Writing Ionic Formulas Ionic Compounds An ionic compound







- Slides: 12

Writing Ionic Formulas

Ionic Compounds § An ionic compound is always made up of a metal and a nonmetal. § Ionic compounds can be binary (made of just two elements), or contain one or more polyatomic ions as part of it (many different elements). § To determine the formula for an ionic compound, we will use the criss-cross method.

The Criss-Cross Method § The criss-cross method is an easy way to determine the formula of any ionic compound. § To write an ionic formula, you need the charge of each ion. § The charge is found by looking at your periodic table for most ions.

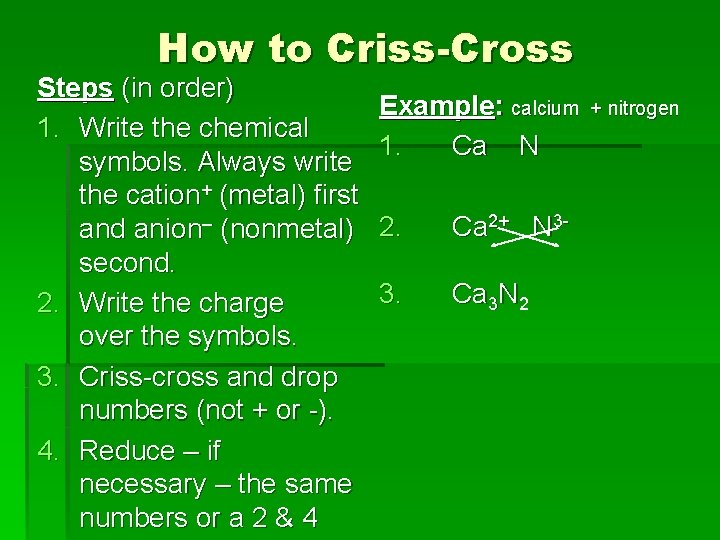
How to Criss-Cross Steps (in order) 1. Write the chemical symbols. Always write the cation+ (metal) first and anion– (nonmetal) second. 2. Write the charge over the symbols. 3. Criss-cross and drop numbers (not + or -). 4. Reduce – if necessary – the same numbers or a 2 & 4 Example: calcium 1. Ca N 2. Ca 2+ N 3 - 3. Ca 3 N 2 + nitrogen

How to Criss-Cross Steps (in order) 1. Write the chemical symbols. Always write the cation+ (metal) first and anion– (nonmetal) second. 2. Write the charge over the symbols. 3. Criss-cross and drop numbers (not + or -). 4. Reduce – if necessary – the same numbers or a 2 & 4 Example: aluminum + oxygen 1. Al O 2. Al 3+ O 2 - 3. Al 2 O 3

How to Criss-Cross Steps (in order) 1. Write the chemical symbols. Always write the cation+ (metal) first and anion– (nonmetal) second. 2. Write the charge over the symbols. 3. Criss-cross and drop numbers (not + or -). 4. Reduce – if necessary – the same numbers or a 2 & 4 Example: sodium + sulfur 1. Na S 2. Na 1+ S 2 - 3. Na 2 S 1’s are understood, you do not need to write them!

