Reactions in Aqueous Solutions 1 2 3 4


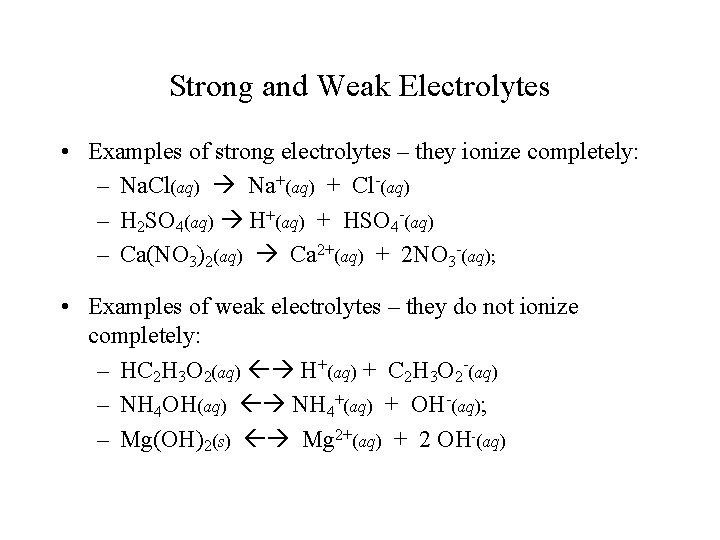

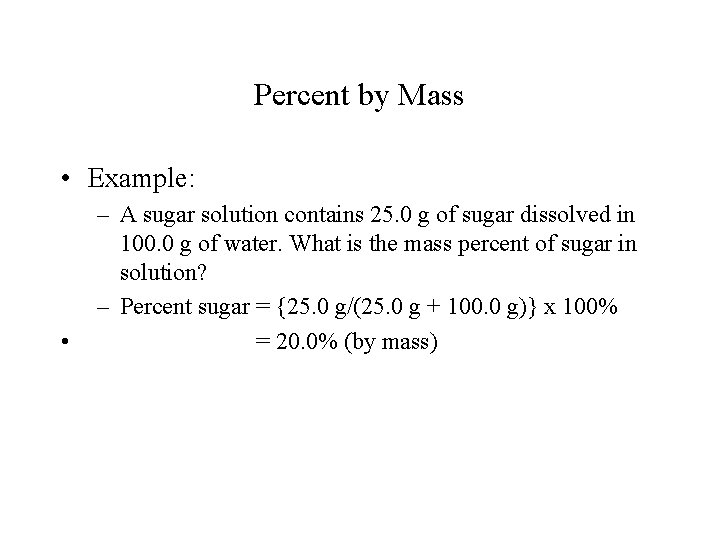










- Slides: 52

Reactions in Aqueous Solutions 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Water as a universal solvent Aqueous solutions - solutions with water as solvent Solubility of ionic compounds in water Solubility of molecular compounds in water Strong, weak, and non-electrolyte solutions Solution compositions - concentrations Types of reactions in aqueous solutions Chemical equations for reactions in aqueous solutions Stoichiometry for reaction in aqueous solution Redox reactions in aqueous solutions.

Water as a Universal Solvent • Water molecule is very polar; • Water interacts strongly with ionic and polar molecules; • Strong interactions enable water to dissolve many solutes – ionic and nonionic; • Solubility of ionic compounds depends on the relative strength of ion-dipole interactions between ions and water molecules and ionic bonds within the compounds; • Many ionic compounds dissolve in water because of strong ion-dipole interactions; • Polar nonionic compounds dissolve in water due to strong dipole-dipole interactions or hydrogen bonding;

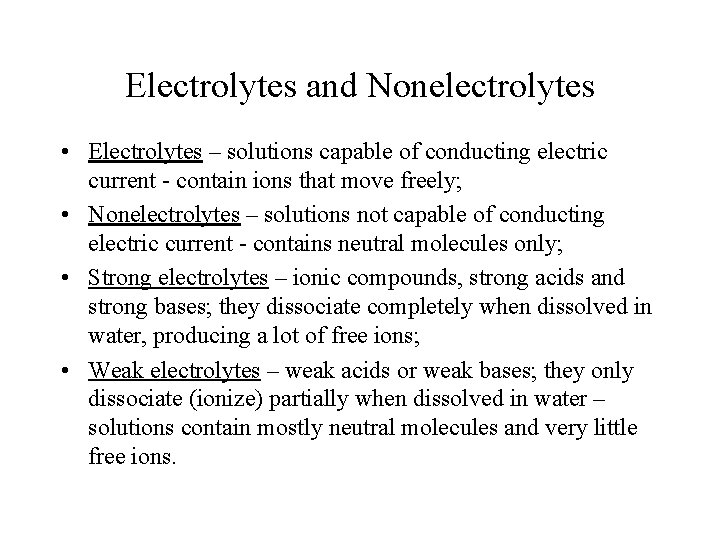
Electrolytes and Nonelectrolytes • Electrolytes – solutions capable of conducting electric current - contain ions that move freely; • Nonelectrolytes – solutions not capable of conducting electric current - contains neutral molecules only; • Strong electrolytes – ionic compounds, strong acids and strong bases; they dissociate completely when dissolved in water, producing a lot of free ions; • Weak electrolytes – weak acids or weak bases; they only dissociate (ionize) partially when dissolved in water – solutions contain mostly neutral molecules and very little free ions.
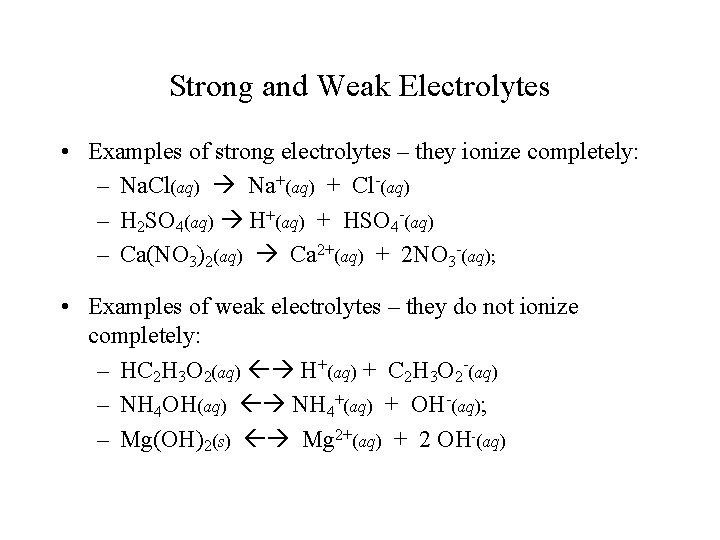
Strong and Weak Electrolytes • Examples of strong electrolytes – they ionize completely: – Na. Cl(aq) Na+(aq) + Cl-(aq) – H 2 SO 4(aq) H+(aq) + HSO 4 -(aq) – Ca(NO 3)2(aq) Ca 2+(aq) + 2 NO 3 -(aq); • Examples of weak electrolytes – they do not ionize completely: – HC 2 H 3 O 2(aq) H+(aq) + C 2 H 3 O 2 -(aq) – NH 4 OH(aq) NH 4+(aq) + OH-(aq); – Mg(OH)2(s) Mg 2+(aq) + 2 OH-(aq)

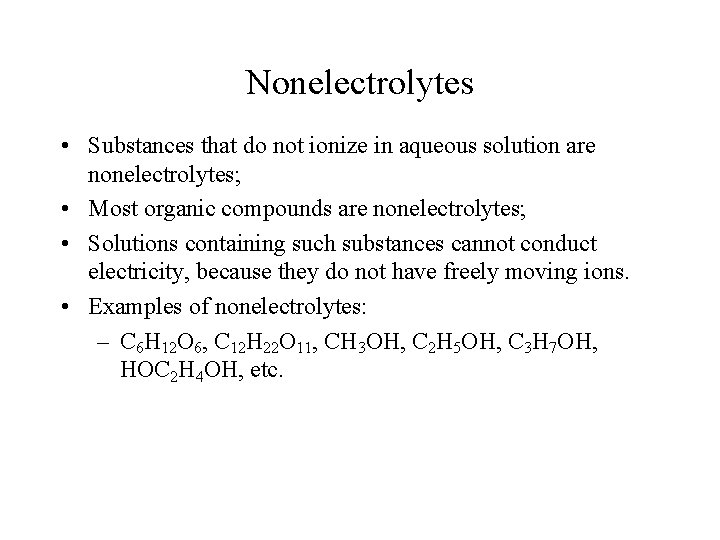
Nonelectrolytes • Substances that do not ionize in aqueous solution are nonelectrolytes; • Most organic compounds are nonelectrolytes; • Solutions containing such substances cannot conduct electricity, because they do not have freely moving ions. • Examples of nonelectrolytes: – C 6 H 12 O 6, C 12 H 22 O 11, CH 3 OH, C 2 H 5 OH, C 3 H 7 OH, HOC 2 H 4 OH, etc.

