Redox Rules Redox Rules to Learn For Ionic







- Slides: 17

Redox Rules! Redox Rules to Learn For Ionic and Covalent bonds

Electronegativity and Redox Rules Definition: Electonegativity is the atom’s ability to attract electrons. Fluorine is the most electronegative element

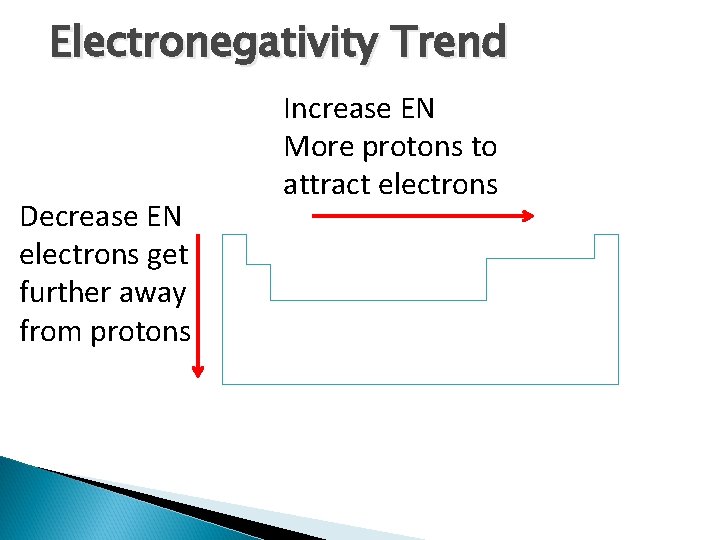
Electronegativity Trend Decrease EN electrons get further away from protons Increase EN More protons to attract electrons

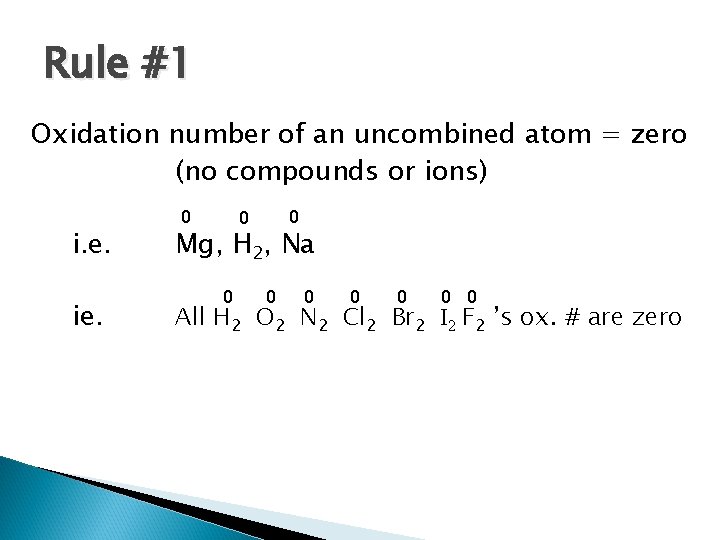
Rule #1 Oxidation number of an uncombined atom = zero (no compounds or ions) i. e. ie. 0 0 0 Mg, H 2, Na 0 0 0 0 All H 2 O 2 N 2 Cl 2 Br 2 I 2 F 2 ’s ox. # are zero

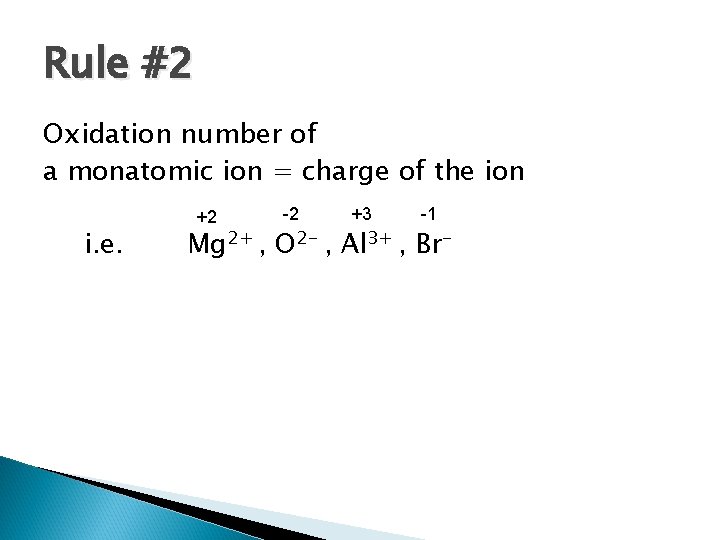
Rule #2 Oxidation number of a monatomic ion = charge of the ion i. e. +2 Mg 2+ , -2 O 2 - , +3 Al 3+ -1 , Br-