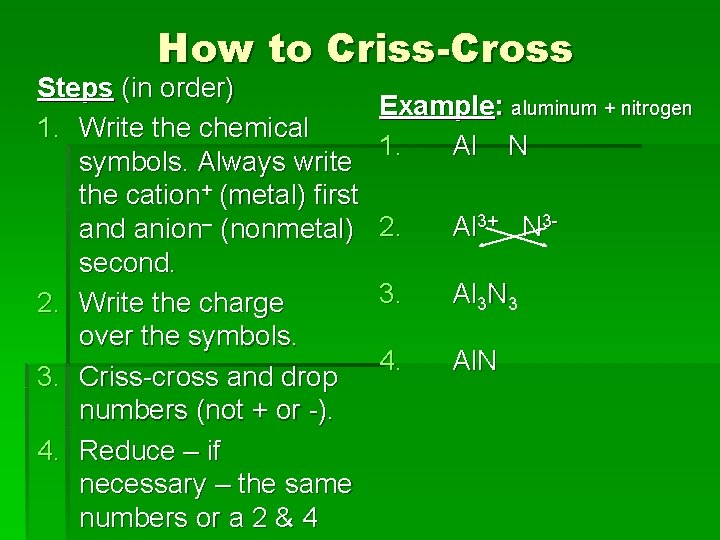
How to Criss-Cross Steps (in order) 1. Write the chemical symbols. Always write the cation+ (metal) first and anion– (nonmetal) second. 2. Write the charge over the symbols. 3. Criss-cross and drop numbers (not + or -). 4. Reduce – if necessary – the same numbers or a 2 & 4 Example: aluminum + nitrogen 1. Al N 2. Al 3+ N 3 - 3. Al 3 N 3 4. Al. N

Try These Formulas Use the criss-cross method to find the formulas for the following. Ions in Compound Chemical Formula § § § § § Beryllium + bromine Lithium + oxygen Potassium + nitrogen Calcium + sulfur Magnesium + nitrogen Aluminum + phosphorus Lithium + chlorine Magnesium + oxygen Calcium + iodine Be. Br 2 Li 2 O K 3 N Ca. S Mg 3 N 2 Al. P Li. Cl Mg. O Ca. I 2

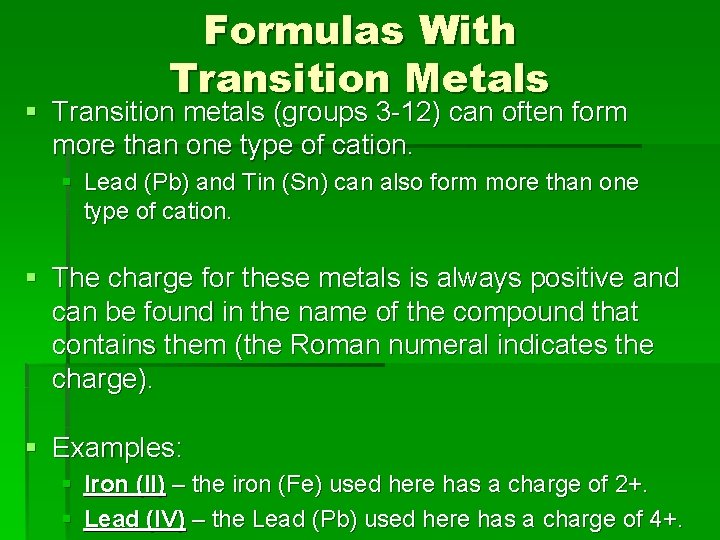
Formulas With Transition Metals § Transition metals (groups 3 -12) can often form more than one type of cation. § Lead (Pb) and Tin (Sn) can also form more than one type of cation. § The charge for these metals is always positive and can be found in the name of the compound that contains them (the Roman numeral indicates the charge). § Examples: § Iron (II) – the iron (Fe) used here has a charge of 2+. § Lead (IV) – the Lead (Pb) used here has a charge of 4+.

