Net Ionic Equations Net Ionic Equations Net ionic






- Slides: 8

Net Ionic Equations

Net Ionic Equations • Net ionic equations are used to show which ions participate in the reaction. • Ions that do NOT participate in the reaction are spectator ions.

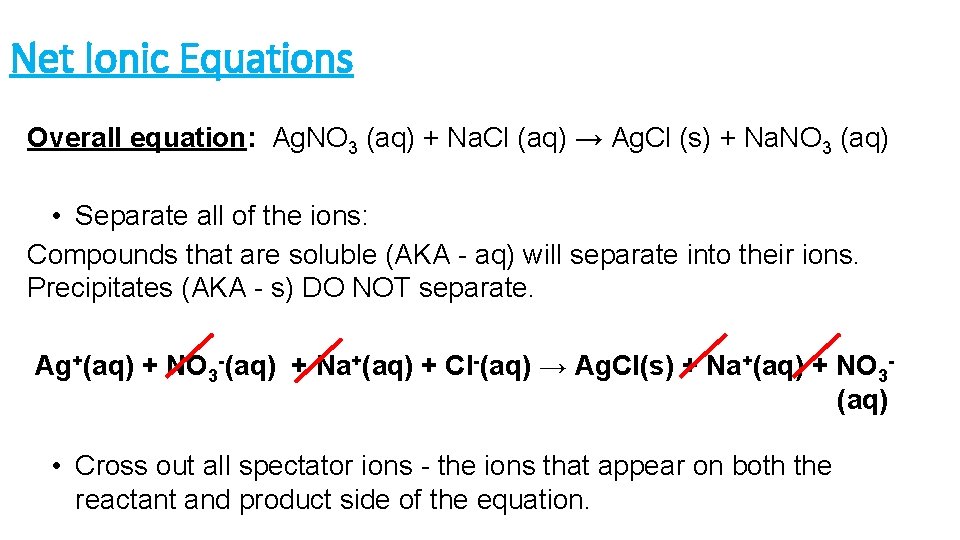
Net Ionic Equations Overall equation: Ag. NO 3 (aq) + Na. Cl (aq) → Ag. Cl (s) + Na. NO 3 (aq) • Separate all of the ions: Compounds that are soluble (AKA - aq) will separate into their ions. Precipitates (AKA - s) DO NOT separate. Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) → Ag. Cl(s) + Na+(aq) + NO 3(aq) • Cross out all spectator ions - the ions that appear on both the reactant and product side of the equation.

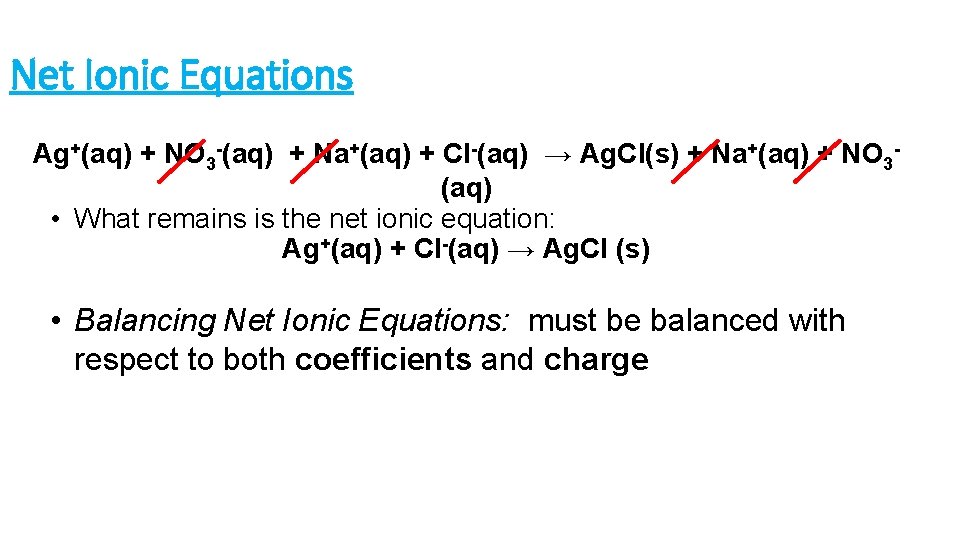
Net Ionic Equations Ag+(aq) + NO 3 -(aq) + Na+(aq) + Cl-(aq) → Ag. Cl(s) + Na+(aq) + NO 3(aq) • What remains is the net ionic equation: Ag+(aq) + Cl-(aq) → Ag. Cl (s) • Balancing Net Ionic Equations: must be balanced with respect to both coefficients and charge

Fe. Cl 3 (aq) + KOH (aq) → a. Predict the products of the following reaction. b. Write the FULL ionic equation. (Yes - the long one) c. Write the NET IONIC EQUATION. d. What are the spectator ions.

ANSWERS Fe. Cl 3 (aq) + KOH (aq) → a. Predict the products of the following reaction. Fe (OH )3+ KCl b. Write the FULL ionic equation. (Yes - the long one) Fe. Cl 3 (aq) + KOH (aq) → Fe (OH)3 (s) + KCL (aq) NOW… BALANCE Fe. Cl 3 + 3 KOH Fe(OH)3 + 3 KCL c. Write the NET IONIC EQUATION. Fe+3 (aq) + Cl-1 (aq) + 3 K+1 (aq) + 3 OH-1 (aq) -- Fe (OH)3 d. What are the spectator ions. Cl -, K+ +1 -1 + 3 K + 3 Cl (s) (aq)

Net Ionic Equations Practice from equations we just did

Today’s Agenda ? ? 1. Turn in Double Replacement lab to the tray ASAP! 2. Have out Study Guide homework to be checked. 3. We are going over the study guide. What questions do you have? 4. Re-Quiz 5. Reactions partner activity. TEST TOMORROW!!!!!