Redox equations Starter What is redox Redox what








- Slides: 19

Redox equations

Starter • What is redox?

Redox – what? Oxidation and reduction happen at the same time There is no net gain or loss of electrons. e You can’t just create them or destroy them!

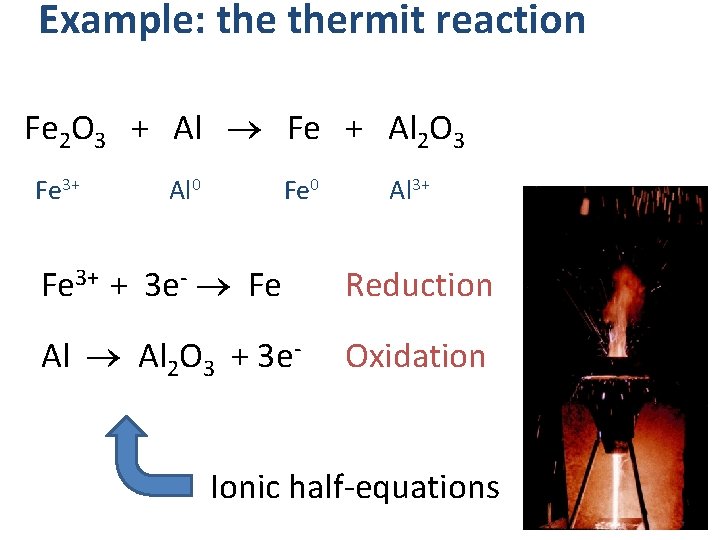
Example: thermit reaction Fe 2 O 3 + Al Fe + Al 2 O 3 Fe 3+ Al 0 Fe 0 Al 3+ Fe 3+ + 3 e- Fe Reduction Al Al 2 O 3 + 3 e- Oxidation Ionic half-equations

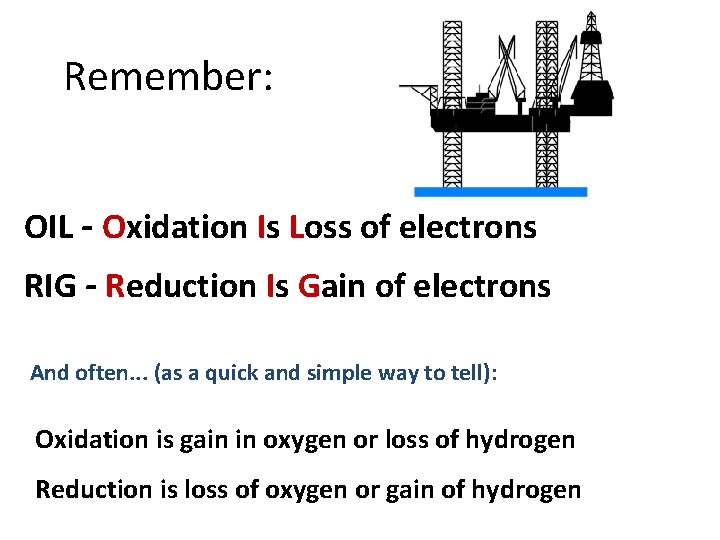
Remember: OIL - Oxidation Is Loss of electrons RIG - Reduction Is Gain of electrons And often. . . (as a quick and simple way to tell): Oxidation is gain in oxygen or loss of hydrogen Reduction is loss of oxygen or gain of hydrogen

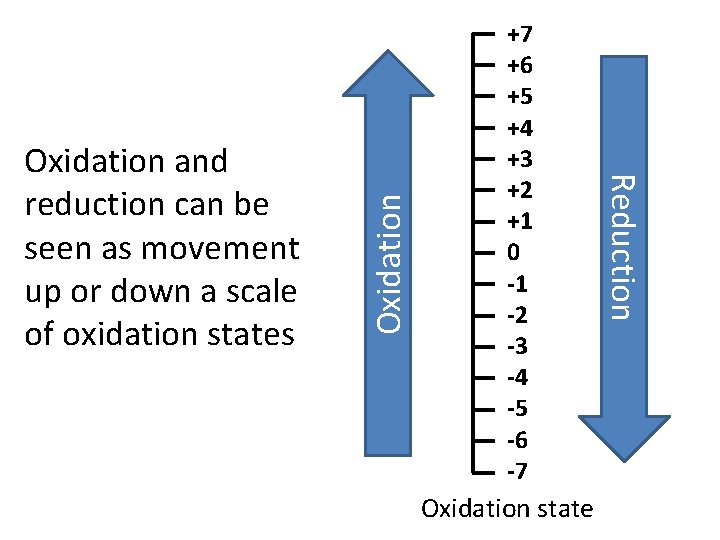
Oxidation state Oxidation Reduction Oxidation and reduction can be seen as movement up or down a scale of oxidation states +7 +6 +5 +4 +3 +2 +1 0 -1 -2 -3 -4 -5 -6 -7