Solution Concentrations • The concentration of a solution may be expressed in: – Percent by mass, percent by volume, or molarity; Percent (by mass) = (Mass of solute/Mass of solution) x 100% Percent (by volume) = (Vol. of solute/Vol. of solution) x 100% Molarity = (Mol of solute/Liter of solution) Mol of solute = Liters of solution x Molarity
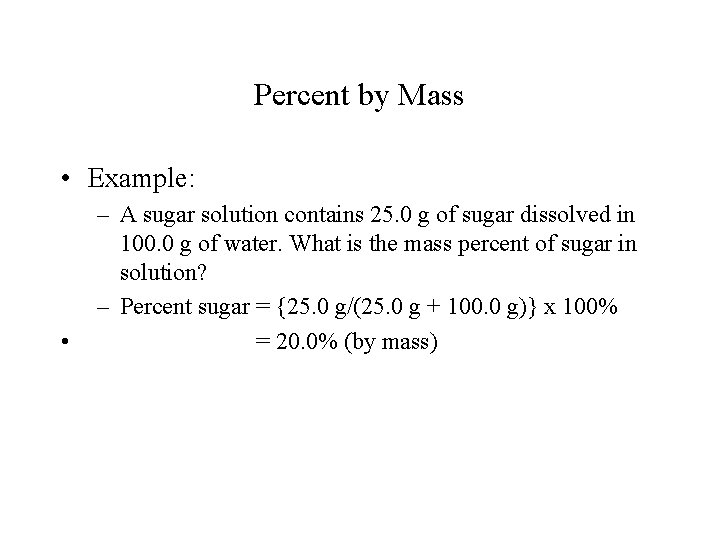
Percent by Mass • Example: • – A sugar solution contains 25. 0 g of sugar dissolved in 100. 0 g of water. What is the mass percent of sugar in solution? – Percent sugar = {25. 0 g/(25. 0 g + 100. 0 g)} x 100% = 20. 0% (by mass)

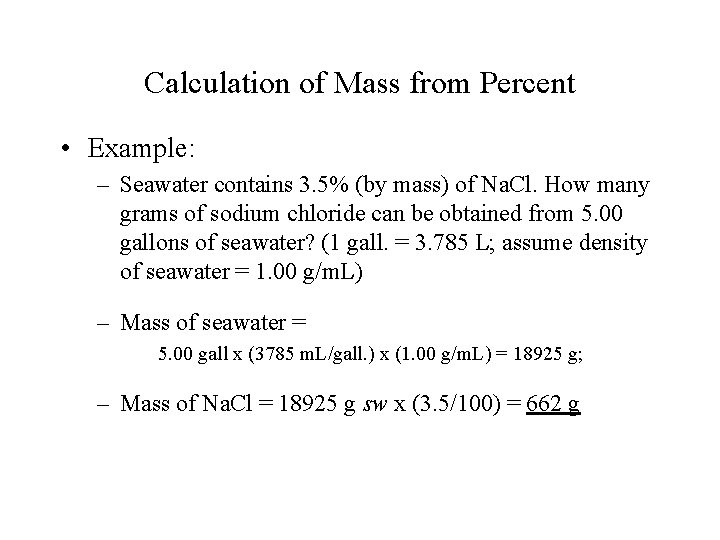
Calculation of Mass from Percent • Example: – Seawater contains 3. 5% (by mass) of Na. Cl. How many grams of sodium chloride can be obtained from 5. 00 gallons of seawater? (1 gall. = 3. 785 L; assume density of seawater = 1. 00 g/m. L) – Mass of seawater = 5. 00 gall x (3785 m. L/gall. ) x (1. 00 g/m. L) = 18925 g; – Mass of Na. Cl = 18925 g sw x (3. 5/100) = 662 g

Percent by Volume • Example: – A solution is prepared by mixing 150. m. L of methanol, 100. m. L of acetone, and 250. m. L of water. What is the volume percent of methanol and acetone in solution? • – Percent methanol = (150. m. L/500. m. L) x 100% = 30. 0% (by volume) – Percent acetone = (100. m. L/500. m. L) x 100% – = 20. 0% (by volume)

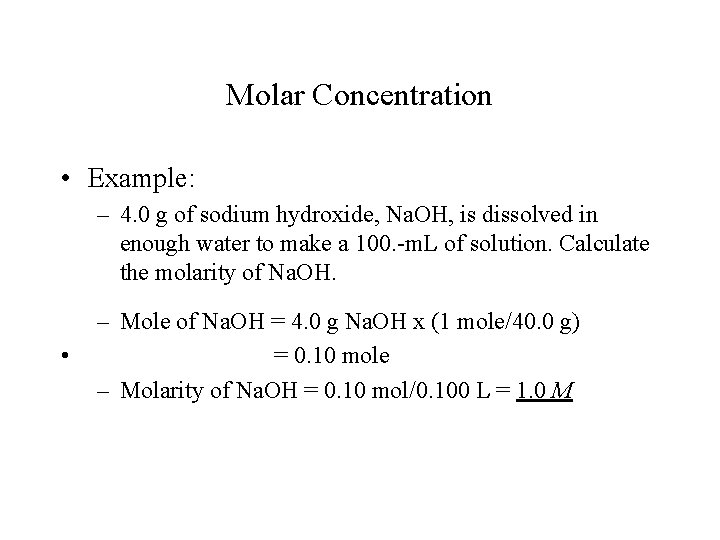
Molar Concentration • Example: – 4. 0 g of sodium hydroxide, Na. OH, is dissolved in enough water to make a 100. -m. L of solution. Calculate the molarity of Na. OH. • – Mole of Na. OH = 4. 0 g Na. OH x (1 mole/40. 0 g) = 0. 10 mole – Molarity of Na. OH = 0. 10 mol/0. 100 L = 1. 0 M

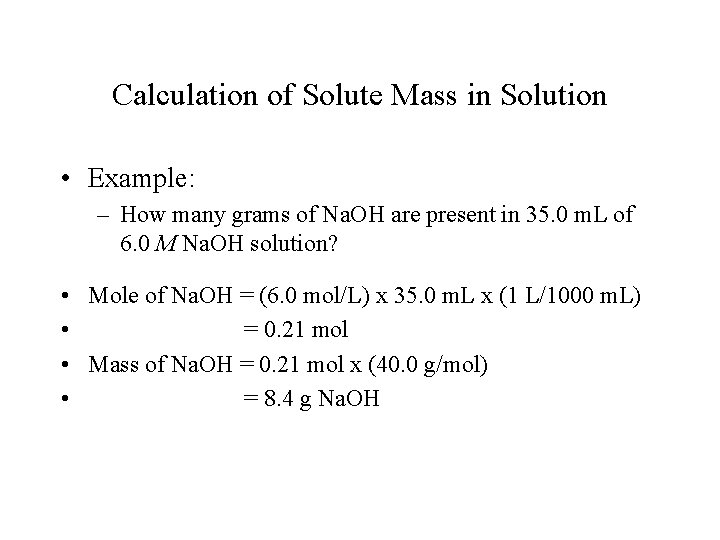
Calculation of Solute Mass in Solution • Example: – How many grams of Na. OH are present in 35. 0 m. L of 6. 0 M Na. OH solution? • Mole of Na. OH = (6. 0 mol/L) x 35. 0 m. L x (1 L/1000 m. L) • = 0. 21 mol • Mass of Na. OH = 0. 21 mol x (40. 0 g/mol) • = 8. 4 g Na. OH

Preparing Solutions from Pure Solids 1. 2. 3. 4. 5. From the volume (in liters) and molarity of solution, calculate the mole and mass of solute needed; Weigh the mass of pure solute accurately; Transfer solute into a volumetric flask of appropriate size; Add deionized water to the volumetric flask, well below the narrow neck, and shake well to dissolve the solute. When completely dissolved, add more distilled water to fill the flask to the mark and mix the solution well.

Preparing Solution from Solid • Example: – Explain how you would prepare 1. 00 L of 0. 500 M Na. Cl solution. • Calculate mass of Na. Cl needed: – Mole of Na. Cl = 1. 00 L x (0. 500 mol/L) = 0. 500 mol – Mass of Na. Cl = 0. 500 mol x (58. 44 g/mol) = 29. 2 g • Preparing the solution: – Weigh 29. 2 g of Na. Cl accurately and transfer into 1 -liter volumetric flask. Fill the flask half way with distilled water, shake well until all solid has dissolved. Fill the flask to the 1 -liter mark with more distilled water and mix the solution well by inverting the flask back and forth several times.

Preparing Solution from Stock 1. 2. 3. 4. Calculate volume of stock solution needed using the formula: Mi. Vi = Mf. Vf (i = initial; f = final) Measure accurately the volume of stock solution and carefully transfer to a volumetric flask of appropriate size; Dilute stock solution with distilled water to the required volume (or to the “mark” on volumetric flask) Mix solution well. (Note: if diluting concentrated acid, place some distilled water in the flask, add the concentrated acid, and then add more distilled water to the required volume. )

Preparing Solution from Stock • Example: – Explain how you would prepare 1. 0 L of 3. 0 M H 2 SO 4 solution from concentrated H 2 SO 4, which is 18 M. • Calculate volume of concentrated H 2 SO 4 needed: – Vol. of conc. H 2 SO 4 = (1. 0 L x 3. 0 M/18 M) = 0. 17 L = 170 m. L • Preparing the solution: – Place some distilled water in the 1 -liter volumetric flask (that would fill the flask to about a quarter full). Measure accurately 170 m. L of conc. H 2 SO 4 and transfer carefully to the volumetric flask that already contains some distilled water. Then fill the flask to the 1 -liter mark with more distilled water and mix the solution well by inverting the flask back and forth several times.

