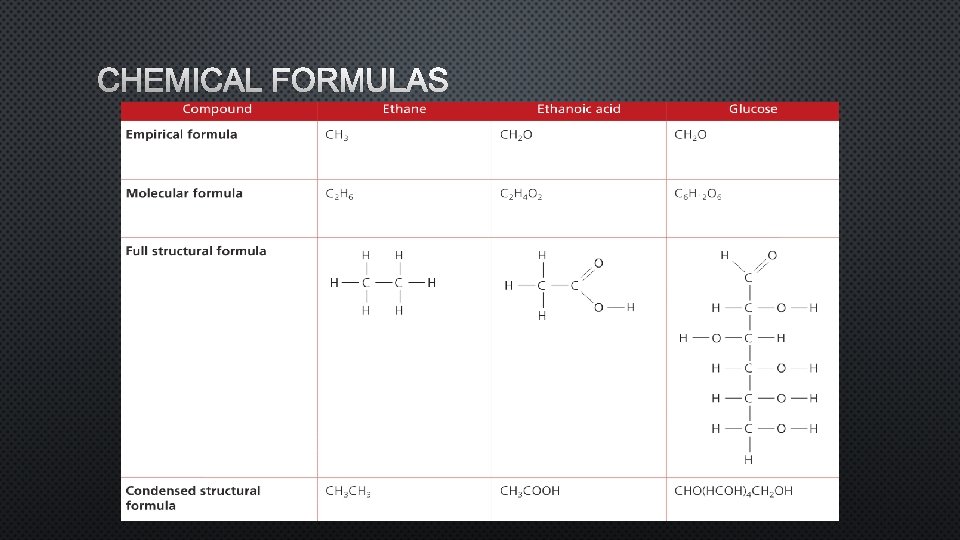
FORMULAS FOR ORGANIC COMPOUNDS • EMPIRICAL FORMULA – A FORMULA THAT SHOWS THE SIMPLEST WHOLE NUMBER RATIO OF THE ATOMS IN A COMPOUND. • MOLECULAR FORMULA - A FORMULA THAT SHOWS THE ACTUAL NUMBER OF ATOMS OF EACH ELEMENT IN A MOLECULE. • FOR EXAMPLE: THE MOLECULAR FORMULA FOR ETHANE ISC 2 H 6. ITS EMPIRICAL FORMULA IS CH 3. • STRUCTURAL FORMULA - A FORMULA THAT SHOWS HOW THE ATOMS ARE BONDED TO EACH OTHER IN A MOLECULE.

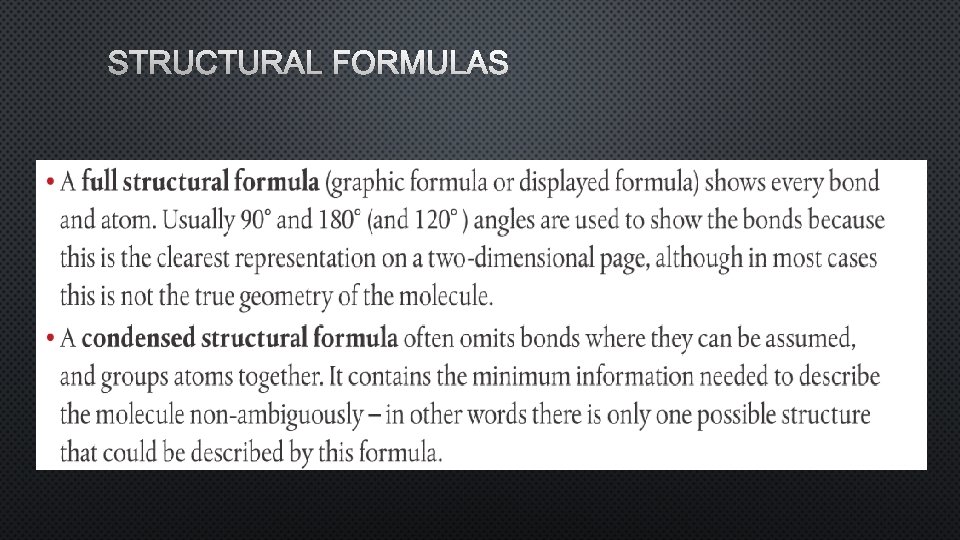
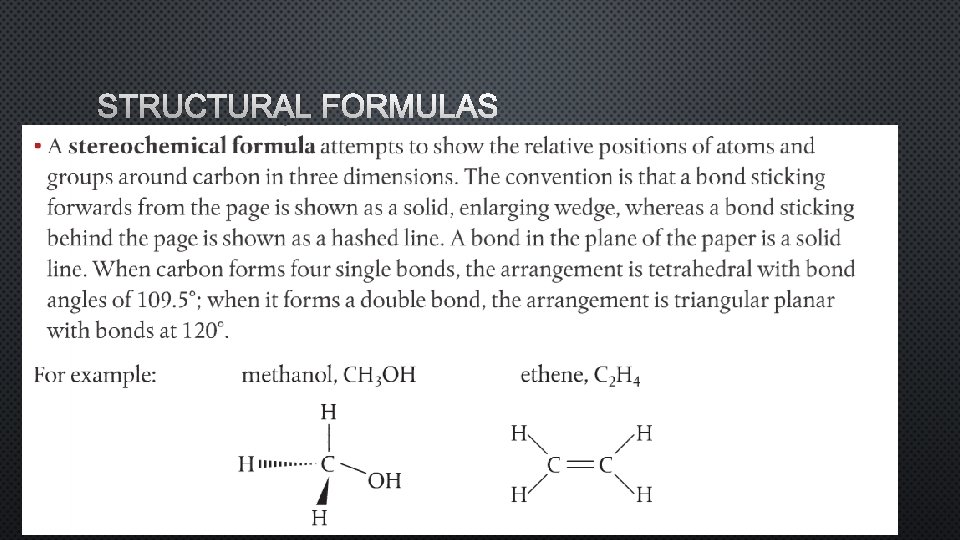
STRUCTURAL FORMULAS