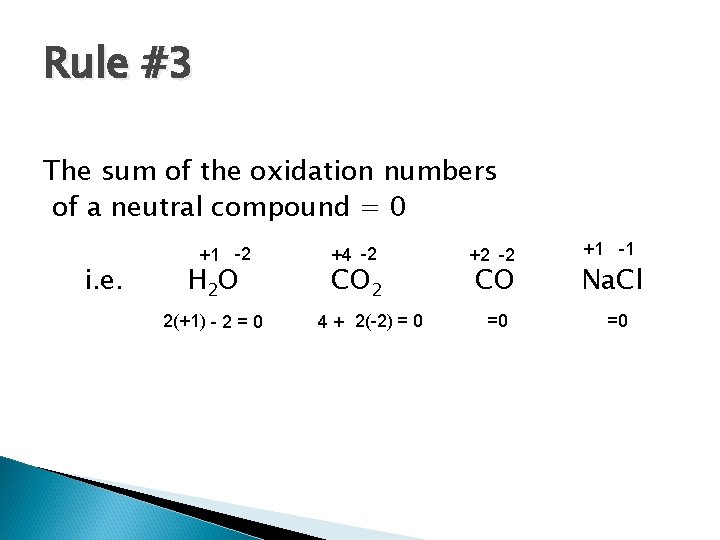
Rule #3 The sum of the oxidation numbers of a neutral compound = 0 i. e. +1 -2 H 2 O 2(+1) - 2 = 0 +4 -2 CO 2 4 + 2(-2) = 0 +2 -2 CO =0 +1 -1 Na. Cl =0

Rule #4 The oxidation number for H is +1 Exception: when bonded to a less electronegative element (metal), then it is -1. i. e. +1 -1 +2 -1 Li. H, Na. H, Mg. H 2 +1 -1 HF, HCl Exception

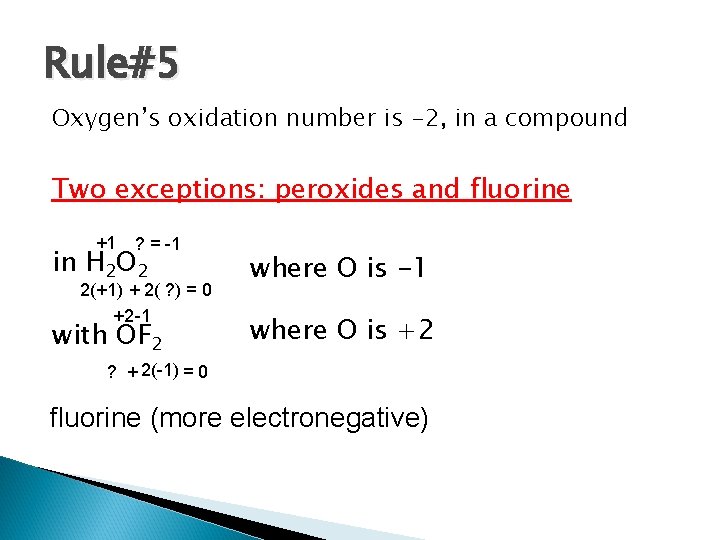
Rule#5 Oxygen’s oxidation number is -2, in a compound Two exceptions: peroxides and fluorine +1 ? = -1 in H 2 O 2 where O is -1 with OF 2 where O is +2 2(+1) + 2( ? ) = 0 +2 -1 ? + 2(-1) = 0 fluorine (more electronegative)