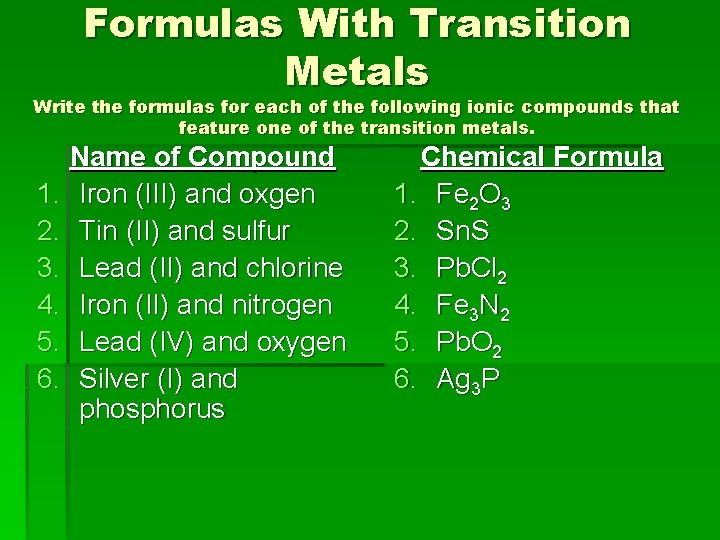
Formulas With Transition Metals Write the formulas for each of the following ionic compounds that feature one of the transition metals. Name of Compound 1. Iron (III) and oxgen 2. Tin (II) and sulfur 3. Lead (II) and chlorine 4. Iron (II) and nitrogen 5. Lead (IV) and oxygen 6. Silver (I) and phosphorus Chemical Formula 1. Fe 2 O 3 2. Sn. S 3. Pb. Cl 2 4. Fe 3 N 2 5. Pb. O 2 6. Ag 3 P

Polyatomic Ionic Compounds § Polyatomic ions are clusters of atoms that stay together as one unit and carry an overall charge. Most polyatomic ions are negatively charged. Parentheses § You may need more than one polyatomic ion in your formula…This means that you will have to use parentheses. Use parentheses whenever you need more than one polyatomic ion in the formula. § Example: Iron (III) nitrate… § Start with symbols and combining charges… Fe 3+ NO 31§ After the criss-cross, you may get one of two possibilities… Fe. NO 33 OR Fe(NO 3)3 § The second option is right because it says you need two nitrate clusters to go with every iron atom. The first option, which did not use brackets, reads as one iron atom with one nitrogen atom and thirty-three oxygen atoms. § Fe(NO 3)3 is the correct formula! Use parentheses!

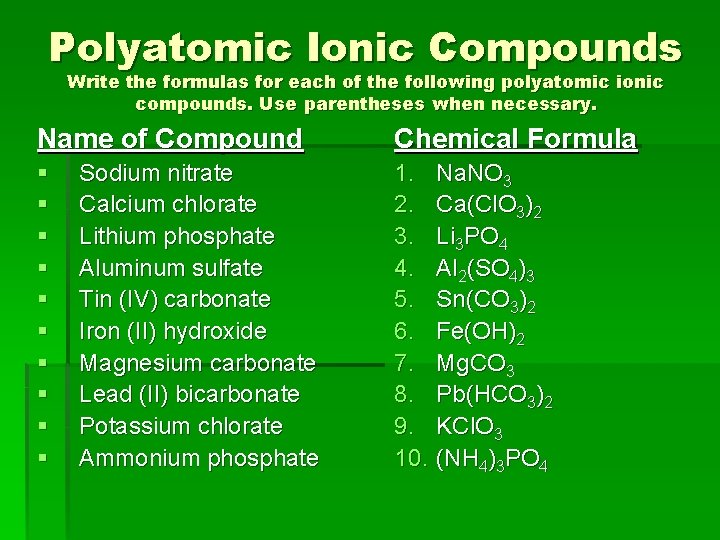
Polyatomic Ionic Compounds Write the formulas for each of the following polyatomic ionic compounds. Use parentheses when necessary. Name of Compound Chemical Formula § § § § § 1. Na. NO 3 2. Ca(Cl. O 3)2 3. Li 3 PO 4 4. Al 2(SO 4)3 5. Sn(CO 3)2 6. Fe(OH)2 7. Mg. CO 3 8. Pb(HCO 3)2 9. KCl. O 3 10. (NH 4)3 PO 4 Sodium nitrate Calcium chlorate Lithium phosphate Aluminum sulfate Tin (IV) carbonate Iron (II) hydroxide Magnesium carbonate Lead (II) bicarbonate Potassium chlorate Ammonium phosphate