STRUCTURAL FORMULAS

CHEMICAL FORMULAS

ISOMERS • ISOMERS ARE COMPOUNDS WITH THE SAME FORMULA, BUT DIFFERENT STRUCTURES. IN OTHER WORDS, THE ATOMS ARE CONNECTED DIFFERENTLY. FOR EXAMPLE: ETHYL ALCOHOL DIMETHYL ETHER C 2 H 6 O CH 3 CH 2 OH CH 3 OCH 3

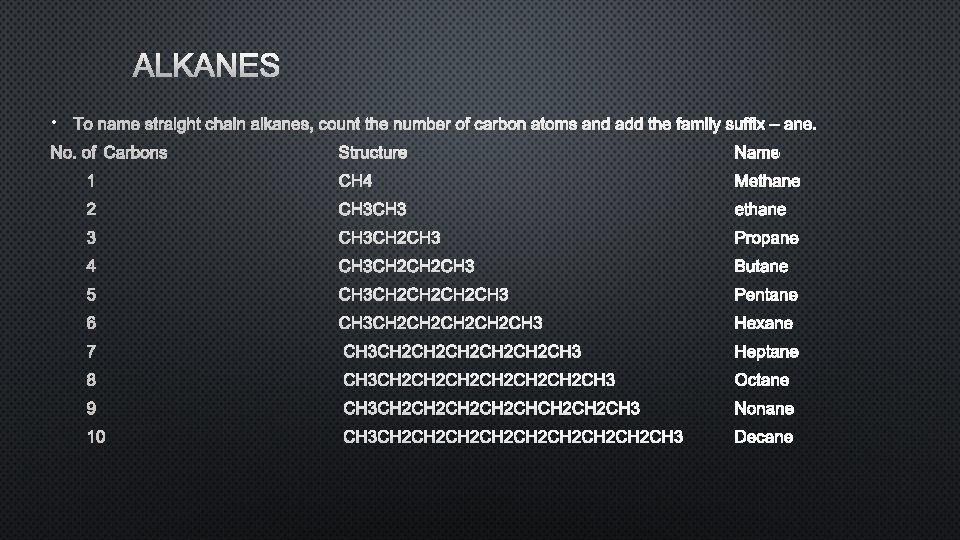
NOMENCLATURE OF ALKANES • ALKANES ARE SATURATED HYDROCARBONS BECAUSE THEY CONTAIN ONLY SINGLE BONDS. THE TERM HYDROCARBON REFERS TO COMPOUNDS THAT ARE MADE OF CARBON AND HYDROGEN. • THE NAME OF ORGANIC COMPOUNDS HAVE THREE PARTS: PREFIX, PARENT, AND SUFFIX, PREFIX – WHERE IS THE FUNCTIONAL GROUP AND THE OTHER SUBSTITUENTS (BRANCHES) LOCATED? PARENT – HOW MANY CARBONS? SUFFIX – WHAT FAMILY DOES THE MOLECULE BELONG TO? THE ALKANES FAMILY SUFFIX IS –ANE.