Reactions in Aqueous Solution 1. Double-Displacement Reactions 1. Precipitation reactions 2. Neutralization (or Acid-Base) reactions 2. Oxidation-Reduction (Redox) Reactions 1. 2. 3. 4. 5. Combination reactions Decomposition reactions Combustion reactions Single-Replacement reactions Reactions involving strong oxidizing reagents

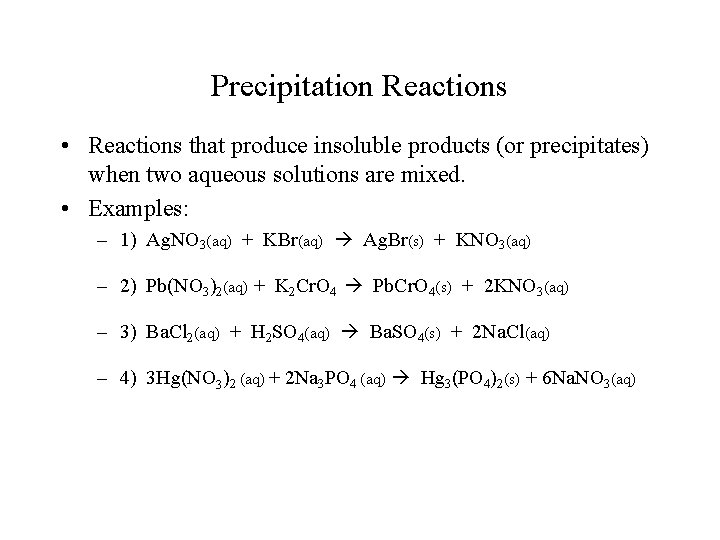
Precipitation Reactions • Reactions that produce insoluble products (or precipitates) when two aqueous solutions are mixed. • Examples: – 1) Ag. NO 3(aq) + KBr(aq) Ag. Br(s) + KNO 3(aq) – 2) Pb(NO 3)2(aq) + K 2 Cr. O 4 Pb. Cr. O 4(s) + 2 KNO 3(aq) – 3) Ba. Cl 2(aq) + H 2 SO 4(aq) Ba. SO 4(s) + 2 Na. Cl(aq) – 4) 3 Hg(NO 3)2 (aq) + 2 Na 3 PO 4 (aq) Hg 3(PO 4)2(s) + 6 Na. NO 3(aq)

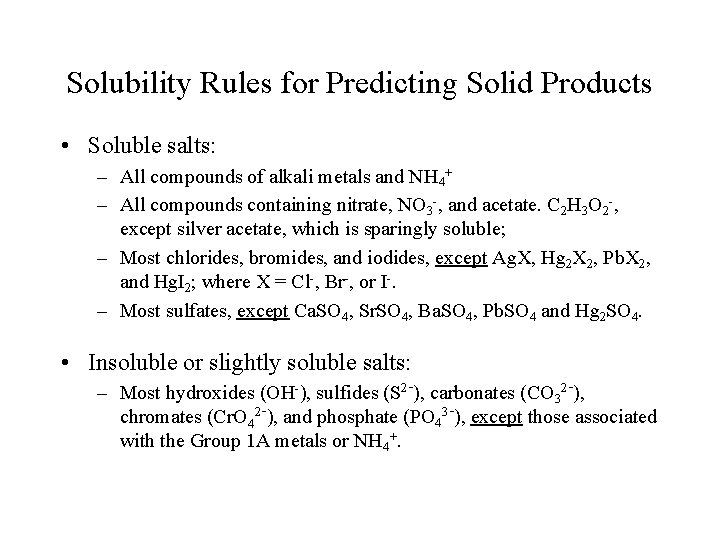
Solubility Rules for Predicting Solid Products • Soluble salts: – All compounds of alkali metals and NH 4+ – All compounds containing nitrate, NO 3 -, and acetate. C 2 H 3 O 2 -, except silver acetate, which is sparingly soluble; – Most chlorides, bromides, and iodides, except Ag. X, Hg 2 X 2, Pb. X 2, and Hg. I 2; where X = Cl-, Br-, or I-. – Most sulfates, except Ca. SO 4, Sr. SO 4, Ba. SO 4, Pb. SO 4 and Hg 2 SO 4. • Insoluble or slightly soluble salts: – Most hydroxides (OH-), sulfides (S 2 -), carbonates (CO 32 -), chromates (Cr. O 42 -), and phosphate (PO 43 -), except those associated with the Group 1 A metals or NH 4+.

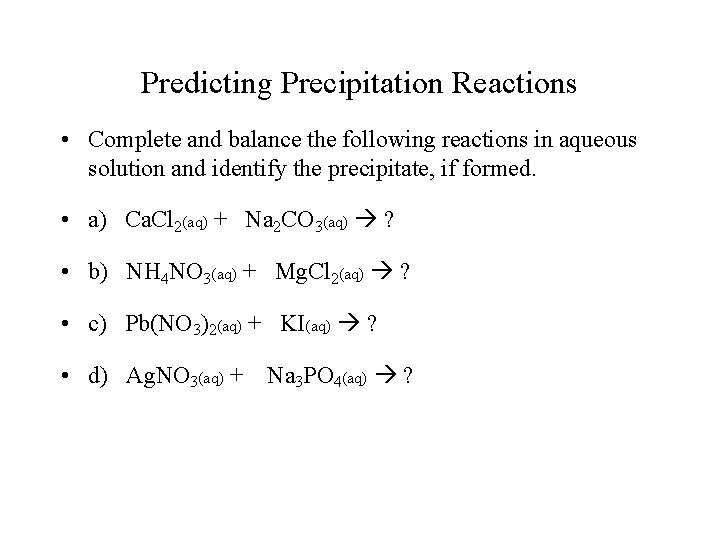
Predicting Precipitation Reactions • Complete and balance the following reactions in aqueous solution and identify the precipitate, if formed. • a) Ca. Cl 2(aq) + Na 2 CO 3(aq) ? • b) NH 4 NO 3(aq) + Mg. Cl 2(aq) ? • c) Pb(NO 3)2(aq) + KI(aq) ? • d) Ag. NO 3(aq) + Na 3 PO 4(aq) ?

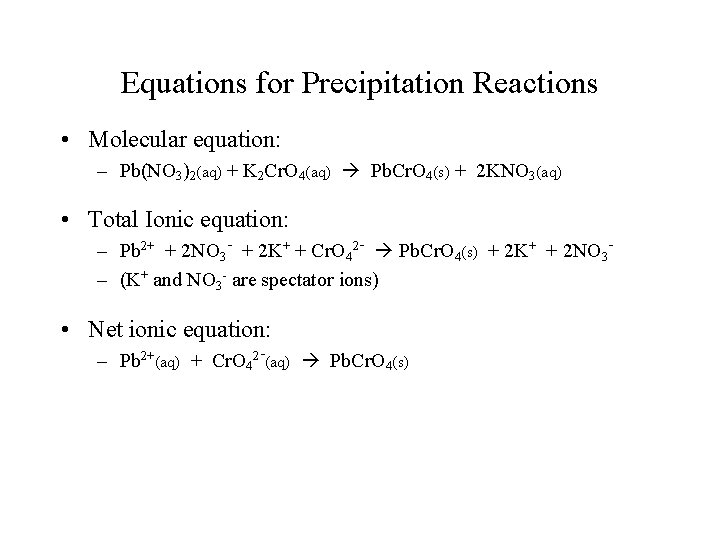
Equations for Precipitation Reactions • Molecular equation: – Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 KNO 3(aq) • Total Ionic equation: – Pb 2+ + 2 NO 3 - + 2 K+ + Cr. O 42 - Pb. Cr. O 4(s) + 2 K+ + 2 NO 3– (K+ and NO 3 - are spectator ions) • Net ionic equation: – Pb 2+(aq) + Cr. O 42 -(aq) Pb. Cr. O 4(s)

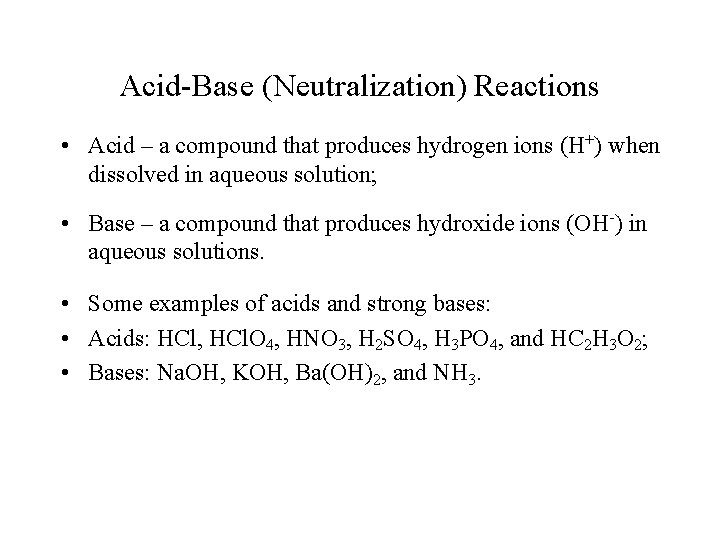
Acid-Base (Neutralization) Reactions • Acid – a compound that produces hydrogen ions (H+) when dissolved in aqueous solution; • Base – a compound that produces hydroxide ions (OH-) in aqueous solutions. • Some examples of acids and strong bases: • Acids: HCl, HCl. O 4, HNO 3, H 2 SO 4, H 3 PO 4, and HC 2 H 3 O 2; • Bases: Na. OH, KOH, Ba(OH)2, and NH 3.

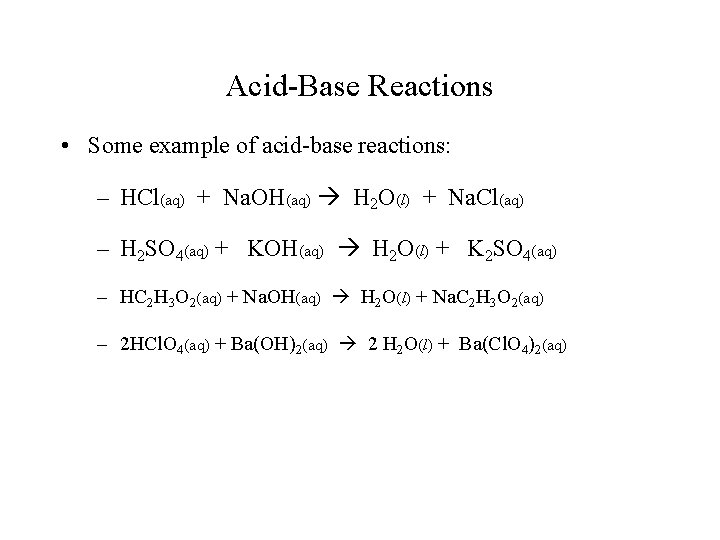
Acid-Base Reactions • Some example of acid-base reactions: – HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq) – H 2 SO 4(aq) + KOH(aq) H 2 O(l) + K 2 SO 4(aq) – HC 2 H 3 O 2(aq) + Na. OH(aq) H 2 O(l) + Na. C 2 H 3 O 2(aq) – 2 HCl. O 4(aq) + Ba(OH)2(aq) 2 H 2 O(l) + Ba(Cl. O 4)2(aq)