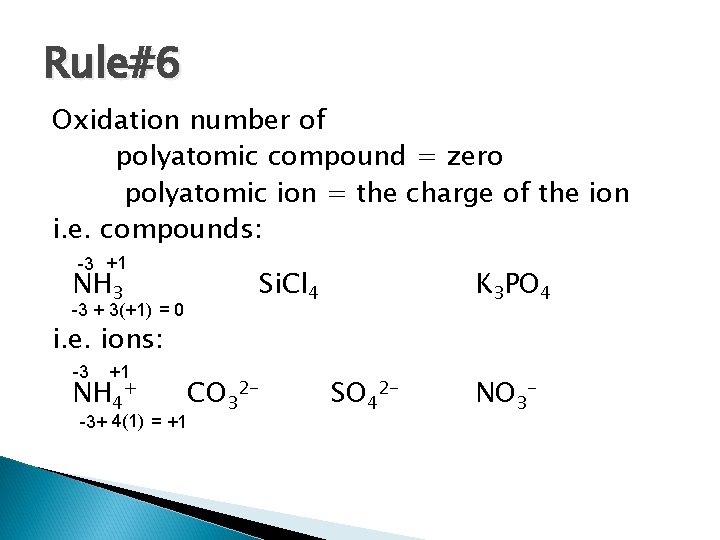
Rule#6 Oxidation number of polyatomic compound = zero polyatomic ion = the charge of the ion i. e. compounds: -3 +1 NH 3 -3 + 3(+1) = 0 Si. Cl 4 K 3 PO 4 i. e. ions: +1 NH 4+ CO 32 -3+ 4(1) = +1 -3 SO 42 - NO 3 -

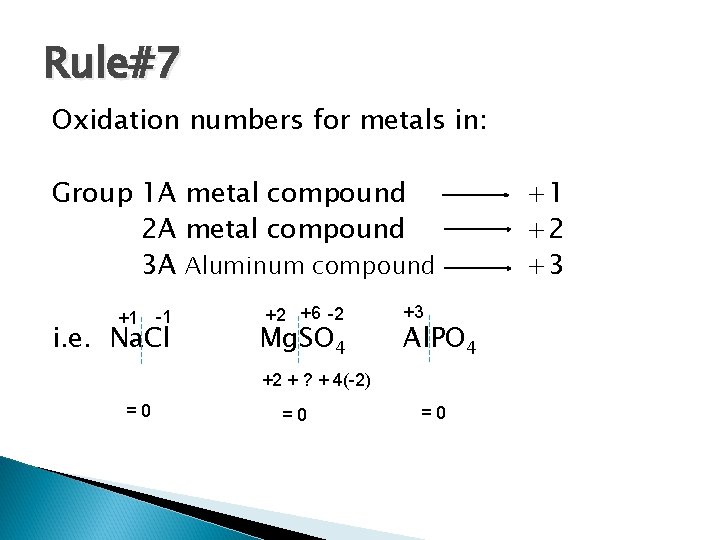
Rule#7 Oxidation numbers for metals in: Group 1 A metal compound 2 A metal compound 3 A Aluminum compound +1 -1 i. e. Na. Cl +2 +6 -2 Mg. SO 4 +3 Al. PO 4 +2 + ? + 4(-2) =0 =0 =0 +1 +2 +3

Rule #8 The most electronegative element fluorine ALWAYS has an oxidation number of -1 when it is bonded to another element. i. e. HF

Question Time Assign oxidation numbers to each element 4. Na. H 5. HCl 1. Cl 2 6. K 3 PO 4 2. Cl- 7. Ca. SO 4 3. Mg. Cl 2 8. KF

Using the Rules Oxidation Number If oxidation number goes up it is oxidized. If oxidation number goes down it is reduced.