ALKANES • TO NAME STRAIGHT CHAIN ALKANES, COUNT THE NUMBER OF CARBON ATOMS AND ADD THE FAMILY SUFFIX – ANE. NO. OF CARBONS STRUCTURE NAME 1 CH 4 METHANE 2 CH 3 ETHANE 3 CH 3 CH 2 CH 3 PROPANE 4 CH 3 CH 2 CH 3 BUTANE 5 CH 3 CH 2 CH 2 CH 3 PENTANE 6 CH 3 CH 2 CH 2 CH 3 HEXANE 7 CH 3 CH 2 CH 2 CH 2 CH 3 HEPTANE 8 CH 3 CH 2 CH 2 CH 2 CH 3 OCTANE 9 CH 3 CH 2 CH 2 CHCH 2 CH 3 NONANE 10 CH 3 CH 2 CH 2 CH 3 DECANE

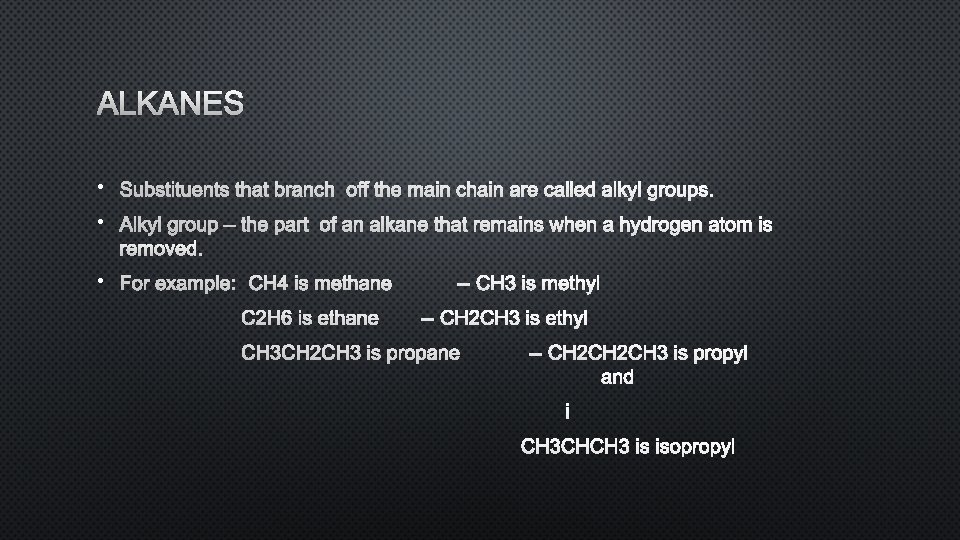
ALKANES • SUBSTITUENTS THAT BRANCH • ALKYL GROUP – THE PART REMOVED. OFF THE MAIN CHAIN ARE CALLED ALKYL GROUPS. OF AN ALKANE THAT REMAINS WHEN A HYDROGEN ATOM IS • FOR EXAMPLE: CH 4 IS METHANE C 2 H 6 IS ETHANE -- CH 3 IS METHYL -- CH 2 CH 3 IS ETHYL CH 3 CH 2 CH 3 IS PROPANE -- CH 2 CH 3 IS PROPYL AND I CH 3 CHCH 3 IS ISOPROPYL

ALKANES • STEP 1 : FIND THE LONGEST CONTINUOUS CHAIN OF CARBONS AND NAME IT ACCORDING TO THE NUMBER OF CARBON ATOMS IT CONTAINS. • STEP 2: NUMBER THE CARBON ATOMS IN THE MAIN CHAIN, BEGINNING AT THE END NEARER TO THE FIRST BRANCH. • STEP 3: IDENTIFY THE BRANCHING SUBSTITUENTS AND NUMBER EACH ACCORDING TO THE CARBON TO WHICH IT IS ATTACHED. • STEP 4. : WRITE THE NAME AS A SINGLE WORD, USING HYPHENS TO SEPARATE NUMBERS FROM PREFIXES AND COMMAS TO SEPARATE NUMBERS. IF TWO OR MORE DIFFERENT SUBSTITUENTS ARE PRESENT, LIST THEM IN ALPHABETICAL ORDER. IF TWO OR MORE IDENTICAL SUBSTITUENTS ARE PRESENT, USE OF THE PREFIXES, DI, TRI, TETRA, ETC.