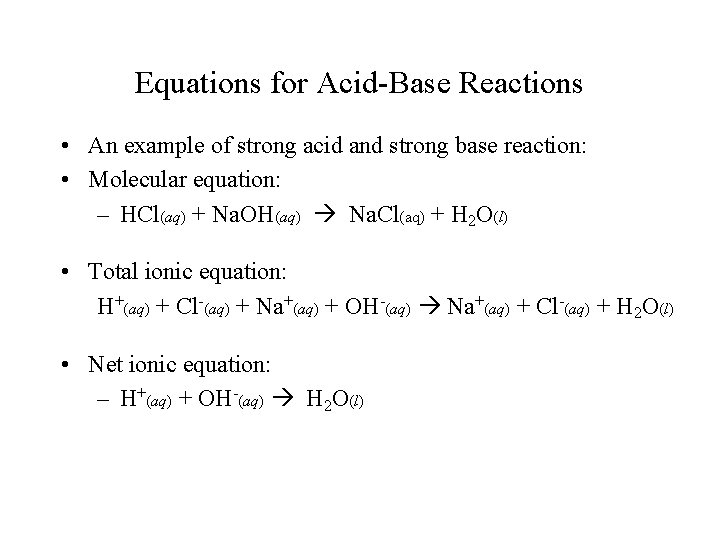
Equations for Acid-Base Reactions • An example of strong acid and strong base reaction: • Molecular equation: – HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) • Total ionic equation: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) Na+(aq) + Cl-(aq) + H 2 O(l) • Net ionic equation: – H+(aq) + OH-(aq) H 2 O(l)

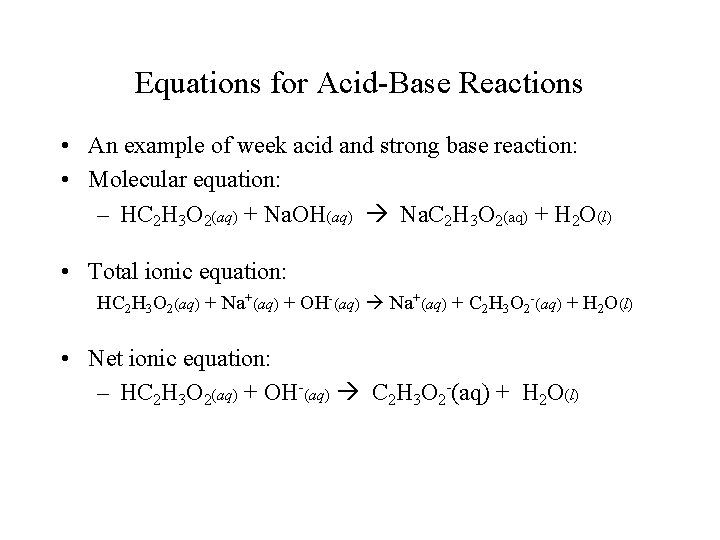
Equations for Acid-Base Reactions • An example of week acid and strong base reaction: • Molecular equation: – HC 2 H 3 O 2(aq) + Na. OH(aq) Na. C 2 H 3 O 2(aq) + H 2 O(l) • Total ionic equation: HC 2 H 3 O 2(aq) + Na+(aq) + OH-(aq) Na+(aq) + C 2 H 3 O 2 -(aq) + H 2 O(l) • Net ionic equation: – HC 2 H 3 O 2(aq) + OH-(aq) C 2 H 3 O 2 -(aq) + H 2 O(l)

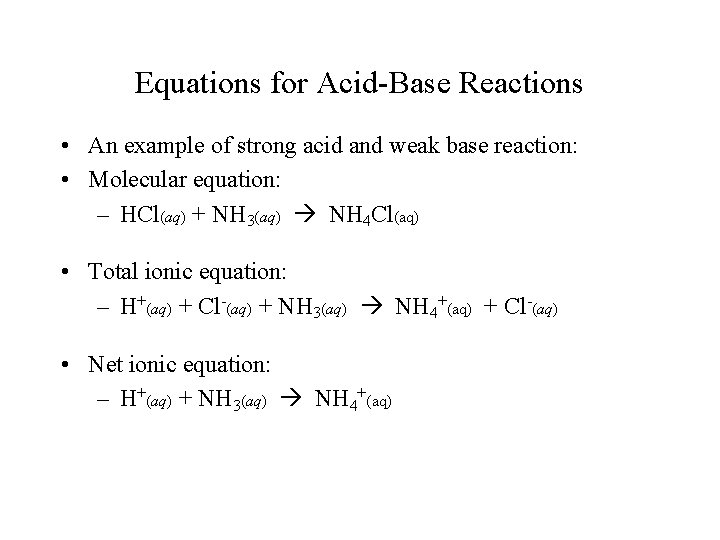
Equations for Acid-Base Reactions • An example of strong acid and weak base reaction: • Molecular equation: – HCl(aq) + NH 3(aq) NH 4 Cl(aq) • Total ionic equation: – H+(aq) + Cl-(aq) + NH 3(aq) NH 4+(aq) + Cl-(aq) • Net ionic equation: – H+(aq) + NH 3(aq) NH 4+(aq)

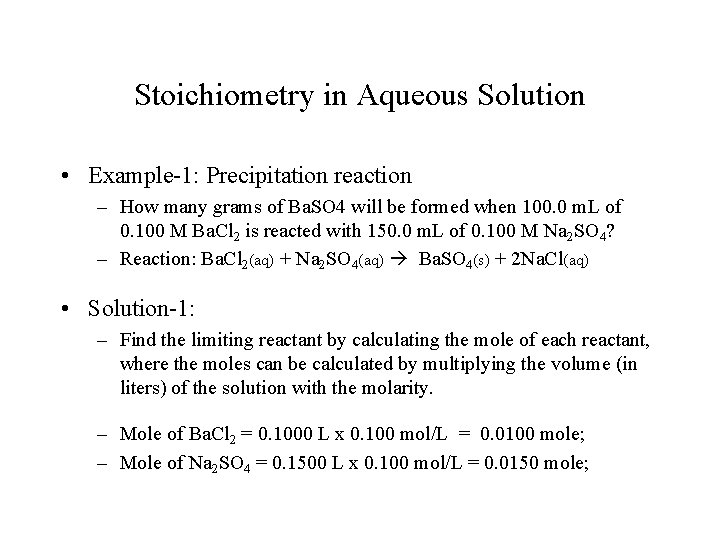
Stoichiometry in Aqueous Solution • Example-1: Precipitation reaction – How many grams of Ba. SO 4 will be formed when 100. 0 m. L of 0. 100 M Ba. Cl 2 is reacted with 150. 0 m. L of 0. 100 M Na 2 SO 4? – Reaction: Ba. Cl 2(aq) + Na 2 SO 4(aq) Ba. SO 4(s) + 2 Na. Cl(aq) • Solution-1: – Find the limiting reactant by calculating the mole of each reactant, where the moles can be calculated by multiplying the volume (in liters) of the solution with the molarity. – Mole of Ba. Cl 2 = 0. 1000 L x 0. 100 mol/L = 0. 0100 mole; – Mole of Na 2 SO 4 = 0. 1500 L x 0. 100 mol/L = 0. 0150 mole;

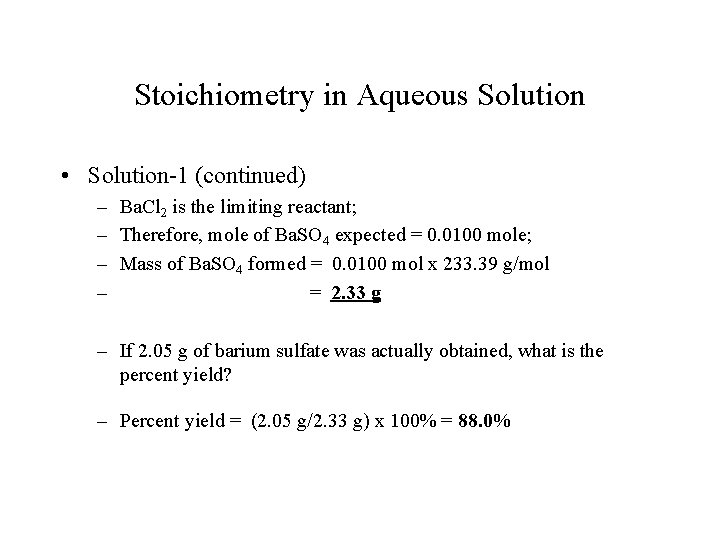
Stoichiometry in Aqueous Solution • Solution-1 (continued) – Ba. Cl 2 is the limiting reactant; – Therefore, mole of Ba. SO 4 expected = 0. 0100 mole; – Mass of Ba. SO 4 formed = 0. 0100 mol x 233. 39 g/mol – = 2. 33 g – If 2. 05 g of barium sulfate was actually obtained, what is the percent yield? – Percent yield = (2. 05 g/2. 33 g) x 100% = 88. 0%