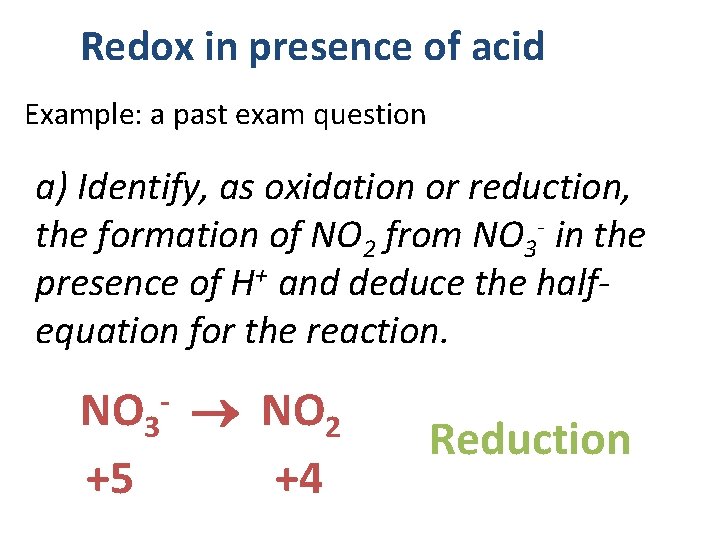
Redox in presence of acid Example: a past exam question a) Identify, as oxidation or reduction, the formation of NO 2 from NO 3 - in the presence of H+ and deduce the halfequation for the reaction. NO 3 NO 2 +5 +4 - Reduction

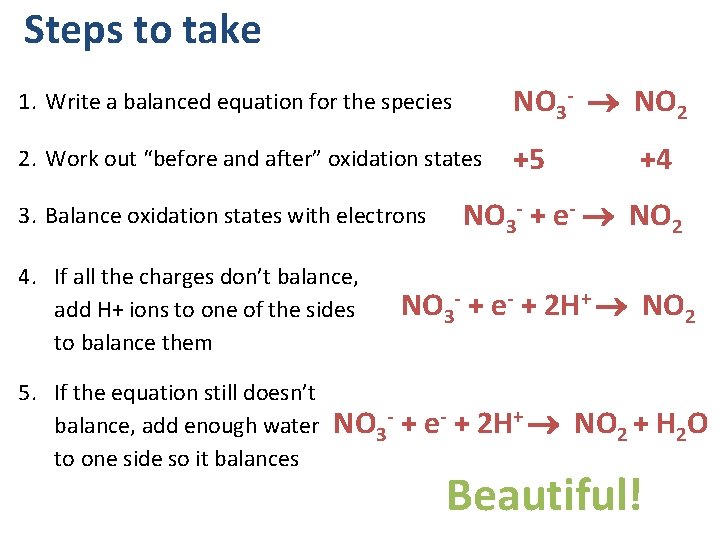
Steps to take 1. Write a balanced equation for the species NO 3 - NO 2 2. Work out “before and after” oxidation states +5 3. Balance oxidation states with electrons 4. If all the charges don’t balance, add H+ ions to one of the sides to balance them 5. If the equation still doesn’t balance, add enough water to one side so it balances +4 NO 3 - + e- NO 2 NO 3 - + e- + 2 H+ NO 2 + H 2 O Beautiful!