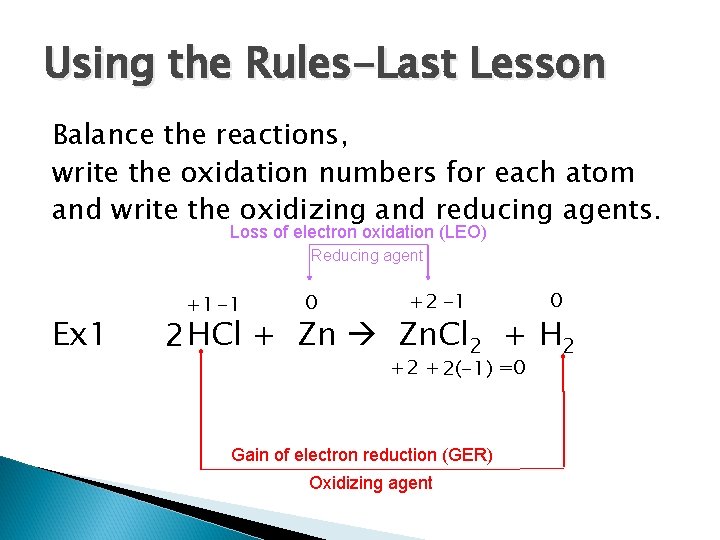
Using the Rules-Last Lesson Balance the reactions, write the oxidation numbers for each atom and write the oxidizing and reducing agents. Loss of electron oxidation (LEO) Reducing agent Ex 1 +1 -1 0 +2 -1 0 2 HCl + Zn Zn. Cl 2 + H 2 +2 + 2(-1) =0 Gain of electron reduction (GER) Oxidizing agent

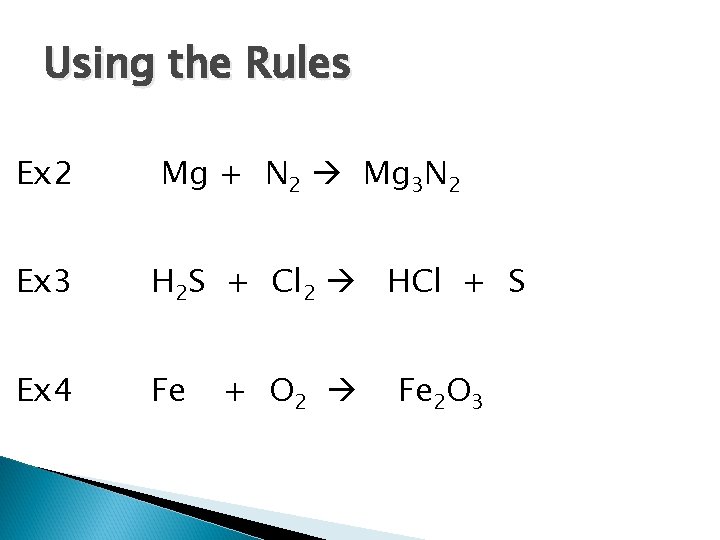
Using the Rules Ex 2 Mg + N 2 Mg 3 N 2 Ex 3 H 2 S + Cl 2 HCl + S Ex 4 Fe + O 2 Fe 2 O 3

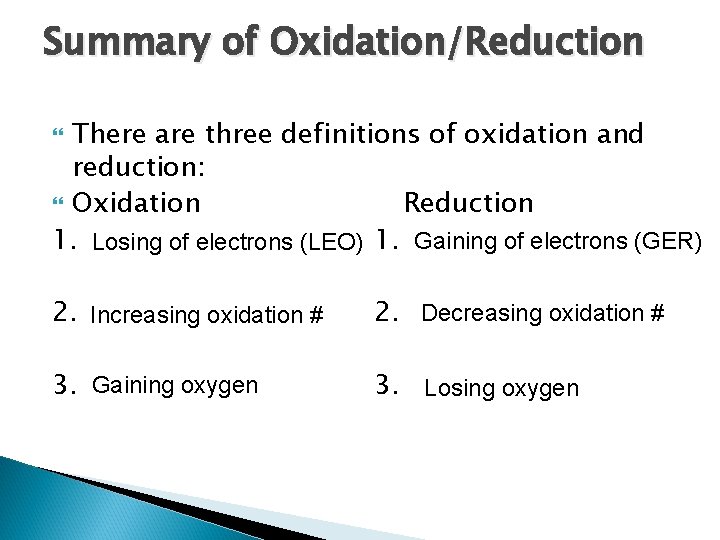
Summary of Oxidation/Reduction There are three definitions of oxidation and reduction: Oxidation Reduction 1. Losing of electrons (LEO) 1. Gaining of electrons (GER) 2. Increasing oxidation # 2. Decreasing oxidation # 3. Gaining oxygen 3. Losing oxygen

Two ways to remember oxidation/reduction (redox) is: L E O goes G E R O S E L E C T R O N S X I D A T I O N A I N L E C T R O N S E D U C T I O N O I L X I D A T I O N S O S I N G R I G E D U C T I O N S A I N G