Acid-Base Titration

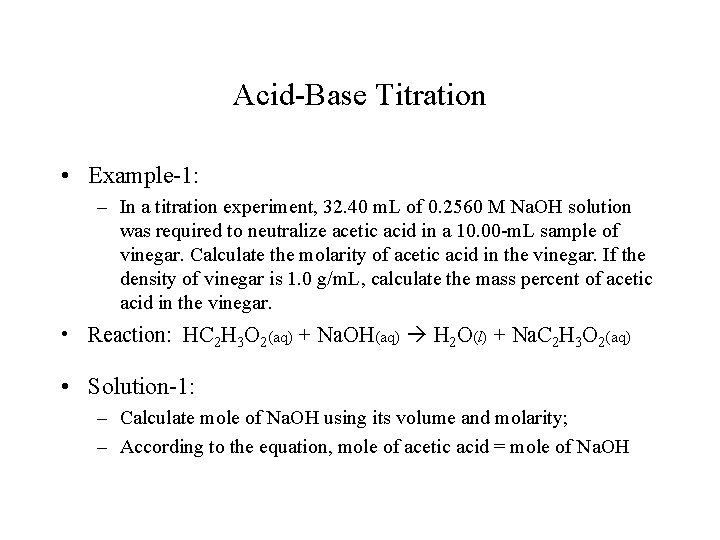
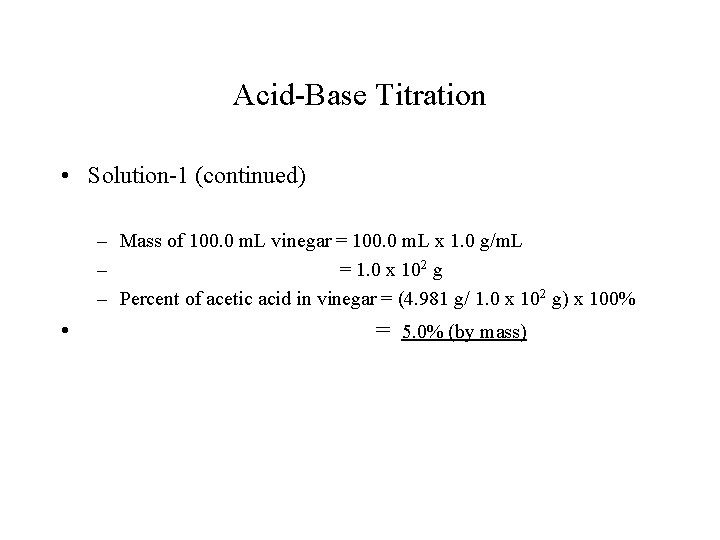
Acid-Base Titration • Example-1: – In a titration experiment, 32. 40 m. L of 0. 2560 M Na. OH solution was required to neutralize acetic acid in a 10. 00 -m. L sample of vinegar. Calculate the molarity of acetic acid in the vinegar. If the density of vinegar is 1. 0 g/m. L, calculate the mass percent of acetic acid in the vinegar. • Reaction: HC 2 H 3 O 2(aq) + Na. OH(aq) H 2 O(l) + Na. C 2 H 3 O 2(aq) • Solution-1: – Calculate mole of Na. OH using its volume and molarity; – According to the equation, mole of acetic acid = mole of Na. OH

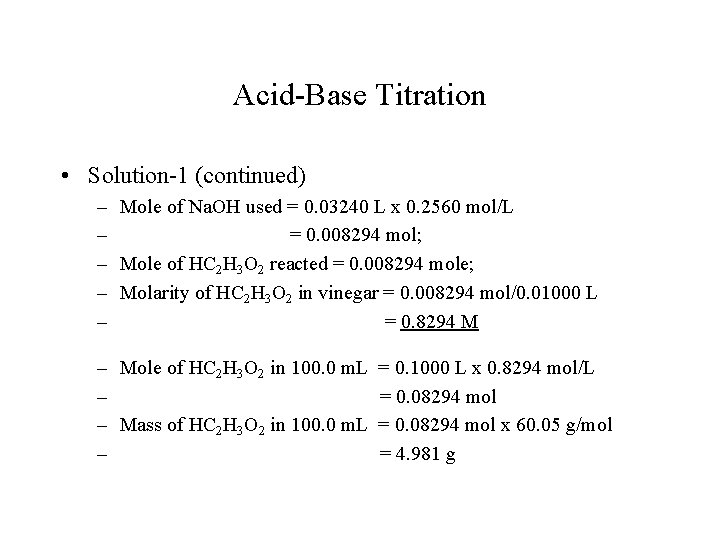
Acid-Base Titration • Solution-1 (continued) – Mole of Na. OH used = 0. 03240 L x 0. 2560 mol/L – = 0. 008294 mol; – Mole of HC 2 H 3 O 2 reacted = 0. 008294 mole; – Molarity of HC 2 H 3 O 2 in vinegar = 0. 008294 mol/0. 01000 L – = 0. 8294 M – Mole of HC 2 H 3 O 2 in 100. 0 m. L – – Mass of HC 2 H 3 O 2 in 100. 0 m. L – = 0. 1000 L x 0. 8294 mol/L = 0. 08294 mol x 60. 05 g/mol = 4. 981 g

Acid-Base Titration • Solution-1 (continued) – Mass of 100. 0 m. L vinegar = 100. 0 m. L x 1. 0 g/m. L – = 1. 0 x 102 g – Percent of acetic acid in vinegar = (4. 981 g/ 1. 0 x 102 g) x 100% • = 5. 0% (by mass)

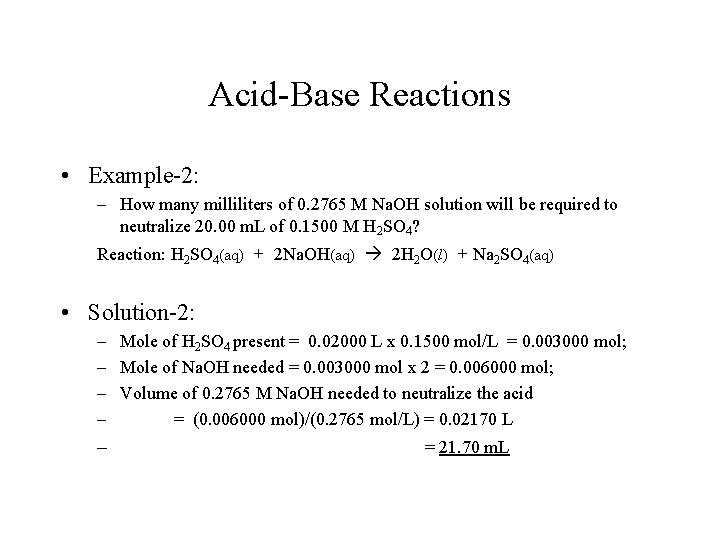
Acid-Base Reactions • Example-2: – How many milliliters of 0. 2765 M Na. OH solution will be required to neutralize 20. 00 m. L of 0. 1500 M H 2 SO 4? Reaction: H 2 SO 4(aq) + 2 Na. OH(aq) 2 H 2 O(l) + Na 2 SO 4(aq) • Solution-2: – Mole of H 2 SO 4 present = 0. 02000 L x 0. 1500 mol/L = 0. 003000 mol; – Mole of Na. OH needed = 0. 003000 mol x 2 = 0. 006000 mol; – Volume of 0. 2765 M Na. OH needed to neutralize the acid – = (0. 006000 mol)/(0. 2765 mol/L) = 0. 02170 L – = 21. 70 m. L

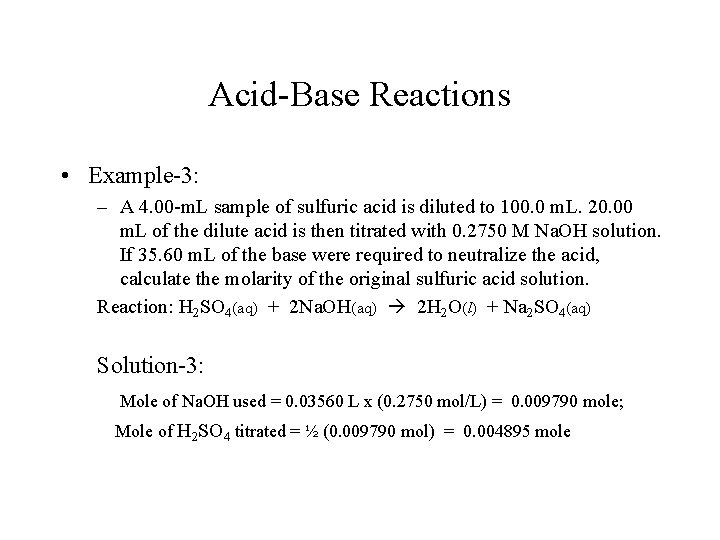
Acid-Base Reactions • Example-3: – A 4. 00 -m. L sample of sulfuric acid is diluted to 100. 0 m. L. 20. 00 m. L of the dilute acid is then titrated with 0. 2750 M Na. OH solution. If 35. 60 m. L of the base were required to neutralize the acid, calculate the molarity of the original sulfuric acid solution. Reaction: H 2 SO 4(aq) + 2 Na. OH(aq) 2 H 2 O(l) + Na 2 SO 4(aq) Solution-3: Mole of Na. OH used = 0. 03560 L x (0. 2750 mol/L) = 0. 009790 mole; Mole of H 2 SO 4 titrated = ½ (0. 009790 mol) = 0. 004895 mole

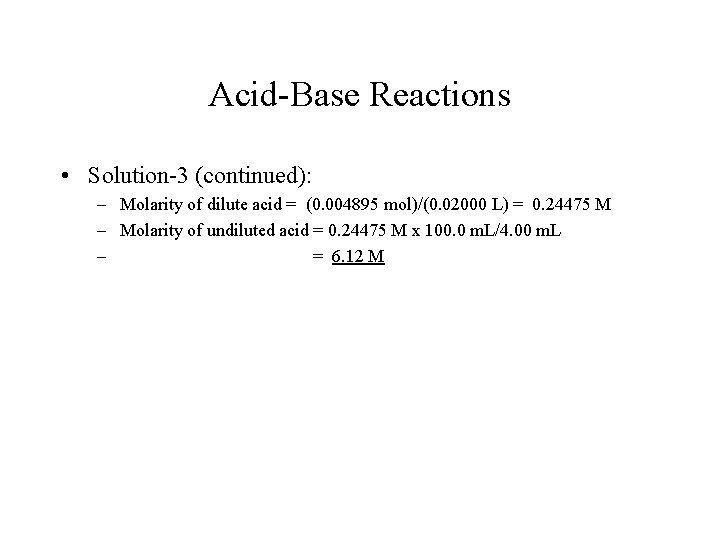
Acid-Base Reactions • Solution-3 (continued): – Molarity of dilute acid = (0. 004895 mol)/(0. 02000 L) = 0. 24475 M – Molarity of undiluted acid = 0. 24475 M x 100. 0 m. L/4. 00 m. L – = 6. 12 M