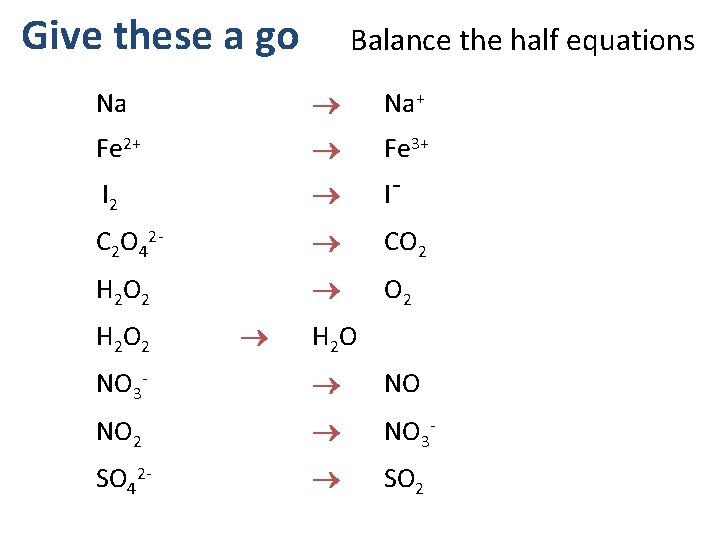
Give these a go Balance the half equations Na Na+ Fe 2+ Fe 3+ I¯ I 2 C 2 O 42 - CO 2 H 2 O 2 O 2 H 2 O 2 H 2 O NO 3 - NO 2 NO 3 - SO 42 - SO 2

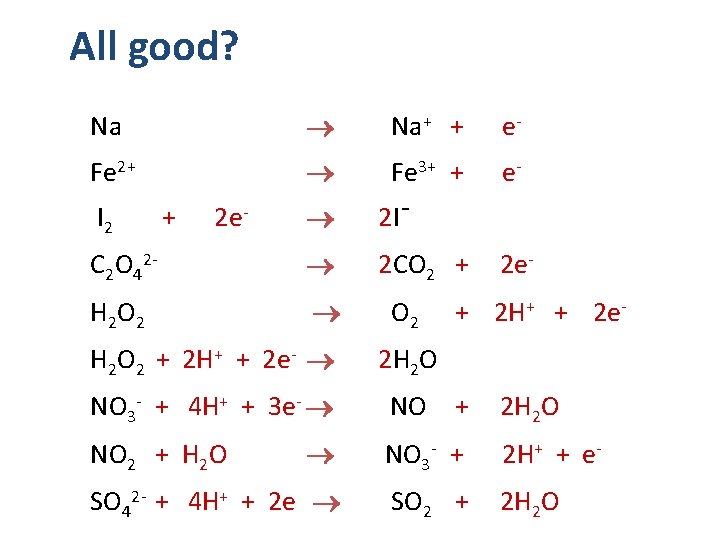
All good? Na+ + e- Fe 2+ Fe 3+ + e- I 2 + 2 e- 2 I¯ C 2 O 42 - 2 CO 2 + 2 e- Na H 2 O 2 O 2 H 2 O 2 + 2 H+ + 2 e- + 2 H + + 2 e- 2 H 2 O NO 3 - + 4 H+ + 3 e- NO + 2 H 2 O NO 2 + H 2 O NO 3 - + 2 H+ + e- SO 42 - + 4 H+ + 2 e SO 2 + 2 H 2 O

Exam Question • 1. Mercury thermometers are not used in some laboratories because of the danger of mercury vapour. This vapour is very easily absorbed through the lungs into the blood. In the blood, mercury reacts with hydrogen peroxide to form mercury(II) oxide. • • Hg + H 2 O 2 → Hg. O + H 2 O The mercury(II) oxide formed accumulates within organs in the body. • Use oxidation numbers to show that the reaction between mercury and hydrogen peroxide is an example of both oxidation and reduction. • [Total 2 marks]

Self marking • 1. Oxidation because oxidation state of Hg changes from 0 to +2 so oxidation (1) Reduction because oxidation number of O changes from – 1 to – 2 (1) • Or • Correct identification of all the oxidation numbers (1) Correct identification of oxidation and reduction (1) 2 • Allow ecf for the identification of oxidation and reduction from wrong oxidation numbers • [2]

Combining half equations Just a mashing together of two half-equations! . . . followed by some satisfying cancelling-out.

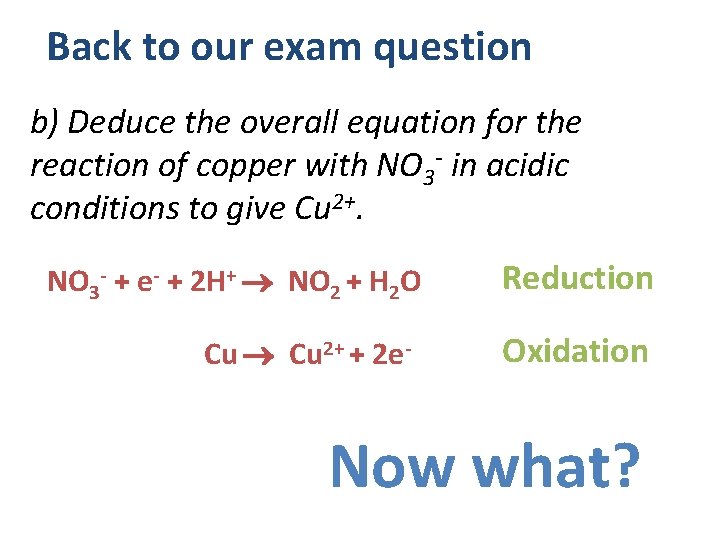
Back to our exam question b) Deduce the overall equation for the reaction of copper with NO 3 - in acidic conditions to give Cu 2+. NO 3 - + e- + 2 H+ NO 2 + H 2 O Cu 2+ + 2 e- Reduction Oxidation Now what?

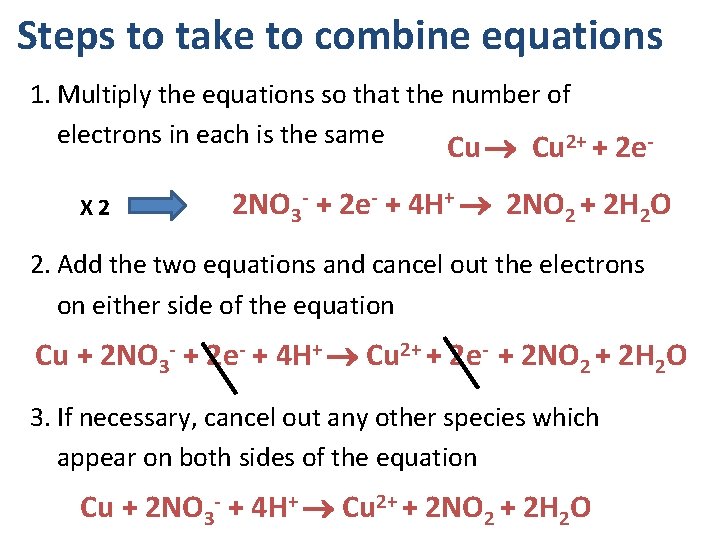
Steps to take to combine equations 1. Multiply the equations so that the number of electrons in each is the same 2+ Cu + 2 e- X 2 2 NO 3 - + 2 e- + 4 H+ 2 NO 2 + 2 H 2 O 2. Add the two equations and cancel out the electrons on either side of the equation Cu + 2 NO 3 - + 2 e- + 4 H+ Cu 2+ + 2 e- + 2 NO 2 + 2 H 2 O 3. If necessary, cancel out any other species which appear on both sides of the equation Cu + 2 NO 3 - + 4 H+ Cu 2+ + 2 NO 2 + 2 H 2 O