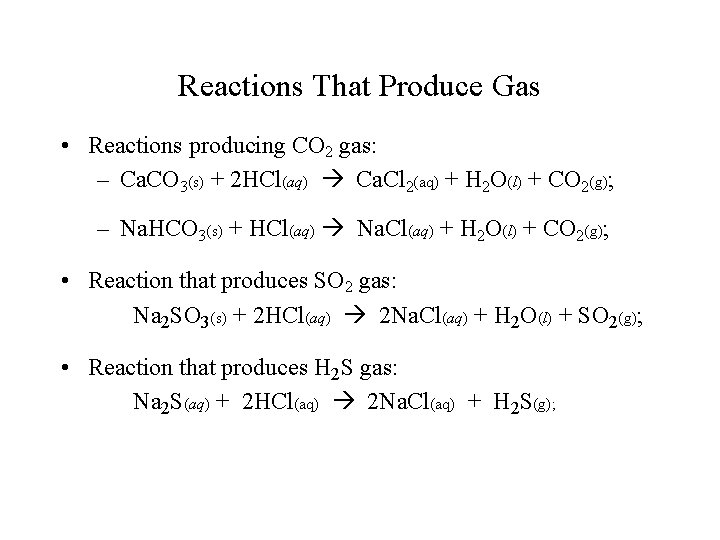
Reactions That Produce Gas • Reactions producing CO 2 gas: – Ca. CO 3(s) + 2 HCl(aq) Ca. Cl 2(aq) + H 2 O(l) + CO 2(g); – Na. HCO 3(s) + HCl(aq) Na. Cl(aq) + H 2 O(l) + CO 2(g); • Reaction that produces SO 2 gas: Na 2 SO 3(s) + 2 HCl(aq) 2 Na. Cl(aq) + H 2 O(l) + SO 2(g); • Reaction that produces H 2 S gas: Na 2 S(aq) + 2 HCl(aq) 2 Na. Cl(aq) + H 2 S(g);

Oxidation-Reduction Reactions • Oxidation loss of electrons and increase in oxidation number; • Reduction gain of electrons and decrease in oxidation number; • Oxidation-reduction (or Redox) reaction one that involves transfer of electrons from one reactant to the other; • Oxidizing agent the reactant that gains electrons and got reduced; • Reducing agent the reactant that loses electrons and got oxidized.

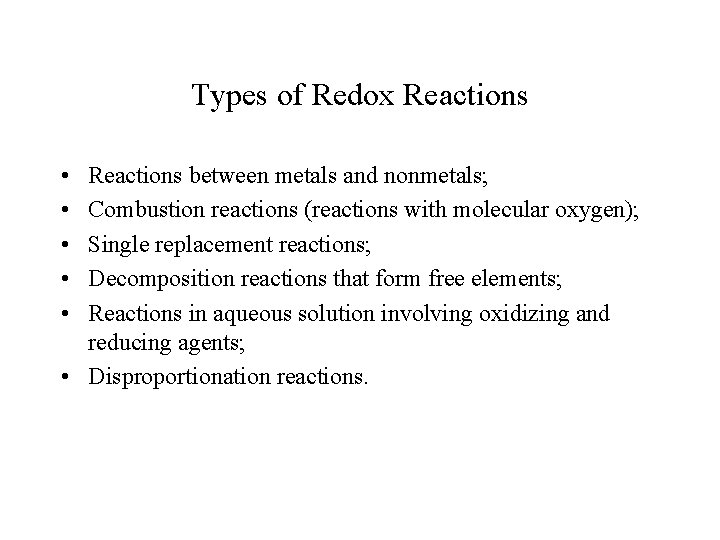
Types of Redox Reactions • • • Reactions between metals and nonmetals; Combustion reactions (reactions with molecular oxygen); Single replacement reactions; Decomposition reactions that form free elements; Reactions in aqueous solution involving oxidizing and reducing agents; • Disproportionation reactions.

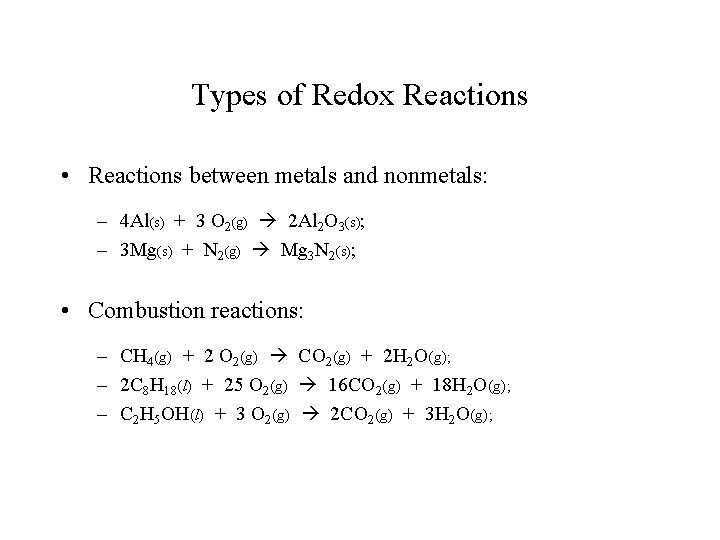
Types of Redox Reactions • Reactions between metals and nonmetals: – 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s); – 3 Mg(s) + N 2(g) Mg 3 N 2(s); • Combustion reactions: – CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g); – 2 C 8 H 18(l) + 25 O 2(g) 16 CO 2(g) + 18 H 2 O(g); – C 2 H 5 OH(l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g);

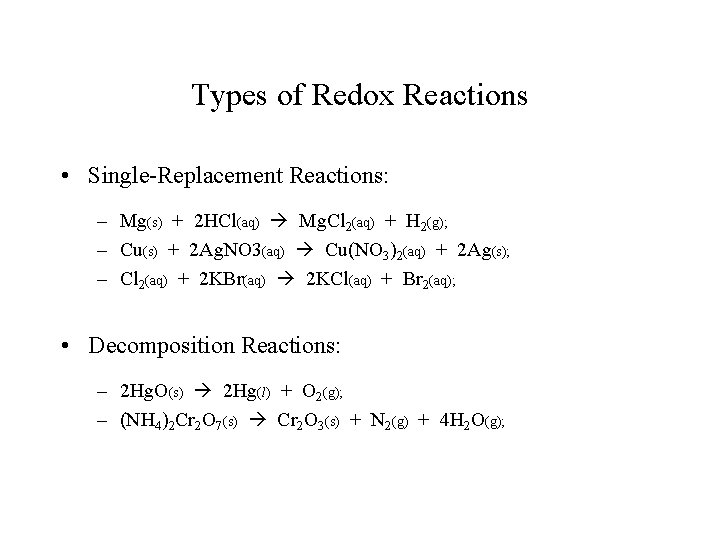
Types of Redox Reactions • Single-Replacement Reactions: – Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g); – Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s); – Cl 2(aq) + 2 KBr(aq) 2 KCl(aq) + Br 2(aq); • Decomposition Reactions: – 2 Hg. O(s) 2 Hg(l) + O 2(g); – (NH 4)2 Cr 2 O 7(s) Cr 2 O 3(s) + N 2(g) + 4 H 2 O(g);

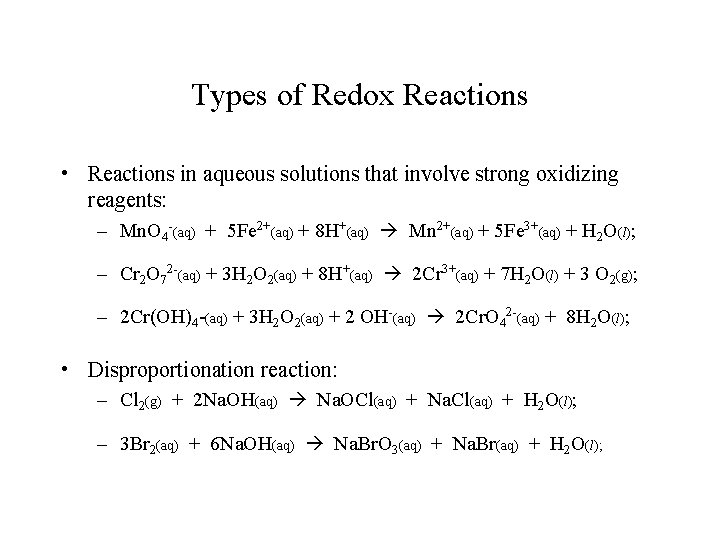
Types of Redox Reactions • Reactions in aqueous solutions that involve strong oxidizing reagents: – Mn. O 4 -(aq) + 5 Fe 2+(aq) + 8 H+(aq) Mn 2+(aq) + 5 Fe 3+(aq) + H 2 O(l); – Cr 2 O 72 -(aq) + 3 H 2 O 2(aq) + 8 H+(aq) 2 Cr 3+(aq) + 7 H 2 O(l) + 3 O 2(g); – 2 Cr(OH)4 -(aq) + 3 H 2 O 2(aq) + 2 OH-(aq) 2 Cr. O 42 -(aq) + 8 H 2 O(l); • Disproportionation reaction: – Cl 2(g) + 2 Na. OH(aq) Na. OCl(aq) + Na. Cl(aq) + H 2 O(l); – 3 Br 2(aq) + 6 Na. OH(aq) Na. Br. O 3(aq) + Na. Br(aq) + H 2 O(l);