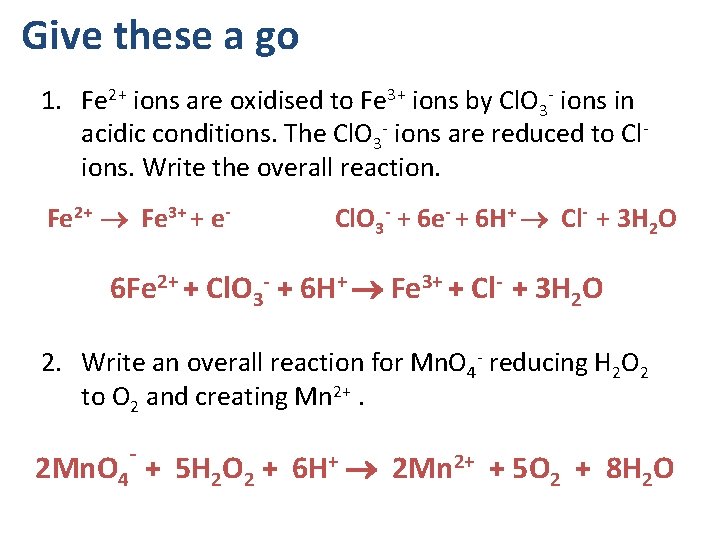
Give these a go 1. Fe 2+ ions are oxidised to Fe 3+ ions by Cl. O 3 - ions in acidic conditions. The Cl. O 3 - ions are reduced to Cl- ions. Write the overall reaction. Fe 2+ Fe 3+ + e- Cl. O 3 - + 6 e- + 6 H+ Cl- + 3 H 2 O 6 Fe 2+ + Cl. O 3 - + 6 H+ Fe 3+ + Cl- + 3 H 2 O 2. Write an overall reaction for Mn. O 4 - reducing H 2 O 2 to O 2 and creating Mn 2+. 2 Mn. O 4¯ + 5 H 2 O 2 + 6 H+ 2 Mn 2+ + 5 O 2 + 8 H 2 O

• 2. An unusual compound of iron has been detected on the surface of the planet Mars. This compound contains the ferrate(VI) ion. • When chlorine is bubbled through a suspension of iron(III) oxide in concentrated aqueous sodium hydroxide, a solution of aqueous sodium ferrate(VI) forms. • The two relevant redox systems are shown below. • Cl 2(aq) + 2 e– → 2 Cl–(aq) • Fe 2 O 3(s) + 10 OH–(aq) → 2 Fe. O 42–(aq) + 5 H 2 O(I) + 6 e– • Construct the redox equation for the reaction between chlorine, iron(III) oxide and hydroxide ions. • [Total 2 marks]

Self marking • 2. Fe 2 O 3 + 3 Cl 2 + 10 OH– → 2 Fe. O 42– + 5 H 2 O + 6 Cl– (2) 2 • Allow one mark if electrons shown Allow one mark if correct reactants and products but not balanced • [2] •

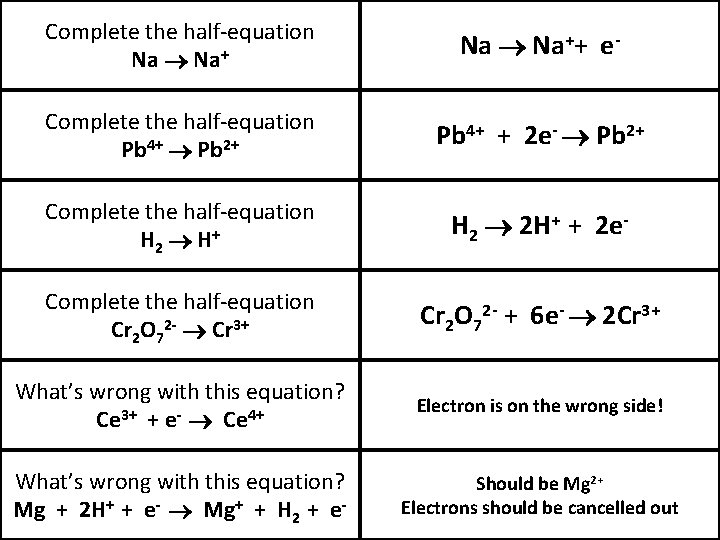
Complete the half-equation Na Na++ e- Complete the half-equation Pb 4+ Pb 2+ Pb 4+ + 2 e- Pb 2+ Complete the half-equation H 2 H+ H 2 2 H+ + 2 e- Complete the half-equation Cr 2 O 72 - Cr 3+ Cr 2 O 72 - + 6 e- 2 Cr 3+ What’s wrong with this equation? Ce 3+ + e- Ce 4+ Electron is on the wrong side! What’s wrong with this equation? Mg + 2 H+ + e- Mg+ + H 2 + e- Should be Mg 2+ Electrons should be cancelled out