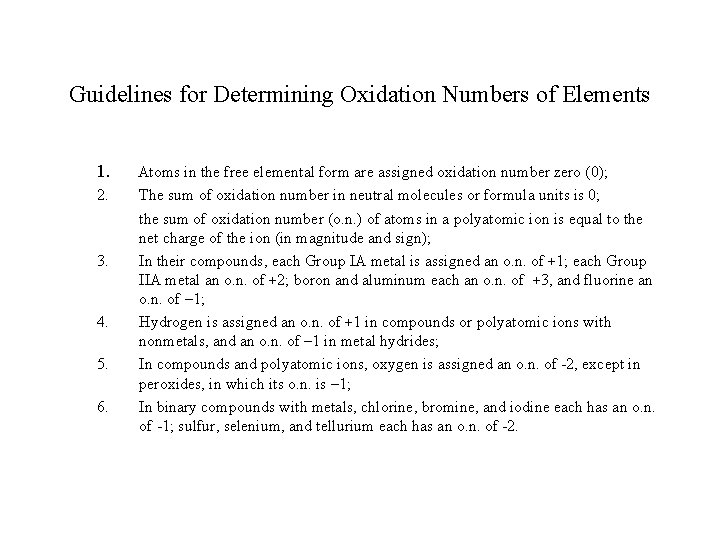
Guidelines for Determining Oxidation Numbers of Elements 1. 2. 3. 4. 5. 6. Atoms in the free elemental form are assigned oxidation number zero (0); The sum of oxidation number in neutral molecules or formula units is 0; the sum of oxidation number (o. n. ) of atoms in a polyatomic ion is equal to the net charge of the ion (in magnitude and sign); In their compounds, each Group IA metal is assigned an o. n. of +1; each Group IIA metal an o. n. of +2; boron and aluminum each an o. n. of +3, and fluorine an o. n. of – 1; Hydrogen is assigned an o. n. of +1 in compounds or polyatomic ions with nonmetals, and an o. n. of – 1 in metal hydrides; In compounds and polyatomic ions, oxygen is assigned an o. n. of -2, except in peroxides, in which its o. n. is – 1; In binary compounds with metals, chlorine, bromine, and iodine each has an o. n. of -1; sulfur, selenium, and tellurium each has an o. n. of -2.

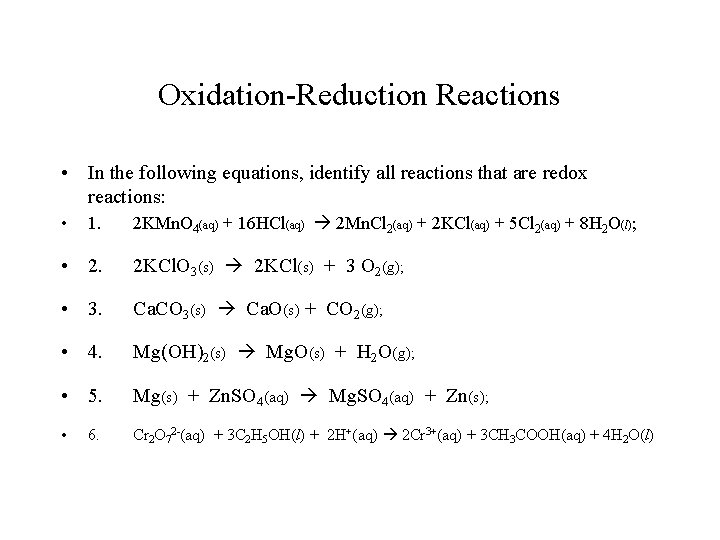
Oxidation-Reduction Reactions • In the following equations, identify all reactions that are redox reactions: • 1. 2 KMn. O 4(aq) + 16 HCl(aq) 2 Mn. Cl 2(aq) + 2 KCl(aq) + 5 Cl 2(aq) + 8 H 2 O(l); • 2. 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g); • 3. Ca. CO 3(s) Ca. O(s) + CO 2(g); • 4. Mg(OH)2(s) Mg. O(s) + H 2 O(g); • 5. Mg(s) + Zn. SO 4(aq) Mg. SO 4(aq) + Zn(s); • Cr 2 O 72 -(aq) + 3 C 2 H 5 OH(l) + 2 H+(aq) 2 Cr 3+(aq) + 3 CH 3 COOH(aq) + 4 H 2 O(l) 6.

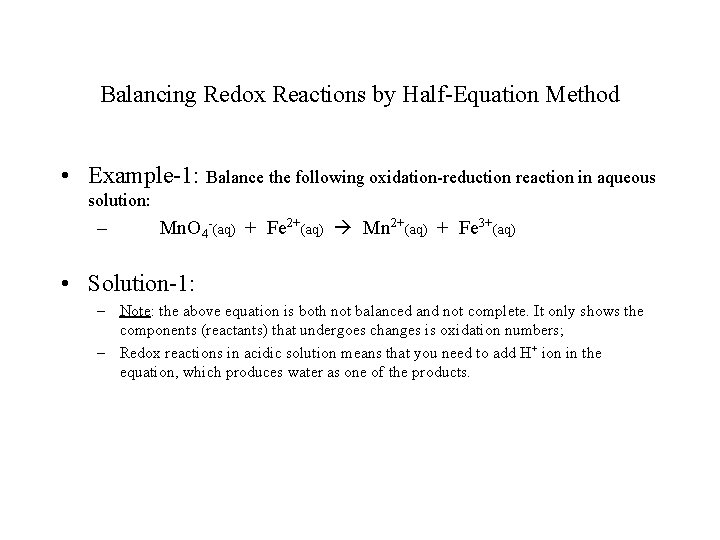
Balancing Redox Reactions by Half-Equation Method • Example-1: Balance the following oxidation-reduction reaction in aqueous solution: – Mn. O 4 -(aq) + Fe 2+(aq) Mn 2+(aq) + Fe 3+(aq) • Solution-1: – Note: the above equation is both not balanced and not complete. It only shows the components (reactants) that undergoes changes is oxidation numbers; – Redox reactions in acidic solution means that you need to add H+ ion in the equation, which produces water as one of the products.

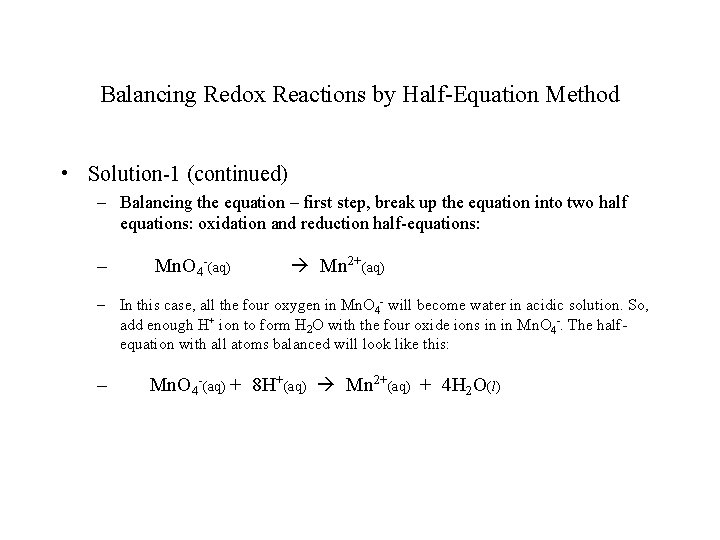
Balancing Redox Reactions by Half-Equation Method • Solution-1 (continued) – Balancing the equation – first step, break up the equation into two half equations: oxidation and reduction half-equations: – Mn. O 4 -(aq) Mn 2+(aq) – In this case, all the four oxygen in Mn. O 4 - will become water in acidic solution. So, add enough H+ ion to form H 2 O with the four oxide ions in in Mn. O 4 -. The halfequation with all atoms balanced will look like this: – Mn. O 4 -(aq) + 8 H+(aq) Mn 2+(aq) + 4 H 2 O(l)

Balancing Redox Reactions by Half-Equation Method • Solution-1 (continued) – Next step, check the total charges on both side of the half-equation; note that they are not equal. Add enough electrons on the side that has more positive charges (or less negative charges), so that the total charges on both sides become equal (in magnitude and sign). – Mn. O 4 -(aq) + 8 H+(aq) + 5 e- Mn 2+(aq) + 4 H 2 O(l); – Now we have a balanced reduction half-equation. – (How do you know it is “reduction” half-equation? )

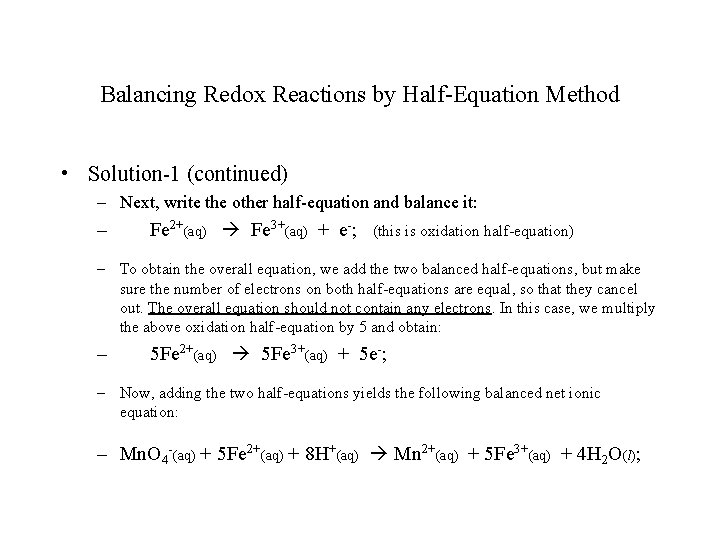
Balancing Redox Reactions by Half-Equation Method • Solution-1 (continued) – Next, write the other half-equation and balance it: – Fe 2+(aq) Fe 3+(aq) + e-; (this is oxidation half-equation) – To obtain the overall equation, we add the two balanced half-equations, but make sure the number of electrons on both half-equations are equal, so that they cancel out. The overall equation should not contain any electrons. In this case, we multiply the above oxidation half-equation by 5 and obtain: – 5 Fe 2+(aq) 5 Fe 3+(aq) + 5 e-; – Now, adding the two half-equations yields the following balanced net ionic equation: – Mn. O 4 -(aq) + 5 Fe 2+(aq) + 8 H+(aq) Mn 2+(aq) + 5 Fe 3+(aq) + 4 H 2 O(l);

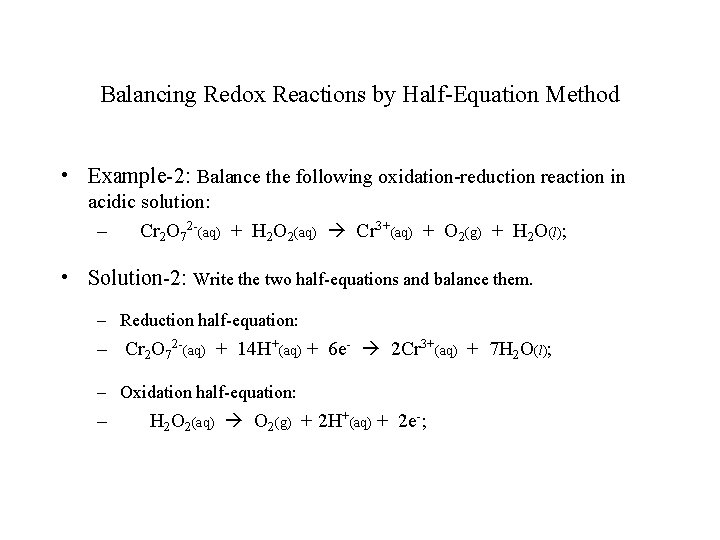
Balancing Redox Reactions by Half-Equation Method • Example-2: Balance the following oxidation-reduction reaction in acidic solution: – Cr 2 O 72 -(aq) + H 2 O 2(aq) Cr 3+(aq) + O 2(g) + H 2 O(l); • Solution-2: Write the two half-equations and balance them. – Reduction half-equation: – Cr 2 O 72 -(aq) + 14 H+(aq) + 6 e- 2 Cr 3+(aq) + 7 H 2 O(l); – Oxidation half-equation: – H 2 O 2(aq) O 2(g) + 2 H+(aq) + 2 e-;

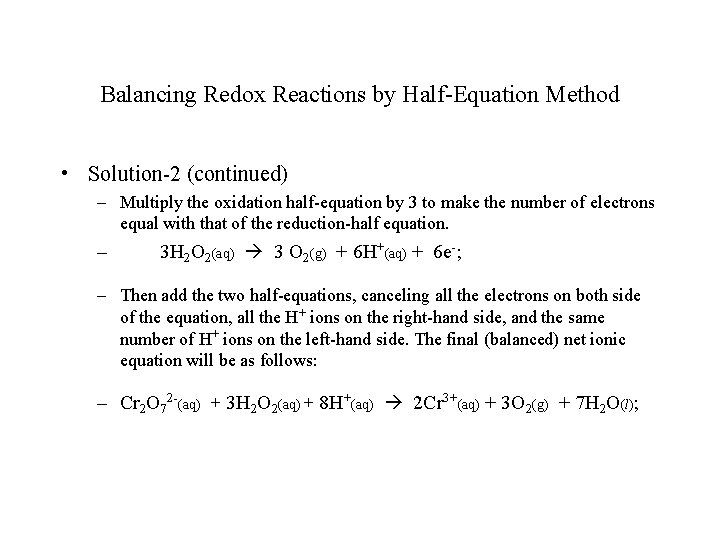
Balancing Redox Reactions by Half-Equation Method • Solution-2 (continued) – Multiply the oxidation half-equation by 3 to make the number of electrons equal with that of the reduction-half equation. – 3 H 2 O 2(aq) 3 O 2(g) + 6 H+(aq) + 6 e-; – Then add the two half-equations, canceling all the electrons on both side of the equation, all the H+ ions on the right-hand side, and the same number of H+ ions on the left-hand side. The final (balanced) net ionic equation will be as follows: – Cr 2 O 72 -(aq) + 3 H 2 O 2(aq) + 8 H+(aq) 2 Cr 3+(aq) + 3 O 2(g) + 7 H 2 O(l);

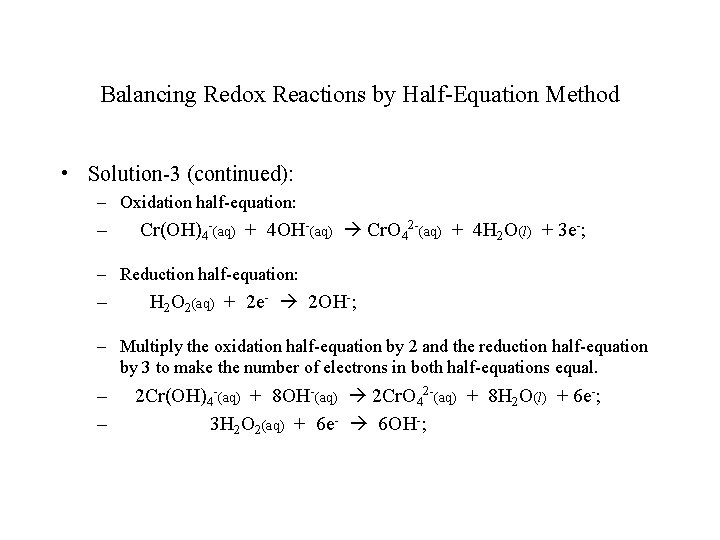
Balancing Redox Reactions by Half-Equation Method • Example-3: Balance the following oxidation-reduction reaction in basic solution: – Cr(OH)4 -(aq) + H 2 O 2(aq) Cr. O 42 -(aq) + H 2 O(l); • Solution-3: – Write the two half-equations and balance them, adding electrons on either side as needed to balance the charges. – Note that this reaction is in basic solution, which means the overall equation should contains OH- ion instead of H+ ions.

Balancing Redox Reactions by Half-Equation Method • Solution-3 (continued): – Oxidation half-equation: – Cr(OH)4 -(aq) + 4 OH-(aq) Cr. O 42 -(aq) + 4 H 2 O(l) + 3 e-; – Reduction half-equation: – H 2 O 2(aq) + 2 e- 2 OH-; – Multiply the oxidation half-equation by 2 and the reduction half-equation by 3 to make the number of electrons in both half-equations equal. – – 2 Cr(OH)4 -(aq) + 8 OH-(aq) 2 Cr. O 42 -(aq) + 8 H 2 O(l) + 6 e-; 3 H 2 O 2(aq) + 6 e- 6 OH-;

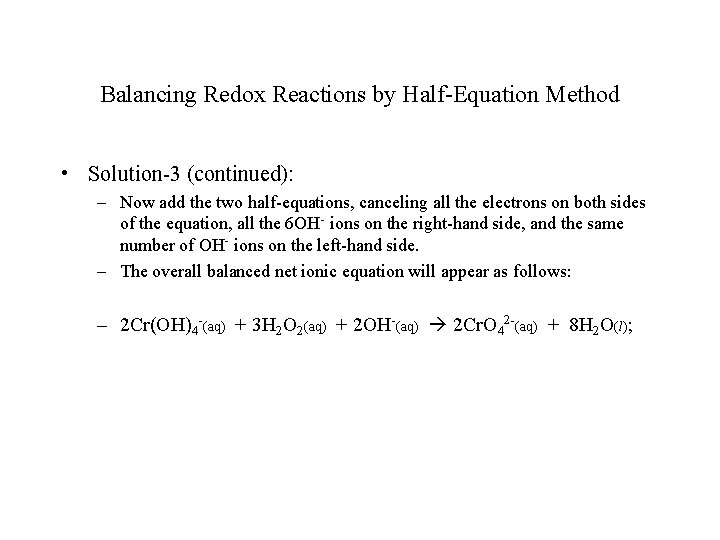
Balancing Redox Reactions by Half-Equation Method • Solution-3 (continued): – Now add the two half-equations, canceling all the electrons on both sides of the equation, all the 6 OH- ions on the right-hand side, and the same number of OH- ions on the left-hand side. – The overall balanced net ionic equation will appear as follows: – 2 Cr(OH)4 -(aq) + 3 H 2 O 2(aq) + 2 OH-(aq) 2 Cr. O 42 -(aq) + 8 H 2 O(l);

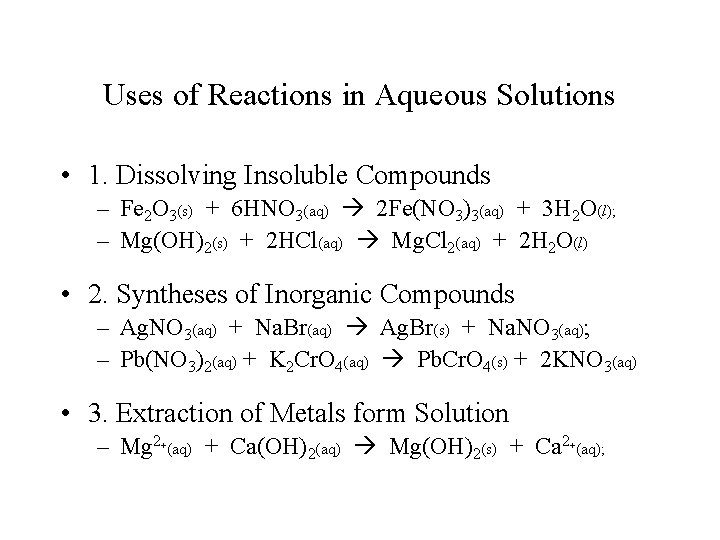
Uses of Reactions in Aqueous Solutions • 1. Dissolving Insoluble Compounds – Fe 2 O 3(s) + 6 HNO 3(aq) 2 Fe(NO 3)3(aq) + 3 H 2 O(l); – Mg(OH)2(s) + 2 HCl(aq) Mg. Cl 2(aq) + 2 H 2 O(l) • 2. Syntheses of Inorganic Compounds – Ag. NO 3(aq) + Na. Br(aq) Ag. Br(s) + Na. NO 3(aq); – Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 KNO 3(aq) • 3. Extraction of Metals form Solution – Mg 2+(aq) + Ca(OH)2(aq) Mg(OH)2(s) + Ca 2+(aq);